Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. The Dose of Methyl Donors and BPA
2.3. Sample Collection
2.4. Analysis of Intestinal Morphology
2.5. Analysis of Intestinal Enzyme Activity
2.6. RT-PCR Analysis
2.7. MassARRAY-Quantitative DNA Methylation Analysis
2.8. Statistical Analysis
3. Results
3.1. Intestinal Index
3.2. Intestinal Morphology
3.3. Disaccharidase Activity
3.4. Nutrient Transporter Gene Expression
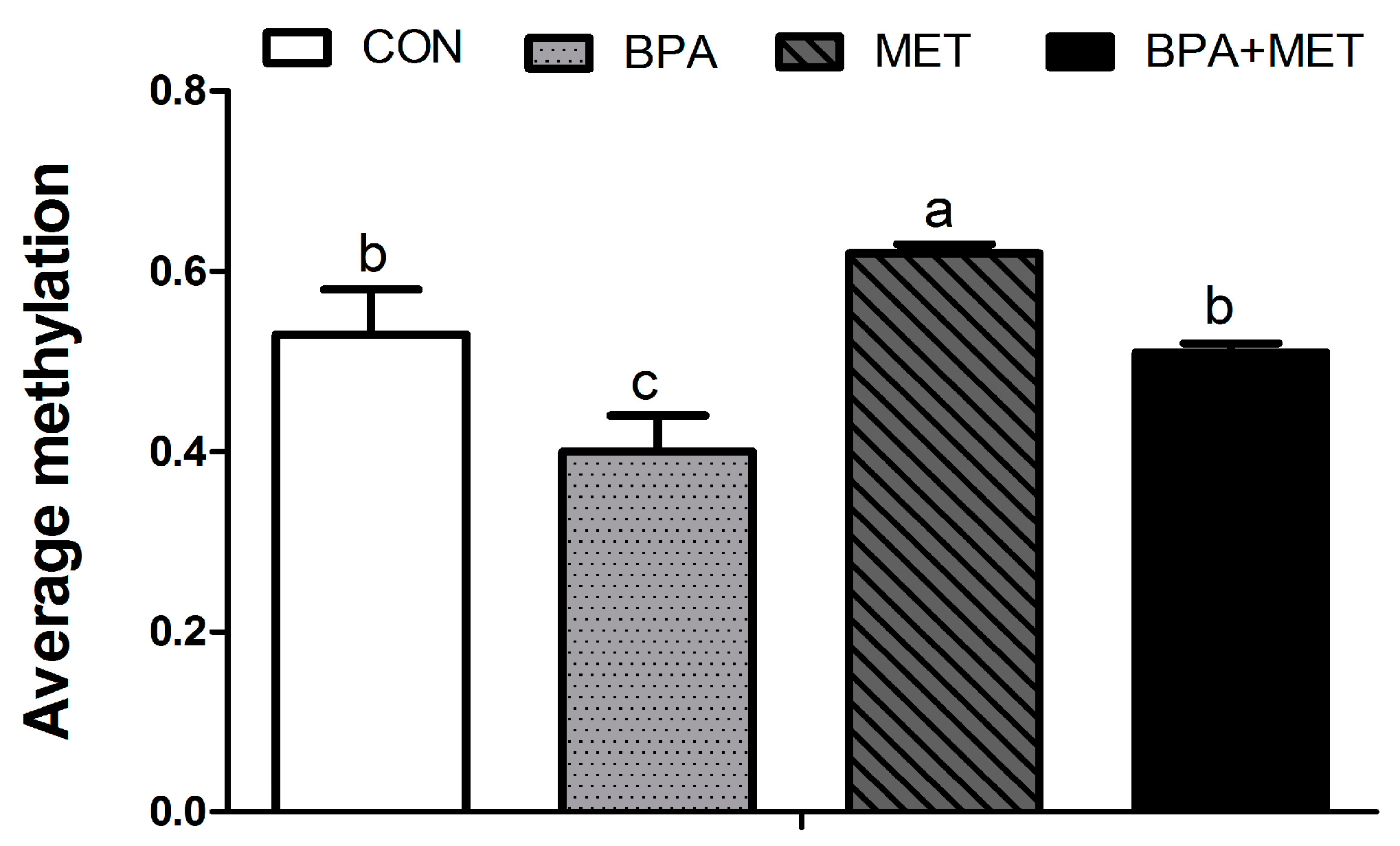
3.5. CpG Methylation
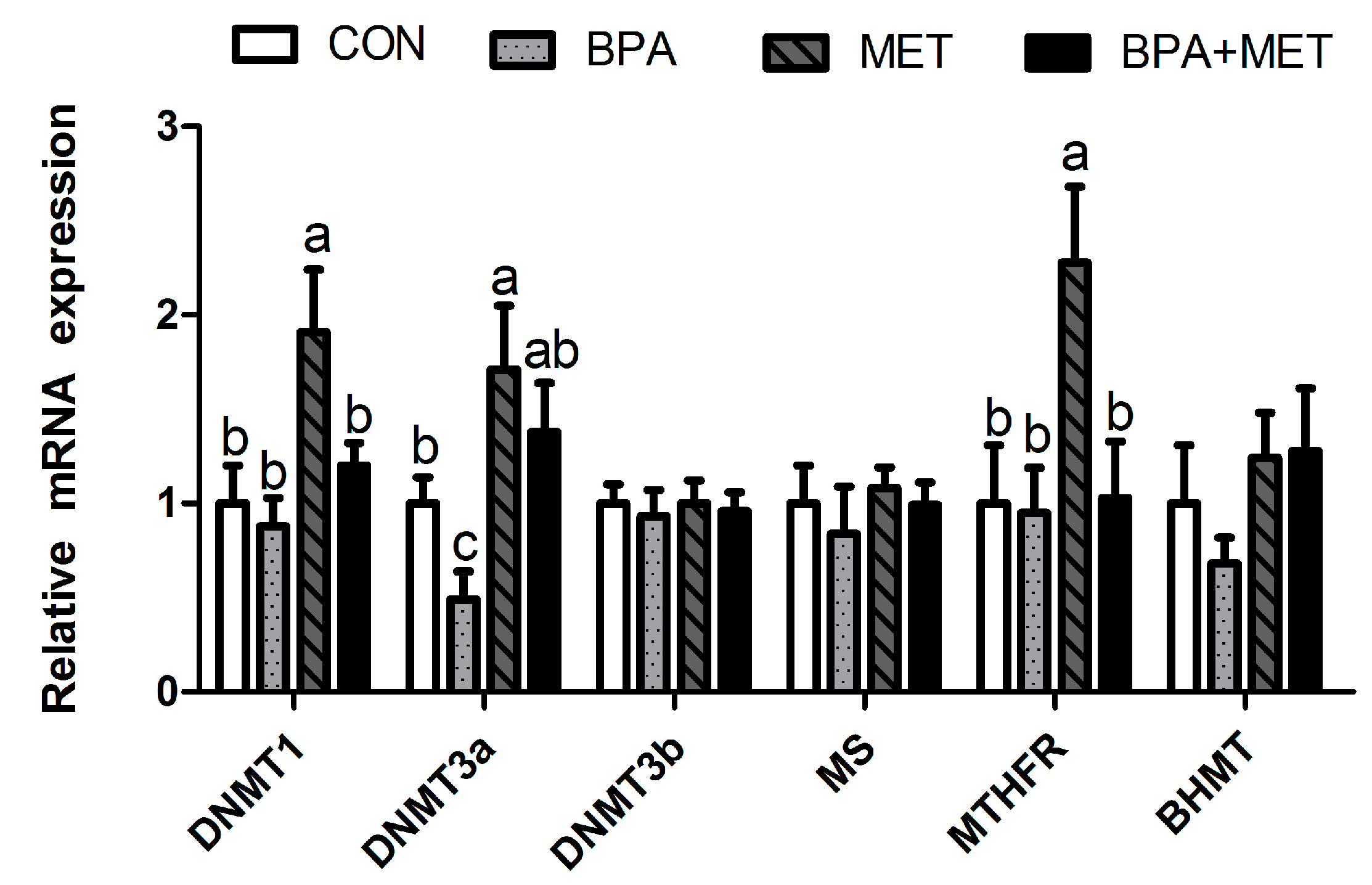
3.6. Methylation-Related Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Brede, C.; Fjeldal, P.; Skjevrak, I.; Herikstad, H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Brotons, J.A.; Olea-Serrano, M.F.; Villalobos, M.; Pedraza, V.; Olea, N. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 1995, 103, 608. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J., Jr. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, A703. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Takamoto, M.; Sugane, K. Exposure to bisphenol A prenatally or in adulthood promotes TH2 cytokine production associated with reduction of CD4+CD25+ regulatory T cells. Environ. Health Perspect. 2008, 116, 514. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S. Bisphenol a: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xia, W.; Wang, D.Q.; Wan, Y.J.; Xu, B.; Chen, X.; Li, Y.Y.; Xu, S.Q. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia 2013, 56, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Kundakovica, M.; Gudsnuka, M.K.; Franksa, B.; Madrida, J.; Millerb, R.L.; Pererab, F.P.; Champagnea, F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Itoh, K.; Yaoi, T.; Fujiwara, Y.; Sugimoto, T.; Fushiki, S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of bisphenol A. J. Neurosci. Res. 2006, 84, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Bromer, J.G.; Zhou, Y.; Taylor, M.B.; Doherty, L.; Taylor, H.S. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010, 24, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed]
- Cropley, J.E.; Suter, C.M.; Beckman, K.B.; Martin, D.I. Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc. Natl. Acad. Sci. USA 2006, 103, 17308–17312. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Travisano, M.; Tahiliani, K.G. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. FASEB J. Off. Publ. Federation Am. Soc. Exp. Biol. 2007, 21, 3380–3385. [Google Scholar] [CrossRef] [PubMed]
- Matte, J.J.; Girard, C.L.; Brisson, G.J. The role of folic acid in the nutrition of gestating and lactating primiparous sows. Livest. Prod. Sci. 1992, 32, 131–148. [Google Scholar] [CrossRef]
- van Wettere, W.H.E.J.; Herde, P.; Hughes, P.E. Supplementing sow gestation diets with betaine during summer increases litter size of sows with greater numbers of parities. Anim. Reprod. Sci. 2012, 132, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kornegay, E.; Meacham, T. Evaluation of supplemental choline for reproducing sows housed in total confinement on concrete or in dirt lots. J. Anim. Sci. 1973, 37, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Stockland, W.; Blaylock, L. Choline requirement of pregnant sows and gilts under restricted feeding conditions. J. Anim. Sci. 1974, 39, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Matte, J.; Guay, F.; Girard, C. Folic acid and vitamin B12 in reproducing sows: New concepts. Can. J. Anim. Sci. 2006, 86, 197–205. [Google Scholar] [CrossRef]
- Li, G.; Chang, H.; Xia, W.; Mao, Z.; Li, Y.; Xu, S. F0 maternal BPA exposure induced glucose intolerance of F2 generation through DNA methylation change in Gck. Toxicol. Lett. 2014, 228, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.; Williams, I.; Aherne, F. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 1996, 62, 131–144. [Google Scholar] [CrossRef]
- Mariadason, J.M.; Nicholas, C.; Kaitlin, E.; Zhuang, M.; Smartt, H.J.; Heerdt, B.G.; Yang, W.; Corner, G.A.; Wilson, A.J.; Klampfer, L. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 2005, 128, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Ota, F.; Hosoi, K.; Kato, M.; Sakai, T.; Satter, M.A. Altered allergic cytokine and antibody response in mice treated with bisphenol A. J. Med. Investig. 2006, 53, 70–80. [Google Scholar] [CrossRef]
- Goto, M.; Takano-Ishikawa, Y.; Ono, H.; Yoshida, M.; Yamaki, K.; Shinmoto, H. Orally administered bisphenol A disturbed antigen specific immunoresponses in the naive condition. Biosci. Biotechnol. Biochem. 2007, 71, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Kagnoff, M.F. Mucosal immunology: New frontiers. Immunol. Today 1996, 17, 57–59. [Google Scholar] [CrossRef]
- Iwanaga, T. The involvement of macrophages and lymphocytes in the apoptosis of enterocytes. Arch. Histol. Cytol. 1995, 58, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.J. Ontogeny of enzymes in the small intestine. Ann. Rev. Physiol. 1985, 47, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, M.; Zabielski, R.; Grenier, B.; Le Normand, L.; Savary, G.; Holst, J.J.; Oswald, I.P.; Metges, C.C.; Guilloteau, P. Steuctural and functional development of small intestine in inteauterin growth retarded porcine offspring born to gilts fed diet with differing protein ratios throughout pregnancy. J. Physiol. Pharmacol. 2012, 63, 225–239. [Google Scholar] [PubMed]
- Osswald, C.; Baumgarten, K.; Stumpel, F.; Gorboulev, V.; Akimjanova, M.; Knobeloch, K.P.; Horak, I.; Kluge, R.; Joost, H.G.; Koepsell, H. Mice without the regulator gene Rsc1A1 exhibit increased Na+-D-glucose cotransport in small intestine and develop obesity. Mol. Biol. Cell 2005, 25, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.H.; Gum, J.R.; Lindstrom, M.M.; McKean, D.; Kim, Y.S. Regional expression and dietary regulation of rat small intestinal peptide and amino acid transporter mRNAs. Biochem. Biophys. Res. Commun. 1995, 216, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Taylor, H.S. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007, 21, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, G.; Nguyen, H.T.T.; Yan, Y.; Charrier-Hisamuddin, L.; Sitaraman, S.V.; Merlin, D. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS ONE 2008, 3, e2476. [Google Scholar] [CrossRef] [PubMed]
- Shimakura, J.; Terada, T.; Katsura, T.; Inui, K. Characterization of the human peptide transporter PEPT1 promoter: Sp1 functions as a basal transcriptional regulator of human PEPT1. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G471–G477. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.M.; Bird, A. CpG islands and the regulation of transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Kundakovic, M.; Champagne, F.A. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav. Immun. 2011, 25, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Bressenot, A.; Pooya, S.; Bossenmeyer-Pourie, C.; Gauchotte, G.; Germain, A.; Chevaux, J.B.; Coste, F.; Vignaud, J.M.; Gueant, J.L.; Peyrin-Biroulet, L. Methyl donor deficiency affects small-intestinal differentiation and barrier function in rats. Br. J. Nutr. 2013, 109, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.J.; Sugawara, M.; Liu, J.C.; Li, H.W.; Ganapathy, V.; Ganapathy, M.E.; Leibach, F.H. cDNA structure, genomic organization, and promoter analysis of the mouse intestinal peptide transporter PEPT1. Biochim. Biophys. Acta 2000, 1492, 145–154. [Google Scholar] [CrossRef]
- Schubeler, D. Function and information content of DNA methylation. Nature 2015, 517, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Homocysteine metabolism. Ann. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Bostom, A.G.; Williams, R.R.; Ellison, R.C.; Eckfeldt, J.H.; Rosenberg, I.H.; Selhub, J.; Rozen, R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996, 93, 7–9. [Google Scholar] [CrossRef] [PubMed]
| Item | Gestation | Lactation |
|---|---|---|
| Ingredients (g/kg) | ||
| Corn | 636.15 | 629.00 |
| Soybean meal | 145.00 | 226.00 |
| Fish meal | 25.00 | |
| Soybean oil | 30.00 | |
| Wheat bran | 180.00 | 50.00 |
| L-lysine·HCl (98%) | 0.80 | 2.70 |
| D-Methionine (98%) | 1.10 | |
| Calcium carbonate | 11.10 | 10.00 |
| Dicalcium phosphate | 13.80 | 10.70 |
| Sodium bicarbonate | 4.00 | |
| Choline (50%) | 1.00 | |
| Salt | 3.00 | 4.00 |
| Vitamin and Mineral Premix * | 10.15 | 6.50 |
| Total | 1000.00 | 1000.00 |
| Nutrient level † | ||
| Digestible energy, Mcal/kg | 3.05 | 3.35 |
| Crude protein, % | 14.00 | 17.50 |
| Total Lysine, % | 0.69 | 1.12 |
| Standard ileal digestible-Lysine, % | 0.60 | 1.00 |
| Total calcium, % | 0.80 | 0.80 |
| Total phosphorus, % | 0.68 | 0.63 |
| Genes | Gene Bank No. | Sequences (5′-3′) |
|---|---|---|
| β-actin | AY550069.1 | Forward: CCAGCACGATGAAGATCAAGA Reverse: AATGCAACTAACAGTCCGCCTA |
| Slc7a9 | NM_001110171.1 | Forward: GAACCCAAGACCACAAATC Reverse: ACCCAGTGTCGCAAGAAT |
| Pept1 | AY180903.1 | Forward: GATGAAATGTGAGCGTATGGG Reverse: AAAGAGGGAGGATCTGGAAAA |
| Sglt1 | NM_001164021.1 | Forward: CCACTTTCCCTATAAAACCTCAC Reverse: CTCCATCAAACTTCCATCCTCAG |
| Glut2 | NM_001097417.1 | Forward:CCTGCTTGGTCTATCTGCTGTG Reverse:TTGATGCTTCTTCCCTTTCTTT |
| LCT | XM_003359430.4 | Forward: TGTGCAGCGGTTTAAGGAGTAT Reverse: CCACAACAAAGGGCTCATTCAG |
| SUC | XM_013990124.1 | Forward: TTATCCGACCCCTTTTGCATGA Reverse: CGAGCATTAGGGACATAGCCTT |
| MGAM | XM_005657730.2 | Forward: AGGCATCCAATTCTTCTGGAGT Reverse: GGCCCCAAATGAGTCATACTGA |
| DNMT1 | DQ060156.1 | Forward: TTTCGTCTCCTTCAAGCGCT Reverse: CCATACTGACCAGCCTGCAA |
| DNMT3a | DQ785811.1 | Forward: AGTGCGTGGATCTCTTGGTG Reverse: TCCTGGTCGTGGTTATTGGC |
| DNMT3b | NM_001162404.1 | Forward: TGAAGAGTCCATCGCTGTTG Reverse: CAATCACCAGGTCAAAGGG |
| MS | AF276463 | Forward: AGCTTTGTTCGCAGTCCAGA Reverse: AAGGTCTCATTTCGGCTGCA |
| MTHFR | AF239166 | Forward: CAGTGGGAAGCAGAGGAAGG Reverse: GCCACACAGACGTCGAAGTA |
| BHMT | NM_001200042.1 | Forward: GAGGCTGTGTGGGCAGTTGAAG Reverse: ACAATGGATGCTCCTGCCTTTACC |
| Assays | Sequences (5′-3′) | Location |
|---|---|---|
| Pept1-2 | Forward: GTGGGGTTAGATTTTTTTAAAATGG Reverse: AAAAAAAACCACATCCACAAATAAA | −968 to −1481 |
| Pept1-10 | Forward: GTTTATTTGGGTGGAGATTGTTTAG Reverse: ACCCTTAACCCAATAAATAAAACCA | −1450 to−1089 |
| Pept1-14 | Forward: AGTTTATATTTGGTGTGGTTGTGGT Reverse: CCCACCTCCCTATATTAACAAAAAA | −1050 to −599 |
| Pept1-20 | Forward: GTTGGGTGTTAGGTATTTTTAAGGG Reverse: AACAAAACCAACTATAAAACTCCCA | −390 to +75 |
| Treatment | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| CON | BPA | MET | BPA + MET | BPA | MET | BPA × MET | |
| Newborn | |||||||
| Body weight, kg | 1.27 ± 0.02 b | 1.38 ± 0.02 a | 1.39 ± 0.01 a | 1.38 ± 0.02 a | 0.04 | 0.01 | 0.01 |
| SI (g) | 41.77 ± 3.11 | 38.78 ± 3.02 | 42.76 ± 4.10 | 43.48 ± 3.12 | 0.70 | 0.33 | 0.53 |
| SI (cm) | 389.49 ± 21.02 | 371.37 ± 23.77 | 370.20 ± 19.54 | 356.05 ± 20.68 | 0.31 | 0.27 | 0.90 |
| SI (g × kg−1 BW) | 32.56 ± 2.09 | 28.08 ± 1.98 | 30.83 ± 3.37 | 31.37 ± 2.72 | 0.32 | 0.69 | 0.21 |
| SI (cm × kg−1 BW) | 306.27 ± 24.02 a | 269.39 ± 15.05 b | 266.32 ± 16.07 b | 258.22 ± 16.51 b | 0.06 | 0.03 | 0.20 |
| SI weight/length (mg/cm) | 106.09 ± 7.02 b | 104.35 ± 7.42 b | 116.14 ± 6.58 a | 122.80 ± 8.00 a | 0.69 | 0.02 | 0.49 |
| Weaning | |||||||
| Body weight, kg | 6.54 ± 0.09 c | 7.01 ± 0.28 b | 7.54 ± 0.13 a | 7.13 ± 0.09 b | 0.86 | 0.00 | 0.01 |
| SI (g) | 190.18 ± 14.05 | 200.28 ± 13.99 | 220.62 ± 12.57 | 192.82 ± 13.78 | 0.77 | 0.62 | 0.44 |
| SI (cm) | 935.67 ± 45.44 | 954.38 ± 49.22 | 964.18 ± 39.49 | 958.73 ± 45.43 | 0.85 | 0.64 | 0.73 |
| SI (g × kg−1 BW) | 29.09 ± 3.22 | 29.85 ± 4.12 | 28.06 ± 5.13 | 28.39 ± 2.79 | 0.82 | 0.60 | 0.93 |
| SI (cm × kg−1 BW) | 143.25 ± 11.54 a | 138.89 ± 14.32 a | 126.91 ± 13.97 b | 134.32 ± 15.07 a,b | 0.81 | 0.05 | 0.35 |
| SI weight/length (mg/cm) | 203.47 ± 27.36 b | 215.85 ± 29.20 a,b | 231.30 ± 30.32 a | 221.60 ± 20.01 a | 0.93 | 0.04 | 0.50 |
| Treatment | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| CON | BPA | MET | BPA + MET | BPA | MET | BPA × MET | |
| Newborn | |||||||
| Duodenum | |||||||
| Villus height (μm) | 479.86 ± 33.57 | 442.69 ± 37.11 | 522.68 ± 39.52 | 512.70 ± 34.79 | 0.70 | 0.37 | 0.83 |
| Crypt depth (μm) | 125.57 ± 11.50 | 126.45 ± 13.47 | 127.25 ± 12.98 | 131.08 ± 14.01 | 0.78 | 0.71 | 0.86 |
| Villus/crypt ratio | 3.87 ± 0.43 | 3.50 ± 0.32 | 3.94 ± 0.27 | 4.12 ± 0.38 | 0.85 | 0.49 | 0.59 |
| Jejunum | |||||||
| Villus height (μm) | 639.99 ± 59.30 b | 649.53 ± 61.37 b | 740.11 ± 60.54 a | 717.39 ± 55.83 a | 0.75 | 0.05 | 0.82 |
| Crypt depth (μm) | 104.73 ± 6.91 | 114.33 ± 7.12 | 102.18 ± 5.90 | 99.11 ± 7.01 | 0.61 | 0.17 | 0.33 |
| Villus/crypt ratio | 5.94 ± 0.62 b | 4.19 ± 0.57 c | 7.99 ± 0.52 a | 8.69 ± 0.71 a | 0.04 | 0.02 | 0.04 |
| Ileum | |||||||
| Villus height (μm) | 579.19 ± 50.11 | 530.19 ± 53.25 | 620.89 ± 51.33 | 653.98 ± 52.17 | 0.92 | 0.31 | 0.61 |
| Crypt depth (μm) | 111.18 ± 13.29 | 122.27 ± 10.12 | 95.33 ± 9.02 | 127.51 ± 9.74 | 0.07 | 0.64 | 0.36 |
| Villus/crypt ratio | 5.58 ± 0.54 b | 5.19 ± 0.62 b | 6.45 ± 0.56 a | 5.86 ± 0.48 a,b | 0.86 | 0.03 | 0.75 |
| Weaning | |||||||
| Duodenum | |||||||
| Villus height (μm) | 287.91 ± 22.89 | 277.98 ± 25.37 | 324.57 ± 21.09 | 308.72 ± 19.88 | 0.73 | 0.36 | 0.94 |
| Crypt depth (μm) | 178.45 ± 10.30 | 171.55 ± 9.02 | 174.37 ± 11.06 | 183.46 ± 13.00 | 0.95 | 0.82 | 0.64 |
| Villus/crypt ratio | 1.74 ± 0.12 | 1.62 ± 0.22 | 1.96 ± 0.15 | 1.89 ± 0.12 | 0.61 | 0.20 | 0.90 |
| Jejunum | |||||||
| Villus height (μm) | 264.71 ± 19.03 | 243.04 ± 21.24 | 331.47 ± 24.01 | 274.58 ± 20.59 | 0.27 | 0.17 | 0.61 |
| Crypt depth (μm) | 132.93 ± 9.77 | 142.21 ± 10.02 | 133.97 ± 8.29 | 120.26 ± 9.15 | 0.84 | 0.34 | 0.30 |
| Villus/crypt ratio | 2.04 ± 0.15 b | 1.51 ± 0.12 c | 2.54 ± 0.12 a | 2.60 ± 0.13 a | 0.02 | 0.03 | 0.48 |
| Ileum | |||||||
| Villus height (μm) | 206.80 ± 39.56 | 233.29 ± 33.47 | 291.58 ± 36.90 | 314.50 ± 35.36 | 0.67 | 0.20 | 0.99 |
| Crypt depth (μm) | 113.45 ± 7.89 | 129.57 ± 8.03 | 122.36 ± 9.76 | 106.86 ± 12.04 | 0.98 | 0.60 | 0.24 |
| Villus/crypt ratio | 1.84 ± 0.08 b | 1.83 ± 0.08 b | 2.58 ± 0.09 a | 1.85 ± 0.010 b | 0.64 | 0.02 | 0.02 |
| Treatment | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| CON | BPA | MET | BPA + MET | BPA | MET | BPA × MET | |
| Newborn Duodenum | |||||||
| Lactase (U/mgprotein) | 189.27 ± 31.20 | 185.04 ± 34.18 | 191.52 ± 36.03 | 187.91 ± 40.85 | 0.91 | 0.94 | 0.99 |
| Maltase (U/mgprotein) | 6.99 ± 1.18 | 6.76 ± 1.04 | 7.70 ± 0.99 | 7.42 ± 1.10 | 0.82 | 0.53 | 0.98 |
| Sucrase (U/mgprotein) | 1.75 ± 0.63 | 1.89 ± 0.77 | 2.43 ± 0.73 | 2.05 ± 0.98 | 0.88 | 0.60 | 0.75 |
| Jejunum | |||||||
| Lactase (U/mgprotein) | 167.50 ± 28.77 b | 166.28 ± 27.43 b | 193.07 ± 30.33 a | 184.18 ± 30.33 a | 0.86 | 0.04 | 0.05 |
| Maltase (U/mgprotein)) | 9.02 ± 1.10 | 8.91 ± 1.10 | 7.46 ± 1.16 | 8.34 ± 1.05 | 0.72 | 0.35 | 0.65 |
| Sucrase (U/mgprotein) | 1.73 ± 0.24 a | 1.00 ± 0.27 b | 1.80 ± 0.23 a | 1.10 ± 0.23 a,b | <0.01 | 0.74 | 0.95 |
| Ileum | |||||||
| Lactase (U/mgprotein) | 29.23 ± 5.03 | 24.33 ± 4.77 | 30.48 ± 4.55 | 31.27 ± 6.16 | 0.69 | 0.43 | 0.59 |
| Maltase (U/mgprotein)) | 8.82 ± 1.10 | 5.86 ± 1.04 | 7.83 ± 0.95 | 8.62 ± 1.17 | 0.08 | 0.28 | 0.75 |
| Sucrase (U/mgprotein) | 1.22 ± 0.19 | 0.92 ± 0.18 | 0.91 ± 0.21 | 0.97 ± 0.22 | 0.58 | 0.52 | 0.38 |
| Weaning Duodenum | |||||||
| Lactase (U/mgprotein)) | 24.55 ± 10.67 | 32.45 ± 11.93 | 46.93 ± 10.67 | 35.13 ± 11.93 | 0.87 | 0.29 | 0.40 |
| Maltase (U/mgprotein) | 49.72 ± 9.44 | 48.05 ± 9.34 | 50.66 ± 12.19 | 45.75 ± 10.56 | 0.76 | 0.95 | 0.88 |
| Sucrase (U/mgprotein) | 7.71 ± 2.18 b | 8.61 ± 2.44 b | 15.84 ± 2.41 a | 12.35 ± 2.82 a | 0.75 | 0.02 | 0.51 |
| Jejunum | |||||||
| Lactase (U/mgprotein) | 48.22 ± 8.98 b | 47.18 ± 11.59 b | 72.03 ± 8.20 a | 67.03 ± 11.59 a,b | 0.85 | 0.05 | 0.05 |
| Maltase (U/mgprotein) | 121.33 ± 23.82 | 102.37 ± 29.17 | 131.34 ± 23.32 | 143.15 ± 29.17 | 0.55 | 0.84 | 0.20 |
| Sucrase (U/mgprotein) | 51.61 ± 13.50 b | 43.53 ± 9.53 b | 81.90 ± 13.50 a | 69.98 ± 13.50 a,b | 0.07 | 0.01 | 0.22 |
| Ileum | |||||||
| Lactase (U/mgprotein) | 5.99 ± 2.51 | 5.13 ± 2.49 | 6.64 ± 2.81 | 6.35 ± 2.61 | 0.56 | 0.50 | 0.97 |
| Maltase (U/mgprotein) | 80.81 ± 18.40 | 50.25 ± 18.40 | 83.58 ± 18.40 | 73.34 ± 16.79 | 0.27 | 0.48 | 0.58 |
| Sucrase (U/mgprotein) | 35.42 ± 11.98 | 29.20 ± 10.32 | 32.33 ± 11.42 | 39.14 ± 10.94 | 0.98 | 0.77 | 0.59 |
| Treatment | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| CON | BPA | MET | BPA + MET | BPA | MET | BPA × MET | ||
| Newborn | ||||||||
| Slc7a9 | 1.00 ± 0.32 | 0.86 ± 0.18 | 1.15 ± 0.18 | 0.97 ± 0.21 | 0.56 | 0.64 | 0.94 | |
| Pept1 | 1.00 ± 0.26 b | 0.54 ± 0.10 c | 2.35 ± 0.22 a | 1.07 ± 0.21 b | <0.01 | <0.01 | 0.11 | |
| Sglt1 | 1.00 ± 0.10 c | 1.16 ± 0.14 b,c | 2.37 ± 0.21 a | 1.59 ± 0.18 b | 0.10 | <0.01 | 0.01 | |
| Glut2 | 1.00 ± 0.23 | 0.73 ± 0.17 | 1.40 ± 0.27 | 0.83 ± 0.18 | 0.09 | 0.31 | 0.54 | |
| LCT | 1.00 ± 0.14 c | 1.24 ± 0.16 b,c | 2.90 ± 0.25 a | 1.89 ± 0.10 b | 0.67 | <0.01 | 0.05 | |
| SUC | 1.00 ± 0.13 b | 0.53 ± 0.08 c | 1.76 ± 0.12 a | 1.35 ± 0.06 a,b | 0.04 | 0.11 | 0.20 | |
| MGAM | 1.00 ± 0.09 | 1.42 ± 0.13 | 1.62 ± 0.17 | 1.22 ± 0.20 | 0.34 | 0.29 | 0.57 | |
| Weaning | ||||||||
| Slc7a9 | 1.00 ± 0.11 | 0.97 ± 0.26 | 0.99 ± 0.17 | 1.02 ± 0.19 | 1.00 | 0.92 | 0.87 | |
| Pept1 | 1.00 ± 0.35 b,c | 0.40 ± 0.10 c | 1.84 ± 0.22 a | 1.37 ± 0.35 a,b | 0.05 | <0.01 | 0.81 | |
| Sglt1 | 1.00 ± 0.18 b | 0.88 ± 0.29 b | 2.14 ± 0.40 a | 1.25 ± 0.31 a,b | 0.13 | 0.03 | 0.24 | |
| Glut2 | 1.00 ± 0.23 | 1.03 ± 0.16 | 1.42 ± 0.32 | 1.30 ± 0.34 | 0.87 | 0.24 | 0.79 | |
| LCT | 1.00 ± 0.13 b | 0.85 ± 0.15 b | 1.73 ± 0.27 a | 0.93 ± 0.09 b | 0.71 | 0.04 | 0.91 | |
| SUC | 1.00 ± 0.12 | 1.02 ± 0.09 | 1.15 ± 0.13 | 1.41 ± 0.07 | 0.95 | 0.89 | 0.09 | |
| MGAM | 1.00 ± 0.09 | 1.18 ± 0.12 | 1.28 ± 0.16 | 1.20 ± 0.21 | 0.77 | 0.65 | 0.81 | |
| CpG Site | Treatment | p-Value | |||||
|---|---|---|---|---|---|---|---|
| CON | BPA | MET | BPA + MET | BPA | MET | BPA × MET | |
| −18 | 0.54 ± 0.03 b | 0.46 ± 0.05 b | 0.70 ± 0.05 a | 0.53 ± 0.05 b | 0.02 | 0.02 | 0.33 |
| −66 | 0.56 ± 0.03 b | 0.19 ± 0.04 c | 0.93 ± 0.04 a | 0.46 ± 0.04 b | <0.01 | <0.01 | 0.23 |
| −93 | 0.87 ± 0.04 a,b | 0.35 ± 0.04 c | 0.94 ± 0.01 a | 0.78 ± 0.06 b | <0.01 | <0.01 | <0.01 |
| −149 | 0.76 ± 0.06 | 0.72 ± 0.02 | 0.77 ± 0.02 | 0.76 ± 0.02 | 0.49 | 0.54 | 0.68 |
| −215 | 0.59 ± 0.07 b | 0.39 ± 0.05 c | 0.89 ± 0.04 a | 0.52 ± 0.03 b,c | <0.01 | <0.01 | 0.12 |
| −278 | 0.87 ± 0.01 | 0.84 ± 0.02 | 0.87 ± 0.06 | 0.84 ± 0.02 | 0.35 | 0.94 | 1.00 |
| −655 | 0.75 ± 0.03 | 0.50 ± 0.17 | 0.74 ± 0.03 | 0.75 ± 0.01 | 0.19 | 0.18 | 0.15 |
| −672 | 0.79 ± 0.03 | 0.61 ± 0.21 | 0.78 ± 0.04 | 0.77 ± 0.02 | 0.38 | 0.49 | 0.44 |
| −688 | 0.36 ± 0.07 b | 0.18 ± 0.06 c | 0.71 ± 0.09 a | 0.51 ± 0.14 b | <0.01 | <0.01 | 0.88 |
| −704 | 0.44 ± 0.13 | 0.18 ± 0.14 | 0.39 ± 0.12 | 0.38 ± 0.13 | 0.32 | 0.59 | 0.37 |
| −721 | 0.63 ± 0.04 b | 0.33 ± 0.04 c | 0.94 ± 0.03 a | 0.83 ± 0.04 a | <0.01 | <0.01 | 0.03 |
| −774 | 0.76 ± 0.06 | 0.50 ± 0.17 | 0.73 ± 0.02 | 0.74 ± 0.03 | 0.22 | 0.29 | 0.17 |
| −1159 | 0.19 ± 0.09 | 0.22 ± 0.13 | 0.19 ± 0.11 | 0.21 ± 0.14 | 0.83 | 0.95 | 0.99 |
| −1169 | 0.93 ± 0.01 | 0.94 ± 0.01 | 0.93 ± 0.01 | 0.91 ± 0.01 | 0.85 | 0.26 | 0.34 |
| −1203 | 0.13 ± 0.07 | 0.15 ± 0.08 | 0.15 ± 0.08 | 0.16 ± 0.09 | 0.87 | 0.82 | 0.99 |
| −1252 | 0.15 ± 0.06 | 0.16 ± 0.01 | 0.20 ± 0.02 | 0.16 ± 0.03 | 0.72 | 0.54 | 0.54 |
| −1352 | 0.77 ± 0.05 a | 0.38 ± 0.03 b | 0.87 ± 0.04 a | 0.43 ± 0.03 b | <0.01 | 0.06 | 0.64 |
| −1417 | 0.10 ± 0.06 | 0.09 ± 0.05 | 0.11 ± 0.06 | 0.07 ± 0.07 | 0.73 | 0.86 | 0.79 |
| −1544 | 0.47 ± 0.05 b | 0.31 ± 0.05 c | 0.64 ± 0.05 a | 041 ± 0.04 a,b | <0.01 | 0.01 | 0.45 |
| −1694 | 0.60 ± 0.03 | 0.61 ± 0.02 | 0.61 ± 0.02 | 0.59 ± 0.02 | 0.88 | 0.80 | 0.52 |
| −1705 | 0.28 ± 0.06 c | 0.20 ± 0.05 c | 0.67 ± 0.04 a | 0.47 ± 0.07 b | 0.03 | <0.01 | 0.35 |
| −1718 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.05 ± 0.02 | 0.82 | 0.94 | 0.34 |
| −1771 | 0.34 ± 0.03 a | 0.23 ± 0.03 b | 0.33 ± 0.05 a,b | 0.26 ± 0.03 a,b | 0.02 | 0.72 | 0.48 |
| −1795 | 0.92 ± 0.01 | 0.92 ± 0.02 | 0.91 ± 0.02 | 0.93 ± 0.03 | 0.72 | 0.90 | 0.72 |
| −1908 | 0.33 ± 0.04 | 0.34 ± 0.01 | 0.32 ± 0.02 | 0.31 ± 0.01 | 0.76 | 0.36 | 0.61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, J.; Mou, D.; Che, L.; Fang, Z.; Feng, B.; Lin, Y.; Xu, S.; Li, J.; Wu, D. Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs. Nutrients 2017, 9, 423. https://doi.org/10.3390/nu9050423
Liu H, Wang J, Mou D, Che L, Fang Z, Feng B, Lin Y, Xu S, Li J, Wu D. Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs. Nutrients. 2017; 9(5):423. https://doi.org/10.3390/nu9050423
Chicago/Turabian StyleLiu, Hong, Jun Wang, Daolin Mou, Lianqiang Che, Zhengfeng Fang, Bin Feng, Yan Lin, Shengyu Xu, Jian Li, and De Wu. 2017. "Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs" Nutrients 9, no. 5: 423. https://doi.org/10.3390/nu9050423





