Vitamin D and Calcium Are Required during Denosumab Treatment in Osteoporosis with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Serum Corrected Calcium and Phosphorus (P) Levels
3.2. Serum Whole PTH and 1,25(OH)2D3
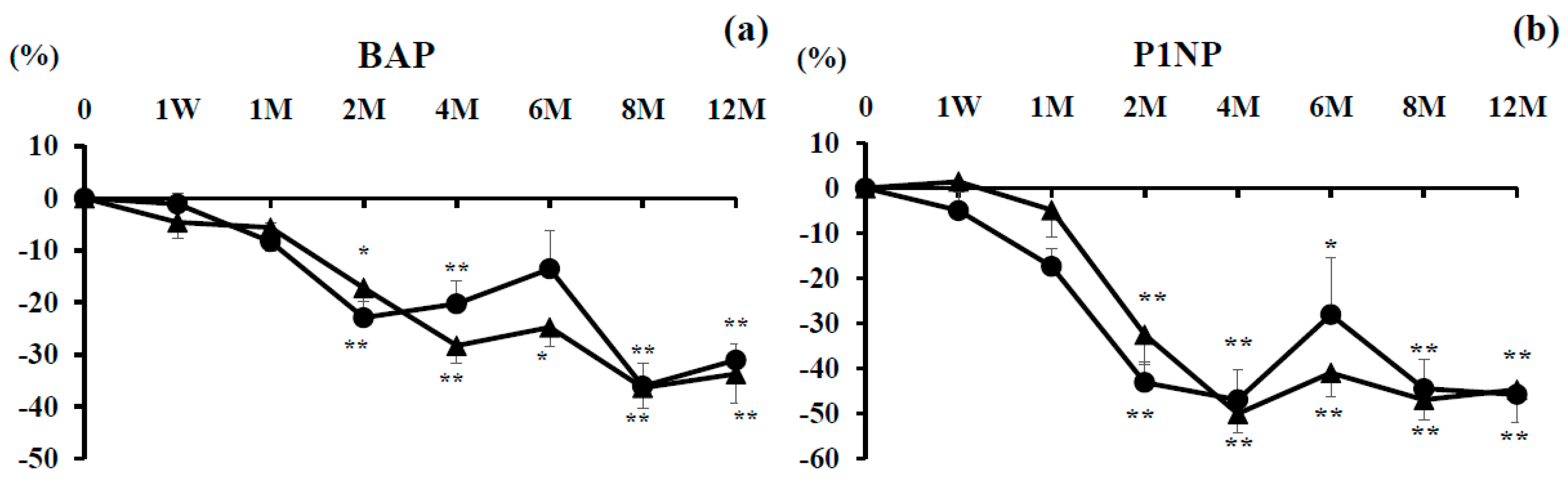
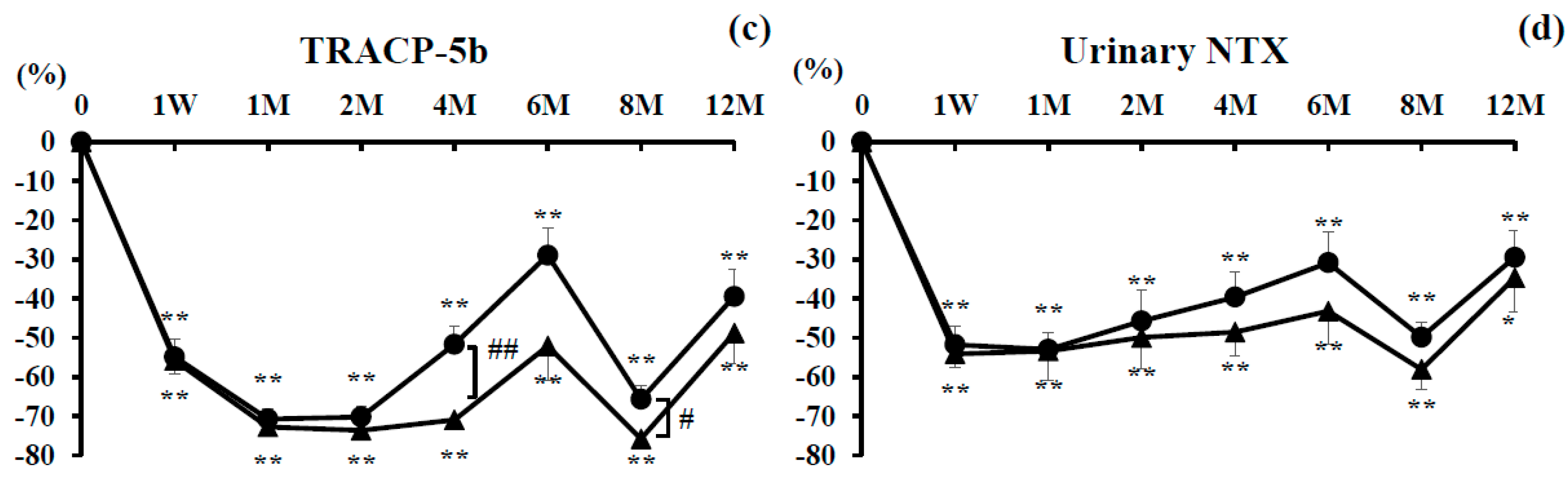
3.3. Bone Turnover Markers
3.3.1. Bone Formation Markers
3.3.2. Bone Resorption Markers
3.3.3. L-BMD and H-BMD
3.3.4. Indicators of RA State
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- McClung, M.R.; Lewiecki, E.M.; Cohen, S.B.; Bolognese, M.A.; Woodson, G.C.; Moffett, A.H.; Peacock, M.; Miller, P.D.; Lederman, S.N.; Chesnut, C.H.; et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2006, 354, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Adachi, J.D.; Lippuner, K.; Zapalowski, C.; Miller, P.D.; Reginster, J.Y.; Törring, O.; Kendler, D.L.; Daizadeh, N.S.; Wang, A.; et al. Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporos. Int. 2015, 26, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Kvien, T.K.; Haugeberg, G.; Uhlig, T.; Falch, J.A.; Halse, J.I.; Lems, W.F.; Dijkmans, B.A.; Woolf, A.D. Data driven attempt to create a clinical algorithm for identification of women with rheumatoid arthritis at high risk of osteoporosis. Ann. Rheum. Dis. 2000, 59, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Arain, S.R.; Riaz, A.; Nazir, L.; Umer, T.P.; Rasool, T. Low bone mineral density among patients with newly diagnosed rheumatoid arthritis. J. Ayub Med. Coll. Abbottabad 2016, 28, 175–178. [Google Scholar] [PubMed]
- Hoes, J.N.; Bultink, I.E.; Lemsm, W.F. Management of osteoporosis in rheumatoid arthritis patients. Expert Opin. Pharmacother. 2015, 16, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Nakamura, T.; Hosoi, T.; Iki, M.; Uenishi, K.; Endo, N.; Ohta, H.; Shiraki, M.; Sugimoto, T.; Suzuki, T.; et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis—Executive summary. Arch. Osteoporos. 2012, 7, 3–20. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [PubMed]
- Bouillon, R.; Okamura, W.H.; Norman, A.W. Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 1995, 16, 200–257. [Google Scholar] [PubMed]
- Shiraki, M.; Kushida, K.; Fukunaga, M.; Kishimoto, H.; Taga, M.; Nakamura, T.; Kaneda, K.; Minaguchi, H.; Inoue, T.; Morii, H.; et al. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. Osteoporos. Int. 1999, 10, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumoto, T.; Sugimoto, T.; Shiraki, M. Dose-response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosis. Osteoporos. Int. 2012, 23, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Diab, D.L.; Watts, N.B. Denosumab in osteoporosis. Expert Opin. Drug Saf. 2014, 13, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Kawazoe, K.; Teraoka, K.; Kujime, T.; Abe, M.; Yasuo, S.; Kazuo, M. Identification of the risk factors associated with hypocalcemia induced by denosumab. Biol. Pharm. Bull. 2013, 36, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Bone, H.G.; De Boer, R.H.; Stopeck, A.; Van Poznak, C.; Damião, R.; Fizazi, K.; Henry, D.H.; Ibrahim, T.; Lipton, A.; et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur. J. Cancer 2015, 51, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Matsumoto, T.; Hosoi, T.; Miki, T.; Gorai, I.; Yoshikawa, H.; Tanaka, Y.; Tanaka, S.; Fukunaga, M.; Sone, T. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: Results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos. Int. 2015, 26, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Soen, S. New Diagnostic Criteria and Guidelines on Osteoporosis. Diagnostic criteria for primary osteoporosis: Year 2012 revision. J. Bone Miner. Metab. 2014, 24, 323–329. [Google Scholar]
- Van der Linden, M.P.; Knevel, R.; Huizinga, T.W.; Van der Helm-van Mil, A.H. Classification of rheumatoid arthritis: Comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis Rheu. 2011, 63, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Nakamura, T.; Fukunaga, M.; Ohta, H.; Hosoi, T.; Uemura, Y.; Kuroda, T.; Miyakawa, N.; Ohashi, Y.; Shiraki, M. Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: The Japanese Osteoporosis Intervention Trial (JOINT)-02. Curr. Med. Res. Opin. 2011, 27, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Majumdar, S.R.; Morin, S.N.; Lix, L.M. Change in Bone Mineral Density Is an Indicator of Treatment-Related Antifracture Effect in Routine Clinical Practice: A Registry-Based Cohort Study. Ann. Intern. Med. 2016, 165, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kamimura, M.; Ikegami, S.; Mukaiyama, K.; Uchiyama, S.; Taguchi, A.; Kato, H. Changes in serum vitamin D and PTH values using denosumab with or without bisphosphonate pre-treatment in osteoporotic patients: A short-term study. BMC Endocr. Disord. 2015, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. The coupling of bone formation to bone resorption: A critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab. Bone Dis. Relat. Res. 1982, 4, 1–6. [Google Scholar] [CrossRef]
- Bellavia, D.; Costa, V.; De Luca, A.; Maglio, M.; Pagani, S.; Fini, M.; Giavaresi, G. Vitamin D Level between Calcium-Phosphorus Homeostasis and Immune System: New Perspective in Osteoporosis. Curr. Osteoporos. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Antoniucci, D.M.; Vittinghoff, E.; Palermo, L.; Black, D.M.; Sellmeyer, D.E. Vitamin D insufficiency does not affect response of bone mineral density to alendronate. Osteoporos. Int. 2009, 20, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Bourke, S.; Bolland, M.J.; Grey, A.; Horne, A.M.; Wattie, D.J.; Wong, S.; Gamble, G.D.; Reid, I.R. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos. Int. 2013, 24, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Heckman, G.A.; Papaioannou, A.; Sebaldt, R.J.; Ioannidis, G.; Petrie, A.; Goldsmith, C.; Adachi, J.D. Effect of vitamin D on bone mineral density of elderly patients with osteoporosis responding poorly to bisphosphonates. BMC Musculoskel. Dis. 2002, 3, 6. [Google Scholar] [CrossRef]
- Roux, C.; Binkley, N.; Boonen, S.; Kiel, D.P.; Ralston, S.H.; Reginster, J.Y.; Pong, A.; Rosenberg, E.; Santora, A. Vitamin D status and bone mineral density changes during alendronate treatment in postmenopausal osteoporosis. Calcif. Tissue Int. 2014, 94, 153–157. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Denosumab (n = 22) | Combination (n = 21) | p-Value |
|---|---|---|---|
| Age (years) | 70.9 ± 1.8 | 70.6 ± 2.3 | 0.9161 |
| Gender (F:M) | 22:0 | 21:00 | |
| BMI (kg/m2) | 20.8 ± 0.9 | 20.0 ± 0.9 | 0.5396 |
| Serum corrected Ca (mg/dL) | 9.4 ± 0.1 | 9.2 ± 0.1 | 0.2156 |
| Serum P (mg/dL) | 3.7 ± 0.1 | 3.5 ± 0.1 | 0.2378 |
| Serum BAP (μg/L) | 13.4 ± 0.6 | 13.8 ± 1.1 | 0.7631 |
| Serum TRACP-5b (mU/dL) | 302.9 ± 16.7 | 312.3 ± 29.7 | 0.7873 |
| Urinary NTX (nmol BCE/mmol/CRE) | 28.4 ± 2.5 | 26.7 ± 1.8 | 0.6015 |
| 1,25(OH)2D3 (pg/mL) | 62.8 ± 4.9 | 60.7 ± 5.7 | 0.7847 |
| P1NP (μg/L) | 37.3 ± 4.2 | 36.0 ± 4.5 | 0.8239 |
| Serum whole PTH (pg/dL) | 29.7 ± 2.9 | 30.1 ± 4.2 | 0.9429 |
| BP use, n (%) | 22 (100) | 21 (100) | |
| Period of BP use | 5.8 ± 1.0 | 5.5 ± 1.0 | 0.8476 |
| L-BMD (g/cm2) | 0.71 ± 0.04 | 0.68 ± 0.02 | 0.4197 |
| H-BMD (g/cm2) | 0.487 ± 0.03 | 0.502 ± 0.02 | 0.6971 |
| MTX, n (mg/week) | 12 (6.7 ± 0.75) | 10 (6.6 ± 0.85) | 0.9536 |
| PSL, n (mg/day) | 2 (4.5 ± 0.5) | 1 (5.0 ± 0.0) | |
| MMP-3 | 92.2 ± 22.2 | 94.3 ± 15.5 | 0.9392 |
| Disease duration (years) | 16.6 ± 2.9 | 18.5 ± 3.2 | 0.6815 |
| DAS28-CRP | 3.1 ± 0.3 | 3.0 ± 0.3 | 0.9090 |
| SDAI | 4.3 ± 1.0 | 4.7 ± 1.0 | 0.7987 |
| HAQ-DI | 0.44 ± 0.2 | 0.39 ± 0.2 | 0.8215 |
| Characteristic | Denosumab (n = 22) | Combination (n = 21) | p-Value |
|---|---|---|---|
| L-BMD (g/cm2) | 0.74 ± 0.03 | 0.72 ± 0.04 | 0.6983 |
| H-BMD (g/cm2) | 0.49 ± 0.02 | 0.54 ± 0.03 | 0.2236 |
| MMP-3 (ng/mL) | 68.6 ± 9.6 | 56.0 ± 8.6 | 0.3518 |
| DAS28-CRP | 2.7 ± 0.3 | 2.0 ± 0.3 | 0.0793 |
| SDAI | 4.6 ± 1.1 | 4.7 ± 1.4 | 0.9327 |
| HAQ-DI | 0.38 ± 0.2 | 0.28 ± 0.1 | 0.6359 |
| Characteristic | Denosumab (n = 22) | Combination (n = 21) | p-Value |
|---|---|---|---|
| L-BMD (%) | 4.8 ± 2.0 | 7.2 ± 2.8 | 0.5069 |
| H-BMD | 1.9 ± 1.0 | 6.0 ± 0.6 | 0.0060 |
| MMP-3 (%) | −4.9 ± 25.6 | −13.0 ± 9.7 | 0.7777 |
| DAS28CRP (%) | −9.7± 6.7 | −24.5 ± 10.4 | 0.2476 |
| SDAI (%) | −7.6 ± 9.7 | −31.1 ± 16.9 | 0.2500 |
| HAQ-DI (%) | −3.6 ± 26.1 | −0.5 ± 3.6 | 0.9095 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, Y.; Suzuki, T.; Yoshida, T.; Yamazaki, H.; Kato, H. Vitamin D and Calcium Are Required during Denosumab Treatment in Osteoporosis with Rheumatoid Arthritis. Nutrients 2017, 9, 428. https://doi.org/10.3390/nu9050428
Nakamura Y, Suzuki T, Yoshida T, Yamazaki H, Kato H. Vitamin D and Calcium Are Required during Denosumab Treatment in Osteoporosis with Rheumatoid Arthritis. Nutrients. 2017; 9(5):428. https://doi.org/10.3390/nu9050428
Chicago/Turabian StyleNakamura, Yukio, Takako Suzuki, Tomohiko Yoshida, Hideshi Yamazaki, and Hiroyuki Kato. 2017. "Vitamin D and Calcium Are Required during Denosumab Treatment in Osteoporosis with Rheumatoid Arthritis" Nutrients 9, no. 5: 428. https://doi.org/10.3390/nu9050428
APA StyleNakamura, Y., Suzuki, T., Yoshida, T., Yamazaki, H., & Kato, H. (2017). Vitamin D and Calcium Are Required during Denosumab Treatment in Osteoporosis with Rheumatoid Arthritis. Nutrients, 9(5), 428. https://doi.org/10.3390/nu9050428





