Eating Like a Rainbow: The Development of a Visual Aid for Nutritional Treatment of CKD Patients. A South African Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Development of the Educational Material
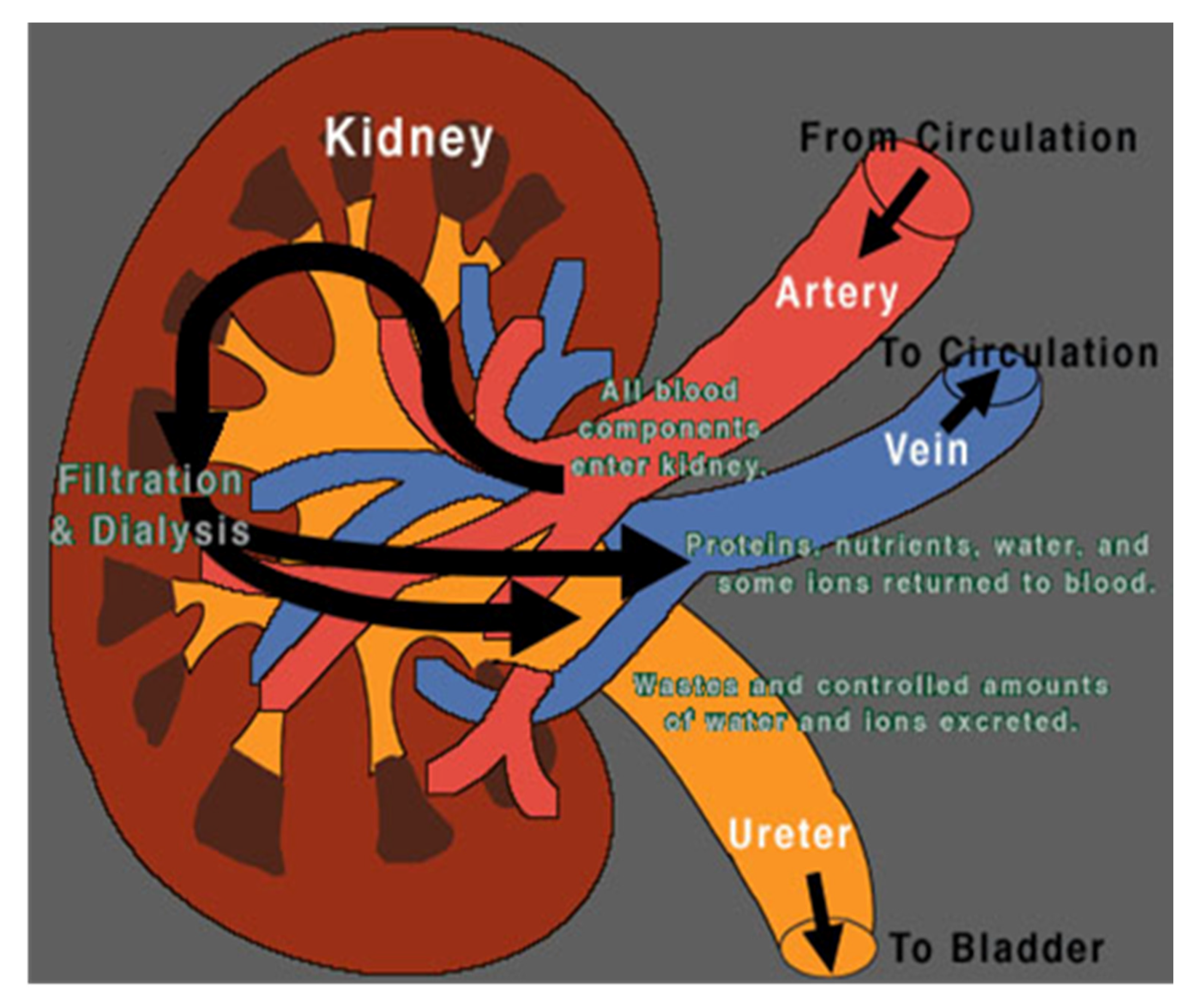
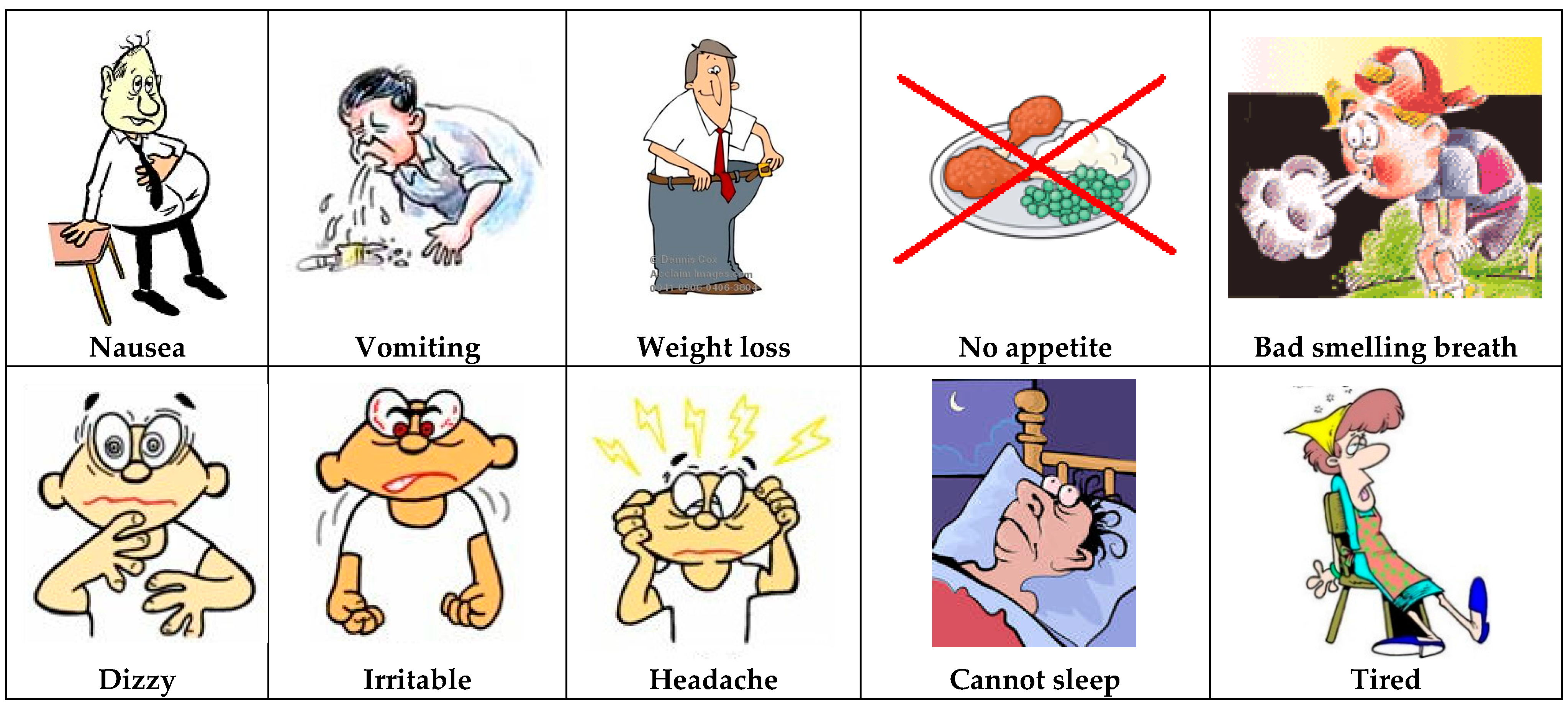
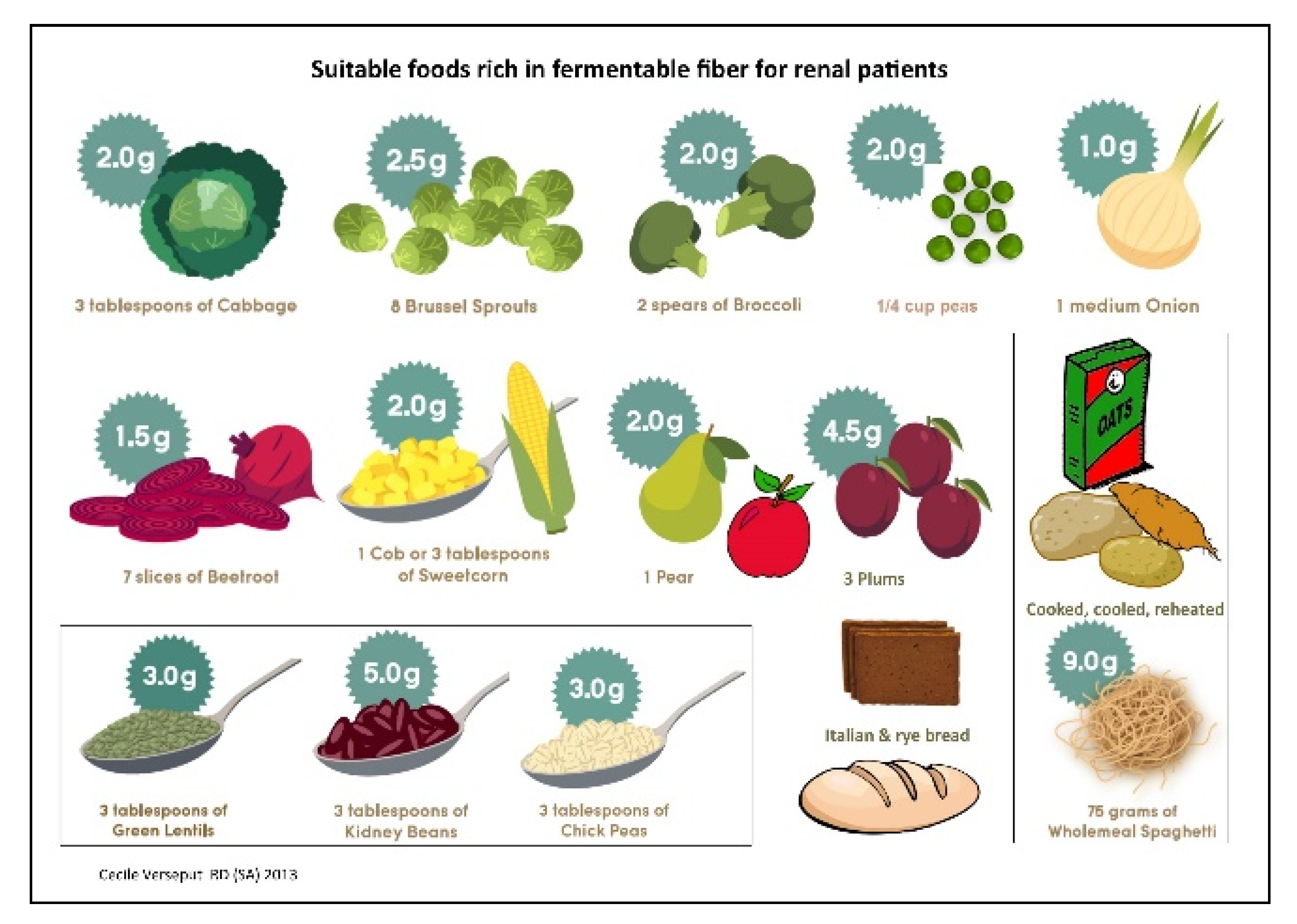
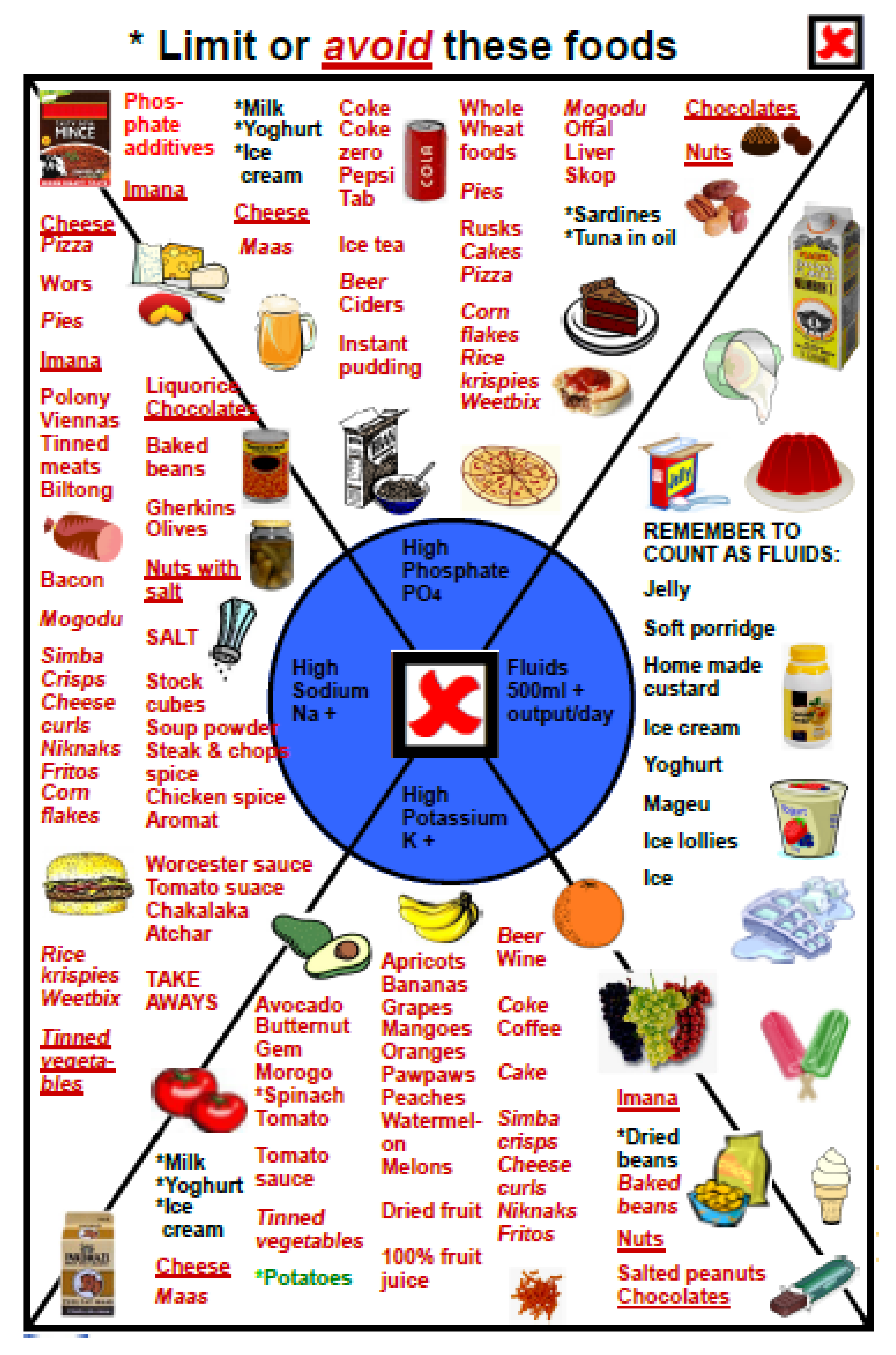
2.3. First Step: Choosing the Symbols
2.4. Second Step: Choosing the Sequence
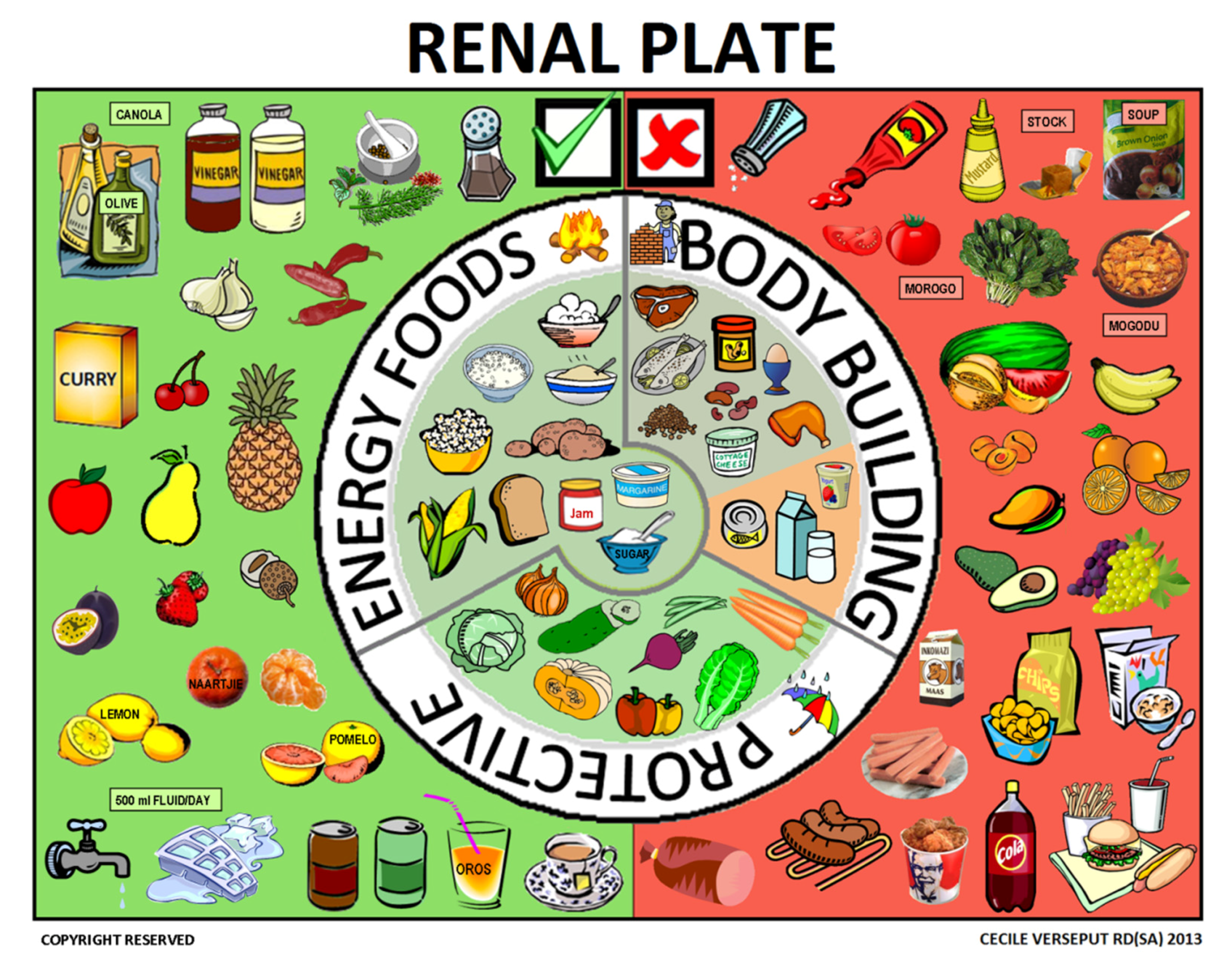
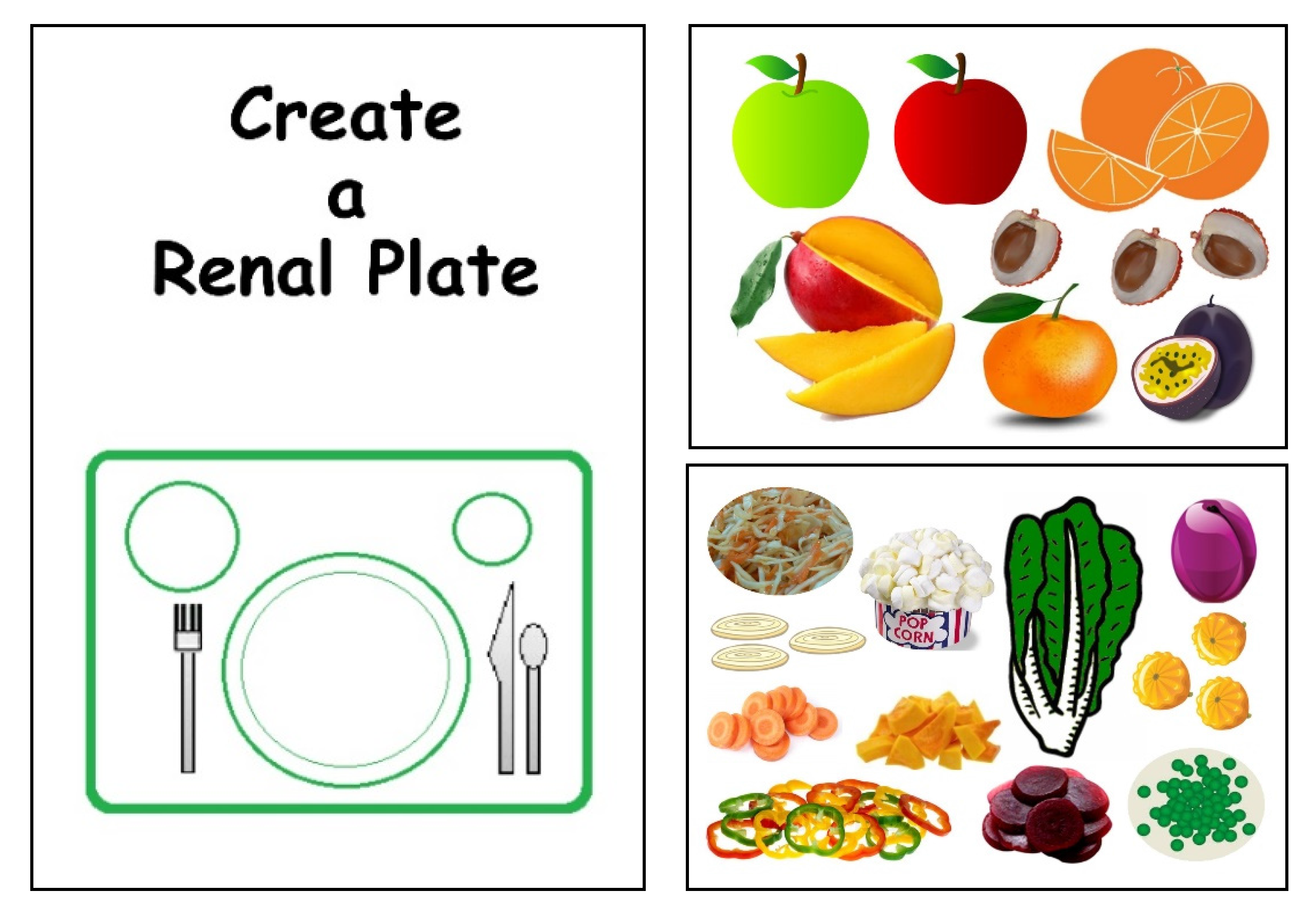
2.5. Third Step: the Renal Plate
3. Results
3.1. The Five-Step Approach
3.2. The Renal Plate
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meyers, A.M. Chronic kidney disease. S. Afr. Med. J. 2015, 105, 232. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.M. Significance, definition, classification and risk factors of chronic kidney disease in South Africa. S. Afr. Med. J. 2015, 105, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Moosa, M.R.; Van der Walt, I.; Naicker, S.; Meyers, A.M. Important causes of chronic kidney disease in South Africa. S. Afr. Med. J. 2015, 105, 2681. [Google Scholar] [CrossRef] [PubMed]
- Ameh, O.I.; Cilliers, L.; Okpechi, I.G. A practical approach to the nutritional management of chronic kidney disease patients in Cape Town, South Africa. BMC Nephrol. 2016, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Mchiza, Z.J.; Steyn, N.P.; Hill, J.; Kruger, A.; Schönfeldt, H.; Nel, J.; Wentzel-Viljoen, W. A review of Dietary Surveys in the Adult South African Population from 2000 to 2015. Nutrients 2015, 7, 8227–8250. [Google Scholar] [CrossRef] [PubMed]
- Schönfeldt, H.C.; Pretorius, B.; Hall, N. The impact of animal source food products on human nutrition & health. S. Afr. J. Anim. Sci. 2013, 41, 394–412. [Google Scholar]
- Eksteen, G.; Mungal-Singh, V. Salt intake in South Africa: A current perspective. J. Endocrinol. Metab. Diabetes S. Afr. 2015, 20, 9–13. [Google Scholar] [CrossRef]
- Gerntholtz, T.; Paget, G.; Hsu, P.; Meyers, A.M. Management of patients with chronic kidney disease. S. Afr. Med. J. 2015, 105, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Katz, I. Strategies for the early detection and management of chronic kidney disease—Tertiary and primary health care working together. Afr. J. Online 2007, 25, 360–365. [Google Scholar]
- Kammoun, K.; Chaker, H.; Mahfoudh, H.; Makhlouf, N.; Jarraya, F.; Hachicha, J. Diet in chronic kidney disease in a Mediterranean African country. BMC Nephrol. 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Ashuntantang, G.E.; Fouda, H.; Folefack Kaze, F.; Halle, M.P.; Tabi-Arrey, C.; Biwole-Sida, M. A practical approach to low protein diets for patients with chronic kidney disease in Cameroon. BMC Nephrol. 2016, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Statistics South Africa. Statistical Release (Revised) P0301.4 Census 2011. Available online: www.statssa.gov.za/publications/P03014/P030142011.pdf (accessed on 15 February 2017).
- Statistics South Africa. Statistical Release (Revised) P0318 General Household Survey 2014. Available online: www.statssa.gov.za/publications/P0318/P03182013.pdf (accessed on 15 February 2017).
- Statistics South Africa. Statistical Release (Revised) P0302 Mid-Year Population Estimates 2014. Available online: www.statssa.gov.za/publications/P0302/P03022014.pdf (accessed on 15 February 2017).
- Lederer, S.; Fischer, M.J.; Gordon, H.S.; Wadwha, A.; Popli, S.; Gordon, E.J. Barriers to effective communication between veterans with chronic kidney disease and their healthcare providers. Clin. Kidney J. 2015, 8, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Devraj, R.; Borrego, M.; Vilay, A.M.; Gordon, E.J.; Pailden, J.; Horowitz, B. Relationship between health literacy and kidney function. Nephrology 2015, 20, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Morony, S.; Flynn, M.; McCaffery, K.J.; Jansen, J.; Webster, A. Readability of written materials for CKD patients: A systematic review. Am. J. Kidney Dis. 2015, 65, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Tuot, D.S.; Cavanaugh, K.L. Evaluating the merits of CKD patient educational materials: Readability is necessary but not sufficient. Am. J. Kidney Dis. 2015, 65, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Tuot, D.S.; Davis, E.; Velasquez, A.; Banerjee, T.; Powe, N.R. Assessment of printed-educational materials for Chronic Kidney Disease. Am. J. Nephrol. 2013, 38, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Lora, C.M.; Gordon, E.J.; Sharp, L.K.; Fischer, M.J.; Gerber, B.S.; Lash, J.P. Progression of CKD in Hispanics: Potential roles of health literacy, acculturation, and social support. Am. J. Kidney Dis. 2011, 58, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Cupisti, A. “Let food be thy medicine…”: Lessons from low-protein diets from around the world. BMC Nephrol. 2017, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vargas, P.A.; Tong, A.; Phoon, R.K.; Chadban, S.J.; Shen, Y.; Craig, J.C. Knowledge deficit of patients with stage 1-4 CKD: A focus group study. Nephrology 2014, 19, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Rak, E.C.; Hooper, S.R.; Belsante, M.J.; Burnett, O.; Layton, B.; Tauer, D.; Mantoo, B.; De Walt, D.; Ferris, M.E. Caregiver word reading literacy and health outcomes among children treated in a pediatric nephrology practice. Clin. Kidney J. 2016, 9, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.D.S.; Roderick, P.J.; Casey, M.; Taal, M.W.; Yuen, H.M. Prevalence and associations of limited health literacy in chronic kidney disease: A systematic review. Nephrol. Dial. Transplant. 2013, 28, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Narva, A.S.; Norton, J.M.; Boulware, L.E. Educating patients about CKD: The path to self-management and patient-centered care. Clin. J. Am. Soc. Nephrol. 2016, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A. Health literacy in Nephrology: Why is it important? Am. J. Kidney Dis. 2013, 62, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Bear, R.A.; Stockie, S. Patient engagement and patient-centered care in the management of advanced chronic kidney disease and chronic kidney failure. Can. J. Kidney Health Dis. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Heal Yourself at Home. Available online: http://www.healyourselfathome.com (accessed on 20 April 2017).
- Choose My Plate. Available online: http://www.choosemyplate.gov/ (accessed on 20 April 2017).
- Half Your Plate. Available online: http://www.halfyourplate.ca/ (accessed on 20 April 2017).
- The Vegan RD. Available online: http://www.theveganrd.com (accessed on 20 April 2017).
- Food Pylramid, Wikipedia. Available online: https://en.wikipedia.org/wiki/Food_pyramid (nutrition) (accessed on 20 April 2017).
- USDA. Food Guide Pyramid: A Guide to Daily Food Choices; USDA: Washington, DC, USA, 1992.
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.N. The silent epidemic—The health effects of illiteracy. N. Engl. J. Med. 2006, 355, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Maxia, S.; Loi, V.; Capizzi, I.; Piccoli, G.B.; Cabiddu, G.; Pani, A. Compliance, illiteracy and low-protein diet: Multiple challenges in CKD and a case of self-empowerment. BMC Nephrol. 2016, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.V.; Patel, Z.M. Role of low protein diet in management of different stages of chronic kidney disease—Practical aspects. BMC Nephrol. 2016, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Chan, M. Protein-controlled versus restricted protein versus low protein diets in managing patients with non-dialysis chronic kidney disease: A single centre experience in Australia. BMC Nephrol. 2016, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, L.; Nerbass, F.B.; Avesani, C.M.; Kamimura, M.A. A practical approach to dietary interventions for nondialysis-dependent CKD patients: The experience of a reference nephrology center in Brazil. BMC Nephrol. 2016, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakagawa, H.; Murakami, Y.; Kitamura, A.; Kiyama, M.; Sakata, K.; Tsuji, I.; Miura, K.; Ueshima, H.; Okamura, T.; et al. Smoking increases the risk of all-cause and cardiovascular mortality in patients with chronic kidney disease. Kidney Int. 2015, 88, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Staplin, N.; Haynes, R.; Herrington, W.G.; Reith, C.; Cass, A.; Fellström, B.; Jiang, L.; Kasiske, B.L.; Krane, V.; Levin, A.; et al. Smoking and adverse outcomes in patients with CKD: The Study of Heart and Renal Protection (SHARP). Am. J. Kidney Dis. 2016, 68, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Laurence, E.C.; Volmink, J.; Esterhuizen, T.M.; Dalal, S.; Holmes, M.D. Risk of cardiovascular disease among teachers in Cape Town: Findings of the South African PaCT pilot study. S. Afr. Med. J. 2016, 106, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Pruszynski, J.; Cai, W.; Simoni, J. Acid retention with reduced glomerular filtration rate increases urine biomarkers of kidney and bone injury. Kidney Int. 2017, 91, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.F.; Zahmatkesh, G.; Ahmadi, E.; Streja, E.; Rhee, C.M.; Gillen, D.L.; De Nicola, L.; Minutolo, R.; Ricardo, A.C.; Kovesdy, C.P.; et al. Associaiation of body mass index with clinical outcomes in non-dialysis-dependent chronic kidney disease: A systematic review and meta-analysis. Cardiorenal Med. 2015, 6, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Whaley-Connell, A.; Sowers, J.R. Obesity and kidney disease: From population to basic science and the search for new therapeutic targets. Kidney Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Klaassen, G.; van Adrichem, E.; Bakker, S.J.; Corpeleijn, E.; Navis, G. Physical inactivity: A risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 2017, 13, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Dharmarajan, S.; Saran, R.; Ríos Burrows, N.; Saydah, S.; Powe, N.R.; CDC CKD Surveillance Team. Food Insecurity, CKD, and Subsequent ESRD in US Adults. Am. J. Kidney Dis. 2017. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verseput, C.; Piccoli, G.B. Eating Like a Rainbow: The Development of a Visual Aid for Nutritional Treatment of CKD Patients. A South African Project. Nutrients 2017, 9, 435. https://doi.org/10.3390/nu9050435
Verseput C, Piccoli GB. Eating Like a Rainbow: The Development of a Visual Aid for Nutritional Treatment of CKD Patients. A South African Project. Nutrients. 2017; 9(5):435. https://doi.org/10.3390/nu9050435
Chicago/Turabian StyleVerseput, Cecile, and Giorgina Barbara Piccoli. 2017. "Eating Like a Rainbow: The Development of a Visual Aid for Nutritional Treatment of CKD Patients. A South African Project" Nutrients 9, no. 5: 435. https://doi.org/10.3390/nu9050435
APA StyleVerseput, C., & Piccoli, G. B. (2017). Eating Like a Rainbow: The Development of a Visual Aid for Nutritional Treatment of CKD Patients. A South African Project. Nutrients, 9(5), 435. https://doi.org/10.3390/nu9050435





