Improvement of Antioxidant Defences and Mood Status by Oral GABA Tea Administration in a Mouse Model of Post-Stroke Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of GABA Tea Extracts
2.3. Total Polyphenol Content
2.4. RP-HPLC-PDA-ESI-MSn Analysis
2.5. RP-HPLC-PDA Analysis
2.6. RP-HPLC-PDA Method Validation
2.7. Determination of Glutamic Acid, Glutamine, γ-Amino Butyric Acid and Theanine
2.8. Animals
2.9. Induction of Stroke
2.10. Tea Extract Administration
2.11. Examination of Stroke-Induced Anhedonia
2.12. Despair Swimming Test (DST) and Tail Suspension Test (TST)
2.13. Anesthesia and Tissue Collection
2.14. Preparation of Tissue Homogenate
2.15. Measurement of Protein Content
2.16. Estimation of Lipid Peroxidation, Reduced Glutathione Level, Superoxide Dismutase and Catalase Activities
2.17. Statistical Analysis
3. Results
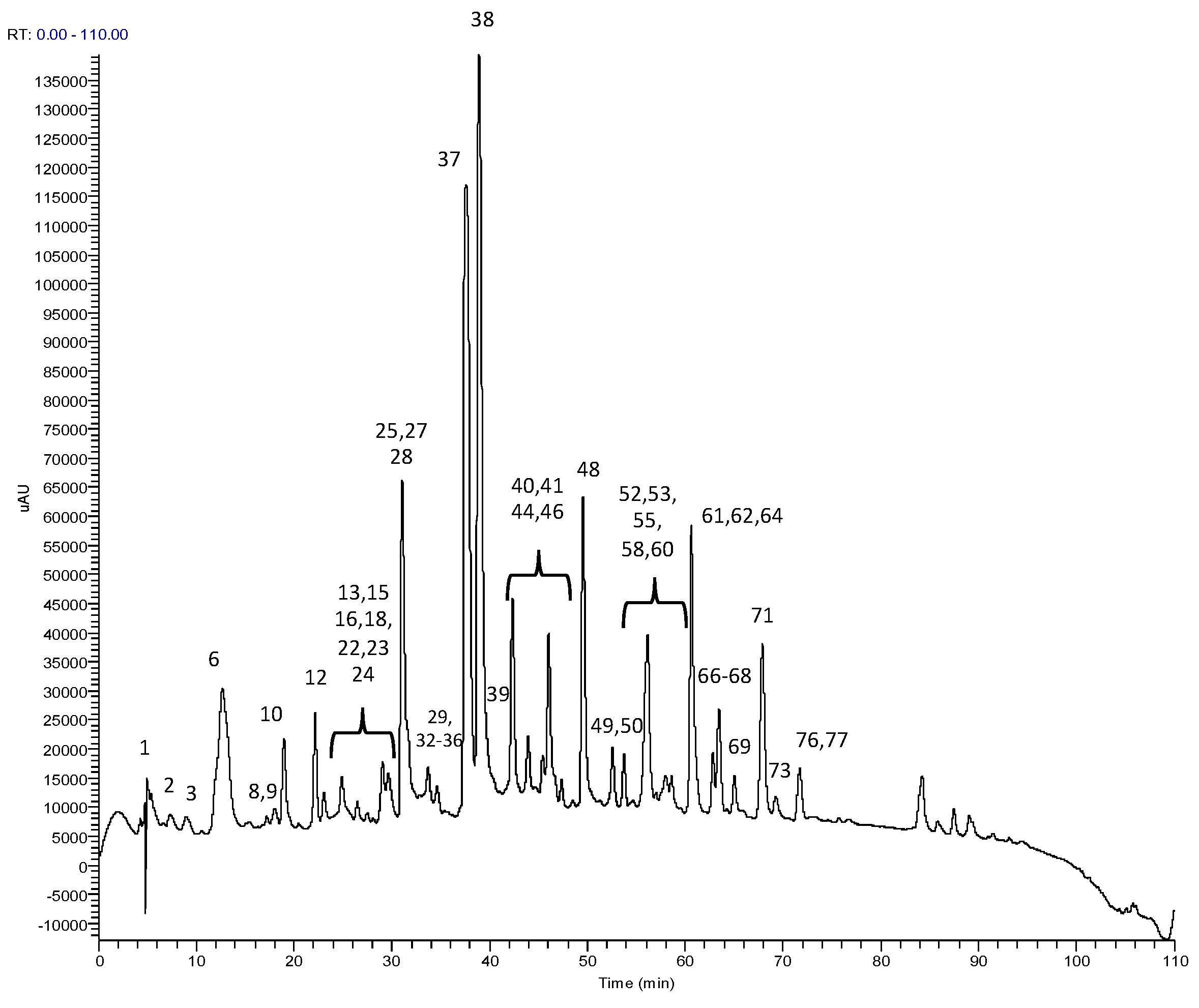
3.1. RP-HPLC-PDA-ESI-MSn Analysis
3.2. RP-HPLC-PDA Method Validation and Bioactives Quantification
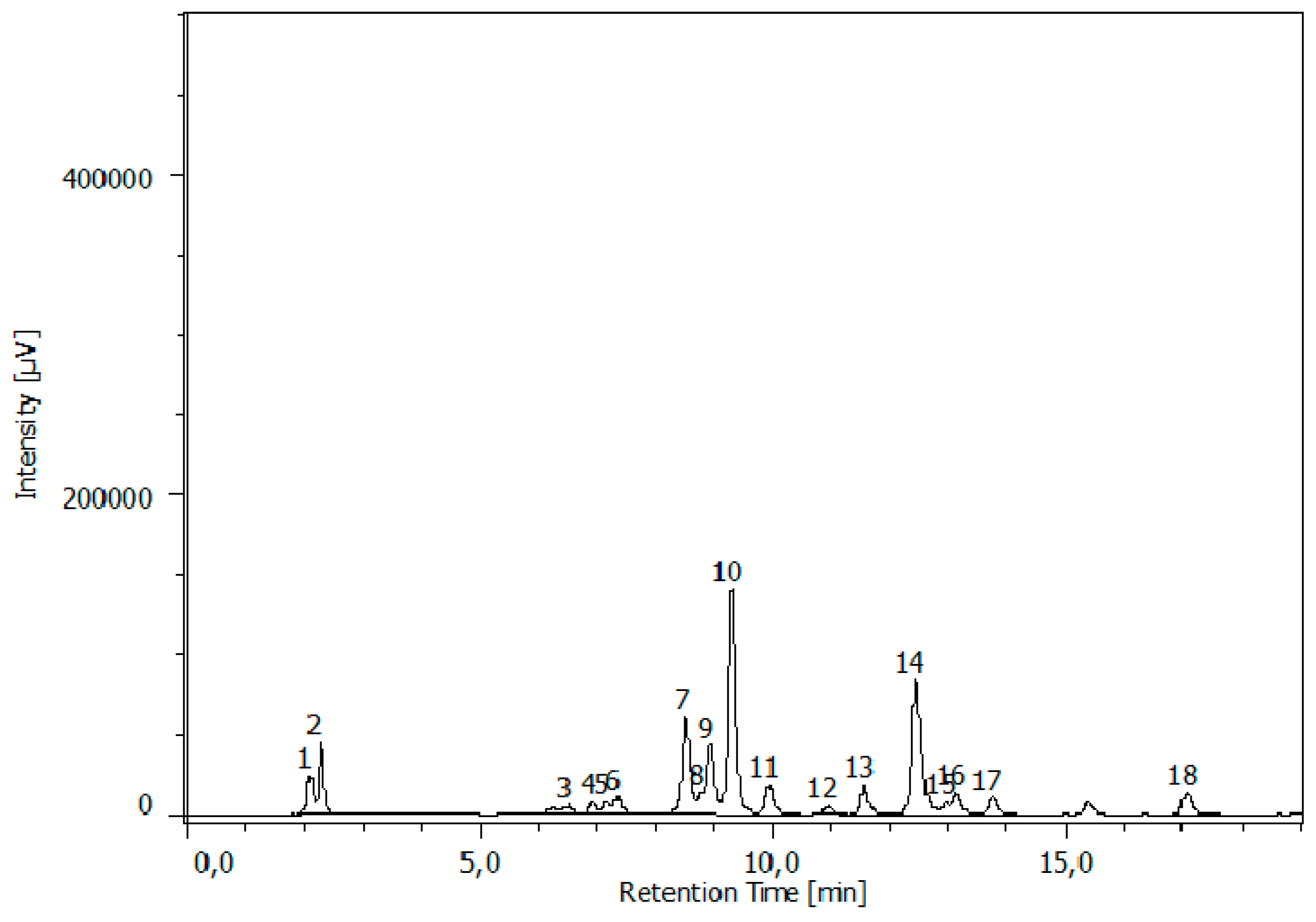
3.3. RP-HPLC-FD Analysis
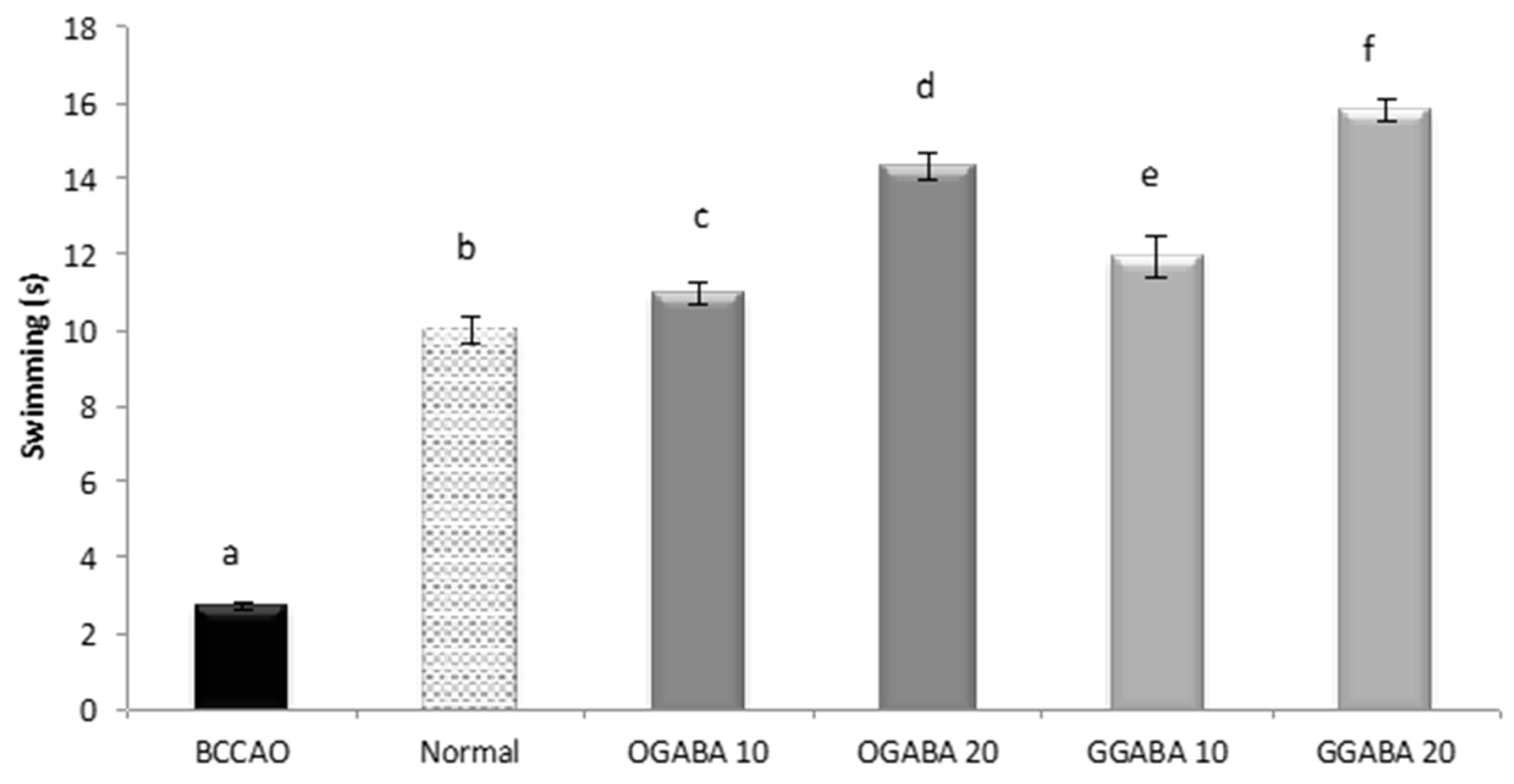
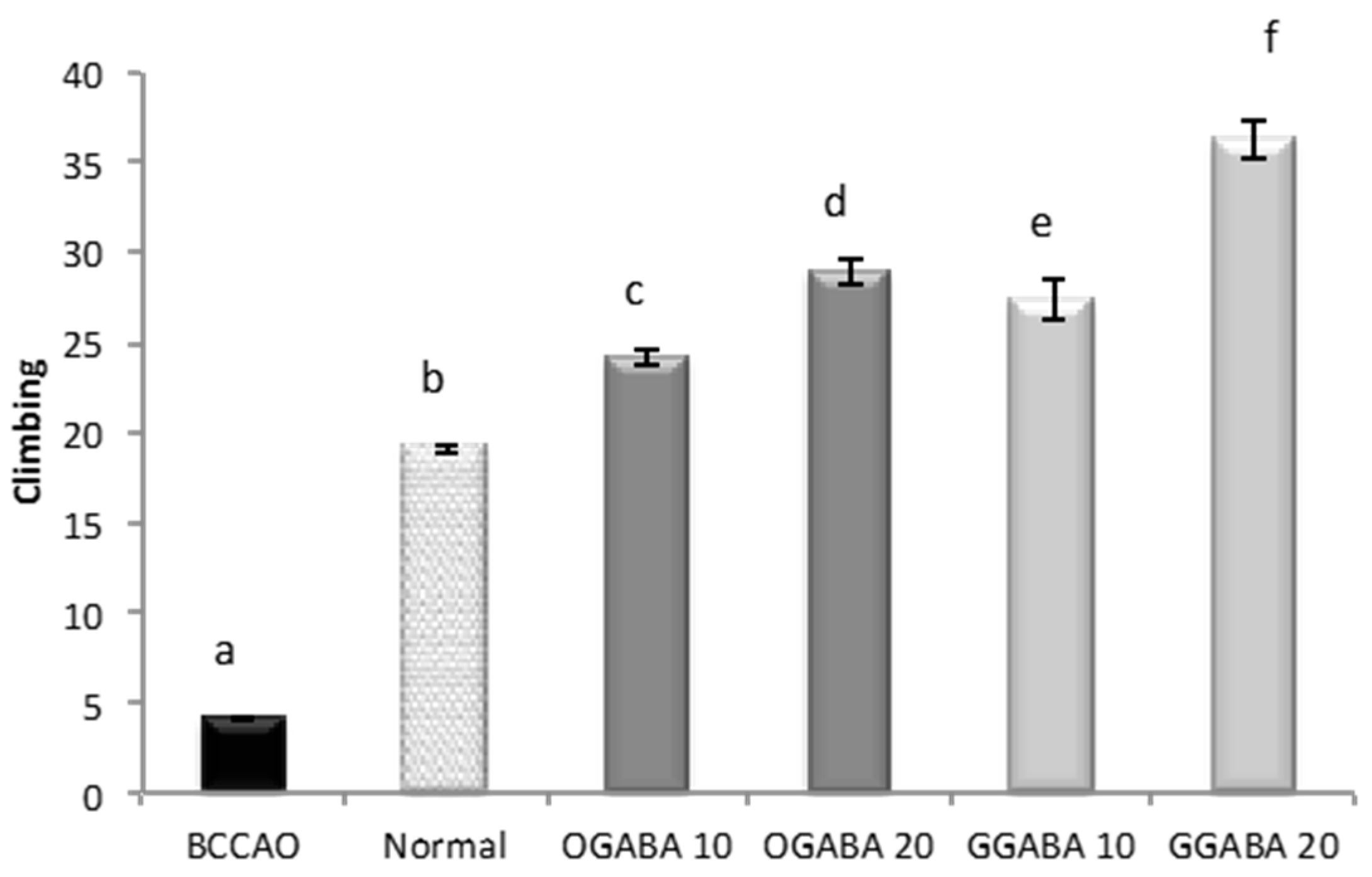
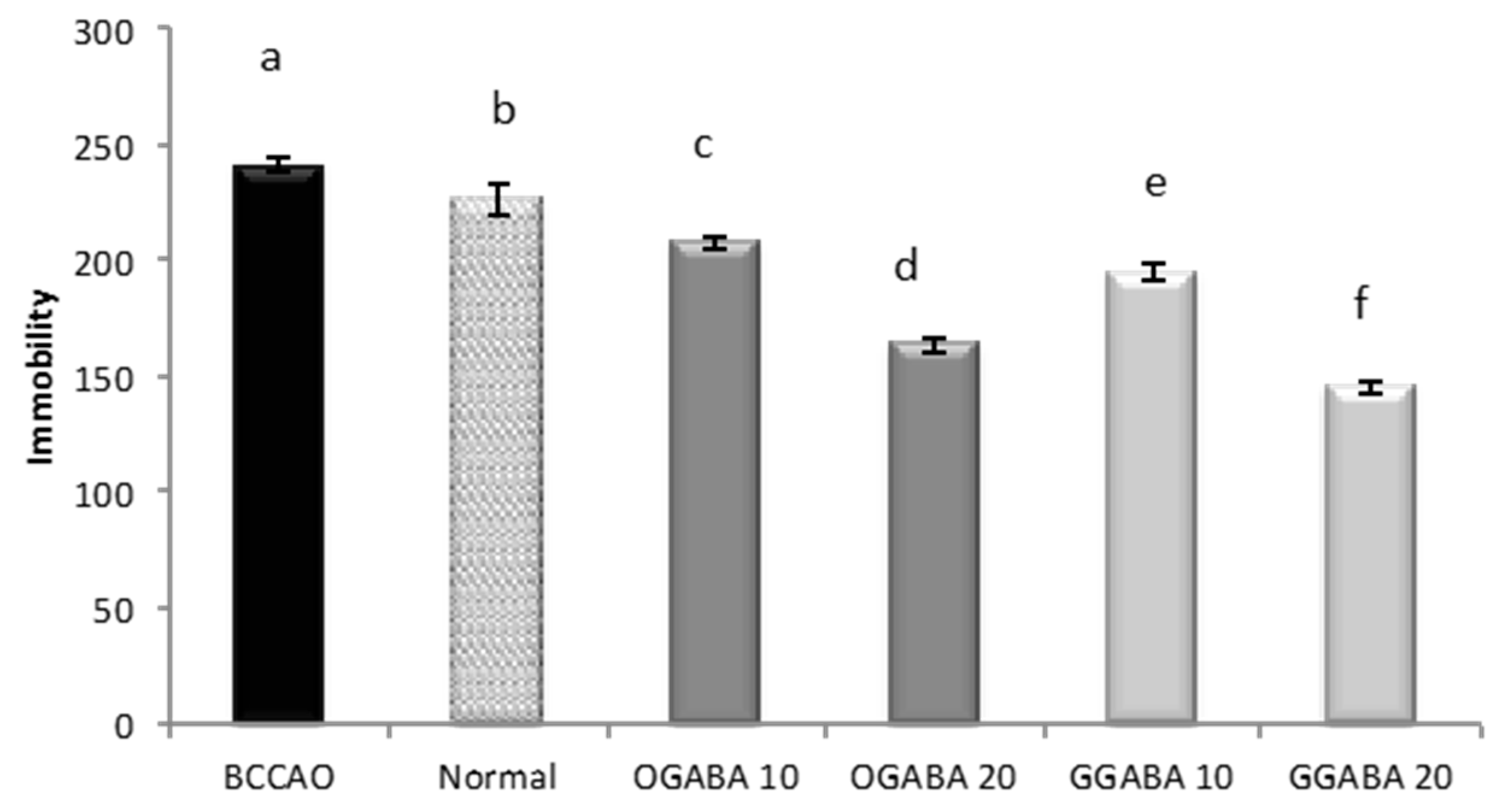
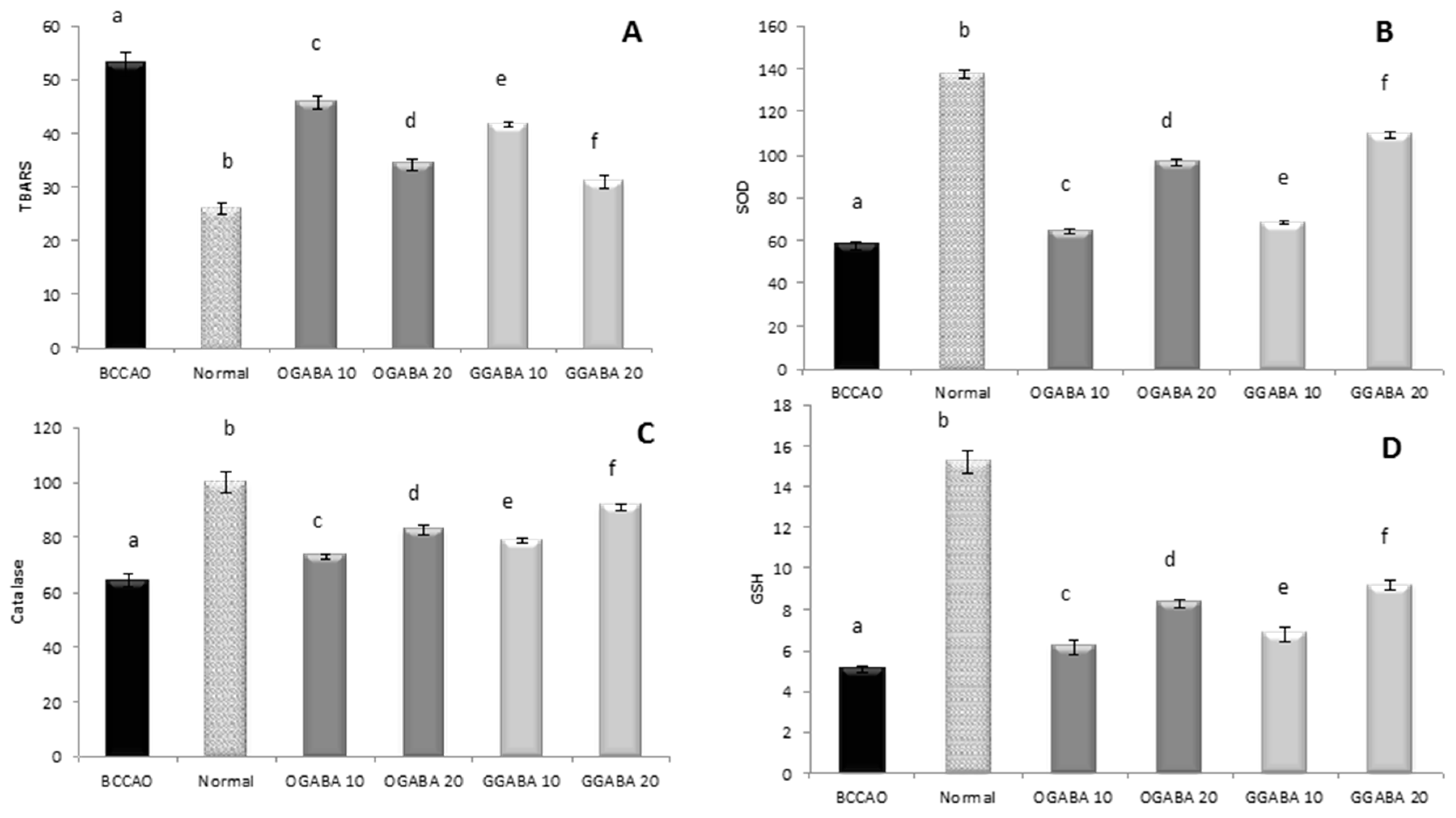
3.4. Antidepressive-Like Activity and In Vivo Antioxidant Effects of GGABA and OGABA Extracts
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Shirley, R.; Ord, E.N.; Work, L.M. Oxidative Stress and the Use of Antioxidants in Stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.G.; Jorge, R.E. Post-Stroke Depression: A Review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.L.; Robinson, R.G.; Andrzejewski, P.; Samuels, J.; Price, T.R. Association of depression with 10-year poststroke mortality. Am. J. Psychiatry 1993, 150, 124–129. [Google Scholar] [PubMed]
- House, A.; Knapp, P.; Bamford, J.; Vail, A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke 2001, 32, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.S.; Ghose, S.S.; Swindle, R.W. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am. J. Psychiatry 2004, 161, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.C.; Mulholland, C.; Trinick, T. Ascorbate and malondialdehyde in stroke patients. Ir. J. Med. Sci. 1994, 163, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Cherubini, A.; Stahl, W.; Senin, U.; Sies, H.; Mecocci, P. Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: Relationship to early outcome. Free Radic. Res. 2002, 36, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Lai, Y.C.; Cheng, T.J.; Lau, M.T.; Hu, M.L. Plasma levels of antioxidant vitamins, selenium, total sulfhydryl groups and oxidative products in ischemic-stroke patients as compared to matched controls in Taiwan. Free Radic. Res. 1998, 28, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Polidori, M.C.; Bregnocchi, M.; Pezzuto, S.; Cecchetti, R.; Ingegni, T.; di Iorio, A.; Senin, U.; Mecocci, P. Antioxidant profile and early outcome in stroke patients. Stroke 2000, 31, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Moylan, S.; Berka, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’Neil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & nitrosative stress in depression: Why so much stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar] [PubMed]
- Herken, H.; Akyol, O.; Yilmaz, H.R.; Tutkun, H.; Savas, H.A.; Ozen, M.E.; Kalenderoglu, A.; Gulec, M. Nitric oxide, adenosine deaminase, xanthine oxidase and superoxide dismutase in patients with panic disorder: Alterations by antidepressant treatment. Hum. Psychopharmacol. 2006, 21, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis/chronic fatigue syndrome: Another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro Endocrinol. Lett. 2011, 32, 133–140. [Google Scholar] [PubMed]
- Behr, G.A.; Moreira, J.C.; Frey, B.N. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: Implications for the pathophysiology of major depressive disorder. Oxid. Med. Cell. Longev. 2012, 2012, 609421. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, M.J.; Miller, G.E. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom. Med. 2006, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yanik, M.; Erel, O.; Kati, M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatr. 2004, 16, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Behravan, E.; Razavi, B.M.; Hosseinzadeh, H. Review of plants and their constituents in the therapy of cerebral ischemia. Phytother. Res. 2014, 28, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Dean, O.M.; Turner, A.; Sureda, A.; Daglia, M.; Nabavi, S.M. Oxidative stress and post-stroke depression: Possible therapeutic role of polyphenols? Curr. Med. Chem. 2015, 22, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Tsushida, T.; Murai, T.; Ohmori, M.; Okamoto, J. Production of a new type of tea containing a high level of γ-aminobutyric acid. Nippon Nogeikagaku Kaishi 1987, 61, 817–822. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Arcidiaco, P.; Nabavi, S.M.; Daglia, M. Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression. Mol. Nutr. Food Res. 2016, 60, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Coppo, E.; Sobolev, A.P.; Rossi, D.; Mannina, L.; Daglia, M. Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis). Food Res. Int. 2014, 63, 182–191. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 10 May 2012).
- Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S.; Moghaddam, A.H.; Sureda, A.; Mirzaei, M. Neuroprotective effects of methyl-3-O-methyl gallate against sodium fluoride-induced oxidative stress in the brain of rats. Cell. Mol. Neurobiol. 2013, 33, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Sureda, A.; Moghaddam, A.H. Bi-3-azaoxoisoaporphine derivatives have antidepressive properties in a murine model of post stroke-depressive like behavior. Curr. Neurovasc. Res. 2013, 10, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Habtemariam, S.; Di Lorenzo, A.; Sureda, A.; Khanjani, S.; Nabavi, S.M.; Daglia, M. Post-Stroke Depression Modulation and in vivo Antioxidant Activity of Gallic Acid and Its Synthetic Derivatives in a Murine Model System. Nutrients 2016, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipidperoxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Bonaventura, J.; Schroeder, W.A.; Fang, S. Human erythrocyte catalase: Animproved method of isolation and a reevaluation of reported properties. Arch. Biochem. Biophys. 1972, 150, 606–617. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Owen, G.N.; Parnell, H.; De Bruin, E.A.; Rycroft, J.A. The combined effects of l-theanine and caffeine on cognitive performance and mood. Nutr. Neurosci. 2008, 11, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Dodd, F.L.; Kennedy, D.O.; Riby, L.M.; Haskell-Ramsay, C.F. A double-blind, placebo-controlled study evaluating the effects of caffeine and l-theanine both alone and in combination on cerebral blood flow, cognition and mood. Psychopharmacology 2015, 232, 2563–2576. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Viña, J.R. How Glutamate Is Managed by the Blood-Brain Barrier. Biology 2016, 5, E37. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; Huang, P.C. Circadian variations in plasma and erythrocyte glutamate concentrations in adult men consuming a diet with and without added monosodium glutamate. J. Nutr. 2000, 130, 1002S–1004S. [Google Scholar] [PubMed]
- Hanson, P.J.; Parsons, D.S. Transport and metabolism of glutamine and glutamate in the small intestine. In Glutamine and Glutamate in Mammals; Kvamme, E., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1988; pp. 235–253. [Google Scholar]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vasconcelos-Moreno, M.P.; Borowsky, C.; Zimmermann, A.B.; Wollenhaupt-Aguiar, B.; Ferrari, P.; de Almeida Fleck, M.P. The effects of exercise on oxidative stress (TBARS) and BDNF in severely depressed inpatients. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 605–613. [Google Scholar] [CrossRef] [PubMed]


| Anions/Cations | mg/L |
|---|---|
| HCO3− | 7.5 |
| F− | <0.1 |
| NO3− | 1.1 |
| SO42− | 3.8 |
| Ca2+ | 2.4 |
| Na+ | 1.7 |
| Total dissolved solids | 23.8 |
| Peak | RT (min) | λ max (nm) | m/z | HPLC-ESI-MS/MS m/z (% of Base Peak) | Proposed Structure |
|---|---|---|---|---|---|
| Xanthines | |||||
| 8 | 22.5 | 222, 273 | 181 + | 137 (100) | theobromine a,b |
| 24 | 38.7 | 230, 270 | 195 + | 138 (100) | caffeine a,b |
| Organic and phenolic acids | |||||
| 1 | 5.2 | 211 | 191 | 127 (100), 85 (80) | quinic acid a,b |
| 4 | 13.6 | 219, 274 | 343 | 191 (100), 169 (10), 125 (2) | galloylquinic acid a,b |
| 11 | 27.5 | 219, 270 | 169 | 125 (100) | Gallic acid a |
| 19 | 34.1 | 211, 330 | 337 | 163 (100), 191 (10), 119 (10), 173 (5) | 3-p-coumaroylquinic acid a,b |
| 27 | 43.5 | 211, 310 | 337 | 191 (100), 173 (25), 163 (10) | 5-p-coumaroylquinic acid a,b |
| 29 | 46.3 | 211, 309 | 337 | 173 (100), 163 (15), 191 (10) | 4-p-coumaroylquinic acid a,b |
| Flavones | |||||
| 30 | 47.7 | 210, 270, 333 | 593 | 473 (100), 353 (25), 383 (15), 503 (30) | dihexosyl-apigenin a,b |
| 32 | 52.9 | 210, 275 | 563 | 503 (70), 473 (100), 443 (80), 383 (45), 353 (50) | 6-C-arabinosyl-8-C-glucosyl apigenin a,b |
| 33 | 54.2 | 210, 275 | 563 | 503 (30), 473 (100), 443 (100), 383 (40), 353 (40) | 6-C-glucosyl-8-C-arabinosyl apigenin a,b |
| 38 | 58.6 | 211, 258, 352 | 577 | 413 (100), 293 (30), 457 (10), 353 (2) | rhamnosyl-hexosylapigenin a |
| 39 | 59.08 | 211, 271, 336 | 593 | 413 (100), 293 (10), 473 (10) | dihexosyl-apigenin a,b |
| 42 | 60.6 | 256, 353 | 533 | 443 (100), 473 (50), 353 (20) | 6,8-C-dipentoside apigenin b |
| Flavan-3-ols | |||||
| 6 | 19.5 | 220, 270 | 305 | 261 (40), 221 (85), 219 (75), 179 (100), 165 (35), 125 (20) | gallocatechin a,b |
| 15 | 31.3 | 240, 270 | 305 | 261 (40), 179 (100), 219 (70), 165 (35) 221 (85), 125 (25) | epigallocatechin a,b |
| 16 | 32.4 | 211, 278 | 289 | 245 (100), 205 (40), 179 (15), 203 (40), 109 (5) | catechin b |
| 25 | 39.2 | 233, 274 | 457 | 331 (70), 169 (100), 305 (35) | epigallocatechingallate a,b |
| 26 | 43.1 | 232, 278 | 289 | 245 (100), 205 (40), 109 (5), 137 (10), 125 (2) | epicatechin a,b |
| 31 | 52.8 | 230, 276 | 441 | 289 (100), 331 (20), 169 (25) | epicatechin-gallate a,b |
| 35 | 55.8 | 219, 270, 352 | 455 | 289 (100), 183 (25) | epicatechina-3-O-(3-metil)-gallato a |
| 37 | 57.2 | 209, 270 | 425 | 273 (100), 169 (40), 125 (5) | epiafzelechin-gallate a,b |
| Theaflavins | |||||
| 49 | 84.8 | 223, 270, 364, 470 | 867 | 715 (65), 697 (100), 679 (15), 527 (45), 545 (25), 559 (20) | theaflavin-digallate a |
| 50 | 84.9 | 213, 269, 367, 478 | 715 | 527 (100), 545 (80), 563 (70), 501 (40), 407 (10) | theaflavin-gallate a |
| 51 | 85.8 | 215, 270, 364 | 563 | 545 (100), 527 (30), 519 (55), 501 (30), 407 (70), 379 (50) | theaflavin a |
| 52 | 86.6 | 213, 269, 367, 478 | 715 | 527 (30), 545 (60), 563 (100), 501 (20), 407 (60) | theaflavin-gallate a |
| Flavonols | |||||
| 34 | 54.5 | 210, 273 | 787 | 316 (100), 769 (30), 359 (20), 625 (15), 725 (20) | myricetinhexosylrutinoside b |
| 36 | 55.9 | 215, 266, 350 | 479 | 316 (100), 317 (30) | myricetinhexoside a,b |
| 41 | 60.4 | 211, 256, 356 | 771 | 301 (100), 609 (10), 463 (2) | quercetinhexosylrutinoside a,b |
| 43 | 62.7 | 207, 267, 356 | 463 | 301 (100) | quercetinhexoside a,b |
| 44 | 63.1 | 211, 256, 357 | 609 | 301 (100), 271 (15) | quercetinrutinoside a,b |
| 45 | 64.5 | 210, 267, 348 | 755 | 285 (100) | kaempferolhexosylrutinoside a,b |
| 46 | 67.3 | 210, 267, 348 | 755 | 285 (100) | kaempferolhexosylrutinoside a,b |
| 47 | 70.7 | 206, 268 | 447 | 285 (40), 284 (100), 327 (20) | kaempferolhexoside a,b |
| 48 | 70.7 | 207, 266, 345 | 593 | 285 (100) | kaempferolrutinoside a |
| 53 | 96.3 | 220, 270 | 885 | 739 (100), 431 (20), 285 (10) | kaempferol-3-O-p-coumaroyl-dirhamnosyl hexoside a |
| Tannins | |||||
| 2 | 7.71 | 216, 267 | 609 | 471 (100), 591 (80), 565 (20), 525 (30) | theasinensin C a |
| 3 | 9.6 | 210, 260 | 331 | 169 (100), 271 (80), 211 (40), 193 (20), 125 (15) | galloylglucosea,b |
| 5 | 18.1 | 211 | 609 | 483 (30), 441 (100), 423 (70), 305 (20), 591 (29) | prodelphinidin a,b |
| 7 | 22.0 | 211, 275 | 761 | 609 (100), 423 (80), 305 (20), 591 (70) | prodelphinidingallate b |
| 9 | 22.8 | 217, 270 | 761 | 609 (40), 591 (100), 453 (10) | theasinensin B a |
| 10 | 24.4 | 211, 278 | 577 | 425 (100), 407 (40), 289 (10), 451 (25) | procyanidin a |
| 12 | 29.4 | 210, 254 | 865 | 739 (15), 695 (100), 577 (45) | (epi)afzelechingallate-(epi)catechingallate a |
| 13 | 29.5 | 226, 275 | 633 | 301 (100), 463 (15) | strictinin a,b |
| 14 | 29.9 | 211, 278 | 577 | 425 (100), 407 (40), 289 (10), 451 (25) | procyanidin a,b |
| 17 | 32.5 | 223, 271 | 913 | 743 (100), 761 (50), 591 (80), 573 (45) | theasinensin C a |
| 18 | 34.0 | 221, 275 | 483 | 271 (100), 331 (20), 169 (10) | digalloylglucose b |
| 20 | 34.2 | 211, 276 | 745 | 559 (65), 407 (100), 619 (90), 577 (65), 441 (40) | (epi)catechin-(epi)gallocatechingallate a |
| 21 | 35.1 | 211, 278 | 577 | 425 (100), 407 (40), 289 (10), 451 (25) | procyanidin a,b |
| 22 | 35.9 | 211, 277 | 729 | 559 (100), 577 (95), 407 (20), 441 (5) 603 (25), 451 (35), 711 (15), 289 (10) | procyanidingallate a,b |
| 23 | 38.4 | 211, 277 | 729 | 407 (80), 577 (80), 711 (20), 559 (100), 28451 (50), 603 (50), 441 (50), 289 (10) | procyanidingallate a,b |
| 28 | 45.8 | 220, 280 | 635 | 465 (100), 483 (70), 313 (20) | trigalloylglucose b |
| Analyte | Concentration Range (μg/mL) | Calibration Curve | R2 |
|---|---|---|---|
| GA | 7–500 | y = 18.941x − 41.578 | 0.9999 |
| caffeine | 10–500 | y = 19.254x − 49.677 | 0.9999 |
| catechin | 25–500 | y = 5.4294x − 84.198 | 0.9998 |
| EGCG | 10–500 | y = 9.6778x − 51.975 | 0.9999 |
| ECG | 10–500 | y = 14.997x − 63.054 | 0.9999 |
| EC | 7–500 | y = 5.6054x − 13.496 | 0.9999 |
| µg/mL | GA | µg/mL | Caffeine | µg/mL | Catechin | µg/mL | EGCG | µg/mL | ECG | µg/mL | EC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| recovery (%) | 2.75 | 105.7 | 12 | 100.6 | 5 | 87.3 | 10 | 90.7 | 5 | 91.1 | 3.75 | 95.9 |
| 5.5 | 106.3 | 24.5 | 101.4 | 10 | 86.1 | 20 | 89.2 | 10 | 88.7 | 7.5 | 93.2 | |
| 11 | 103.1 | 49 | 101.0 | 20 | 87.5 | 40 | 87.8 | 20 | 86.4 | 15 | 91.3 | |
| Intraday precision (RSD %) | 2.75 | 0.5 | 12 | 0.5 | 5 | 0.7 | 10 | 0.1 | 5 | 0.3 | 3.75 | 0.5 |
| 5.5 | 0.3 | 24.5 | 0.1 | 10 | 0.2 | 20 | 0.1 | 10 | 0.3 | 7.5 | 0.3 | |
| 11 | 0.8 | 49 | 0.1 | 20 | 0.9 | 40 | 0.3 | 20 | 0.1 | 15 | 0.2 | |
| Interday precision (RSD %) | 2.75 | 1.2 | 12 | 0.3 | 5 | 1.8 | 10 | 3.4 | 5 | 3.6 | 3.75 | 0.5 |
| 5.5 | 2.3 | 24.5 | 0.3 | 10 | 0.5 | 20 | 4.6 | 10 | 1.9 | 7.5 | 0.4 | |
| 11 | 0.7 | 49 | 0.2 | 20 | 0.8 | 40 | 0.5 | 20 | 1.6 | 15 | 0.2 | |
| LOQ (µg/mL) | 2.6 | 0.4 | 11.5 | 7.2 | 1.2 | 0.6 | ||||||
| LOD (µg/mL) | 0.4 | 0.1 | 3.8 | 2.4 | 0.4 | 0.2 |
| Compound | GGABA | OGABA |
|---|---|---|
| Flavan-3-ols | ||
| catechin | 361.93 ± 3.9 | N.D. |
| epicatechin | 329.80 ± 2.43 | 33.70 ± 0.11 |
| epicatechin-gallate | 208.64 ± 0.65 | 46.67 ± 0.14 |
| epigallocatechingallate | 906.42 ± 0.7 | 94.81 ± 0.30 |
| Amino acids | ||
| GABA | 17.81 ± 0.55 | 22.25 ± 0.65 |
| Glutamic acid | 27.10 ± 0.33 | 15.19 ± 0.45 |
| Glutamine | 10.08 ± 0.10 | 4.39 ± 0.08 |
| Theanine | 90.84 ± 0.87 | 46.15 ± 0.93 |
| gallic acid | N.D. | 59.47 ± 0.31 |
| caffeine | 1039.85 ± 4.32 | 525.05 ± 4.28 |
| TBARS | SOD | Cat | GSH | |
|---|---|---|---|---|
| Swimming | −0.674 a p < 0.001 | 0.448 a p = 0.003 | 0.593 a p < 0.001 | 0.279 p = 0.076 |
| Climbing | −0.595 a p < 0.001 | 0.356 b p = 0.021 | 0.526 a p < 0.001 | 0.188 p = 0.234 |
| Immobility (DST) | 0.483 a p = 0.001 | −0.271 p = 0.088 | −0.366 b p = 0.020 | −0.020 p = 0.901 |
| Immobility (TST) | 0.568 a p < 0.001 | −0.339 b p = 0.029 | −0.468 a p = 0.002 | −0.129 p = 0.415 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Khanjani, S.; Moghaddam, A.H.; Braidy, N.; Nabavi, S.M. Improvement of Antioxidant Defences and Mood Status by Oral GABA Tea Administration in a Mouse Model of Post-Stroke Depression. Nutrients 2017, 9, 446. https://doi.org/10.3390/nu9050446
Daglia M, Di Lorenzo A, Nabavi SF, Sureda A, Khanjani S, Moghaddam AH, Braidy N, Nabavi SM. Improvement of Antioxidant Defences and Mood Status by Oral GABA Tea Administration in a Mouse Model of Post-Stroke Depression. Nutrients. 2017; 9(5):446. https://doi.org/10.3390/nu9050446
Chicago/Turabian StyleDaglia, Maria, Arianna Di Lorenzo, Seyed Fazel Nabavi, Antoni Sureda, Sedigheh Khanjani, Akbar Hajizadeh Moghaddam, Nady Braidy, and Seyed Mohammad Nabavi. 2017. "Improvement of Antioxidant Defences and Mood Status by Oral GABA Tea Administration in a Mouse Model of Post-Stroke Depression" Nutrients 9, no. 5: 446. https://doi.org/10.3390/nu9050446






