A Novel Millet-Based Probiotic Fermented Food for the Developing World
Abstract
:1. Introduction
2. Materials and Methods
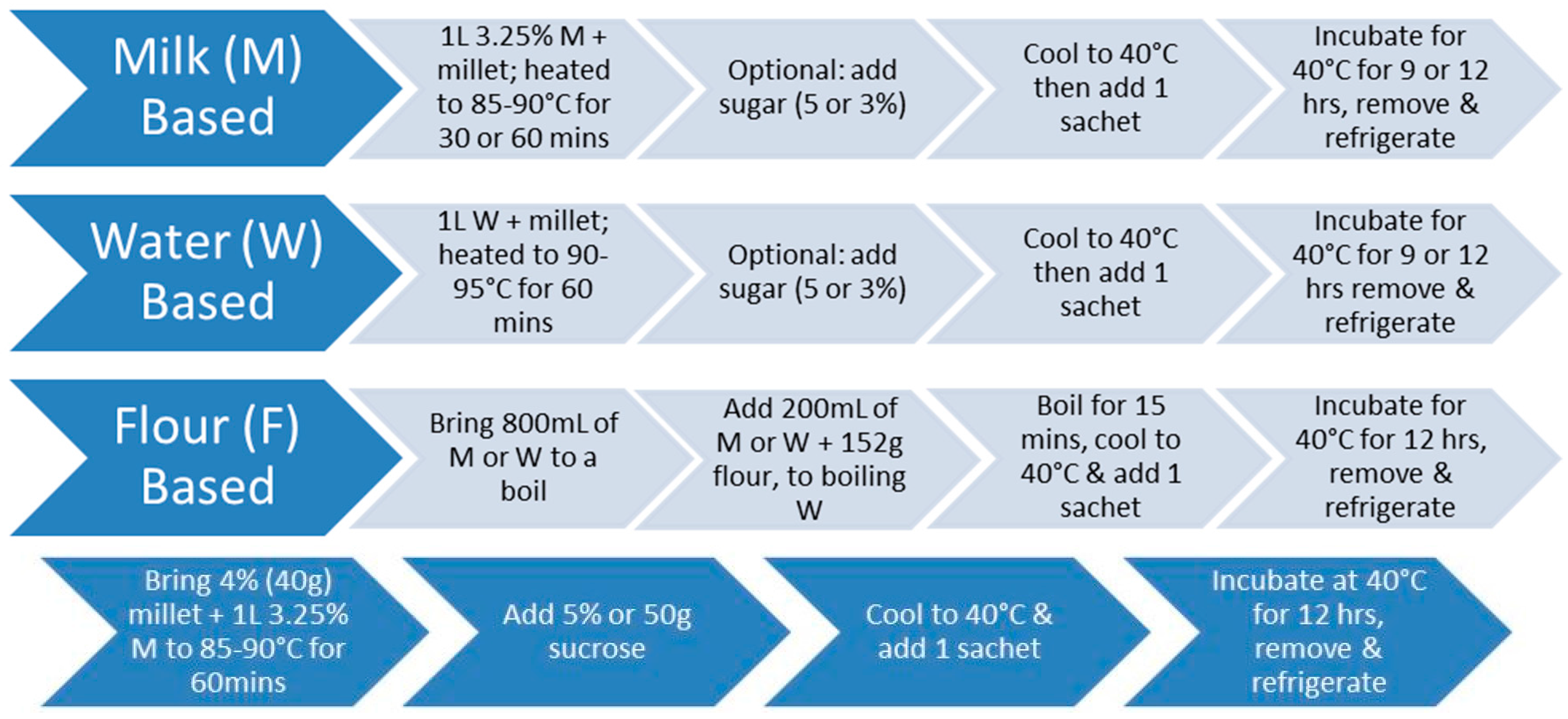
2.1. Cereal Matrix Preparation
2.1.1. Water-Based Millet
2.1.2. Milk-Based Millet
2.1.3. Dried Millet
2.1.4. Flour-Based Millet
2.2. Probiotic Strains and Preparation of Inocula
2.3. Production of Probiotic Fermented Products
2.4. Determination of Acidity
2.5. Bacterial Growth
2.6. Sensory Evaluation
2.7. Metabolite Analysis
2.8. Shelf-Life Tests
2.9. Statistical Analysis
3. Results
3.1. Fiti Sachet Used to Ferment Milk
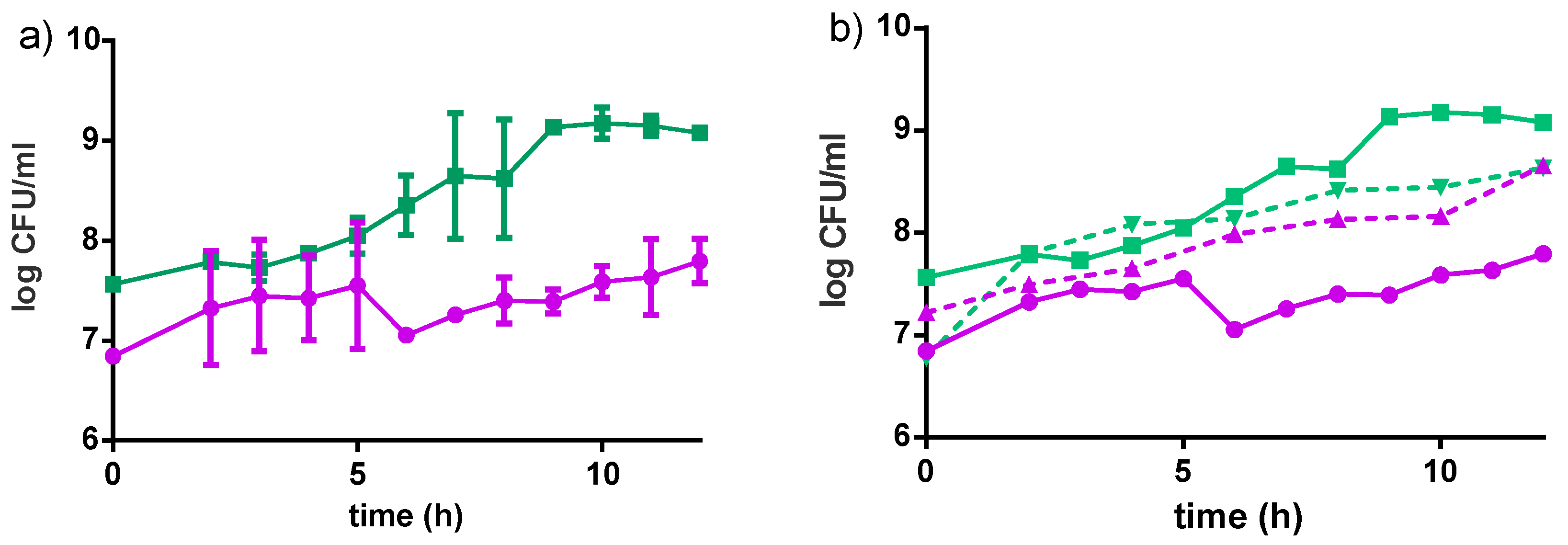
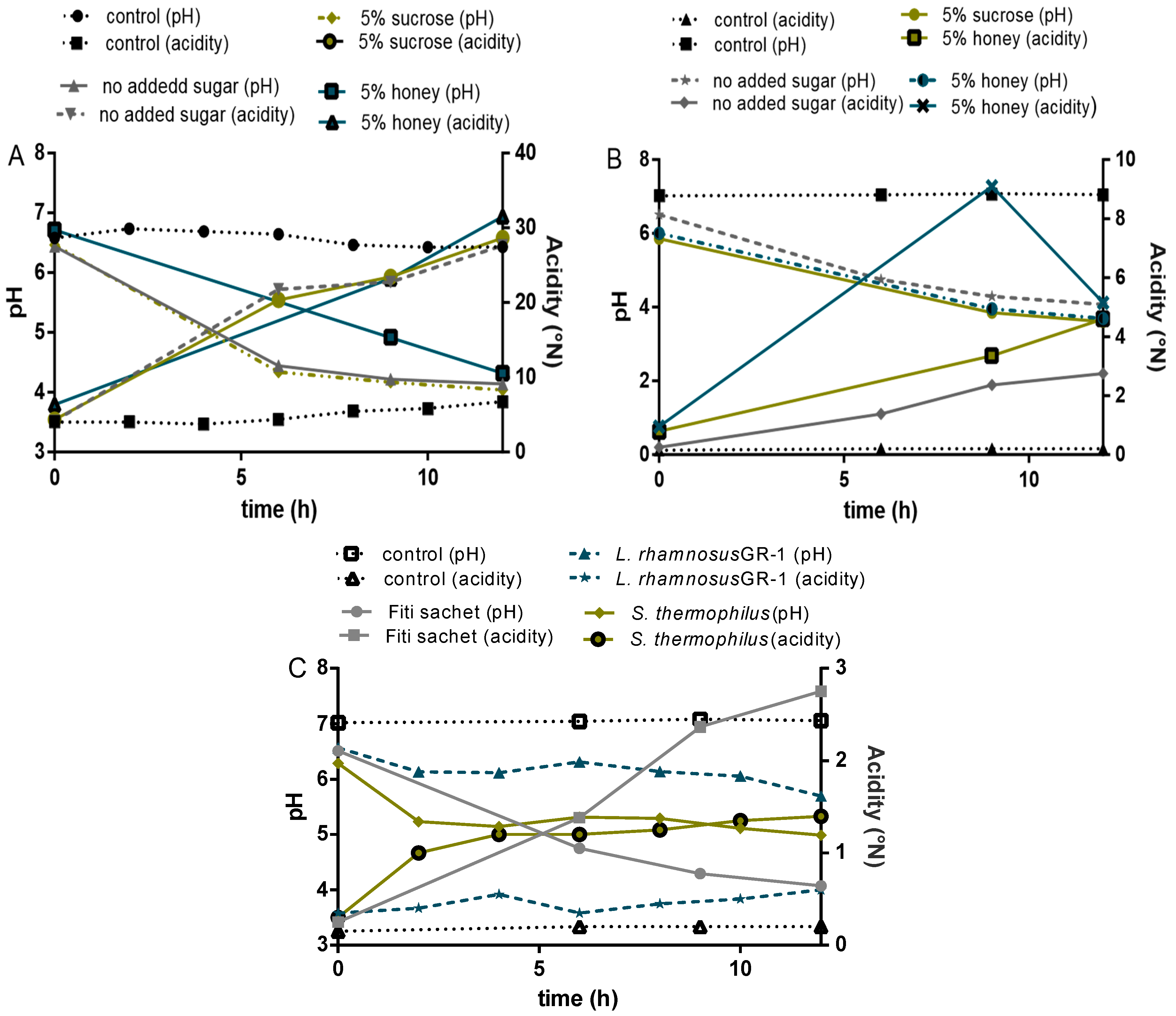
Cell Growth and Acidity Profile
3.2. Fiti Sachet in Millet Media
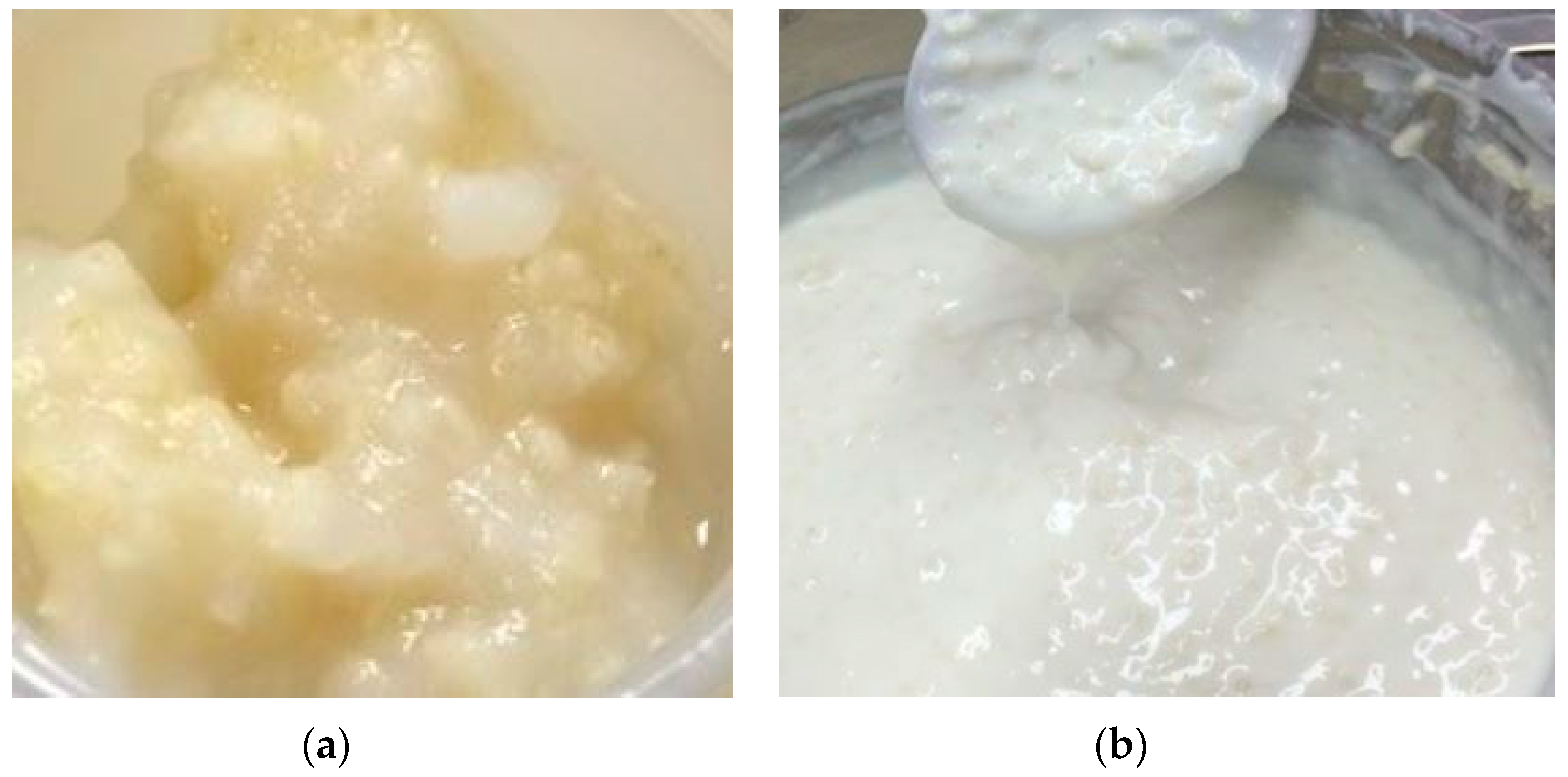
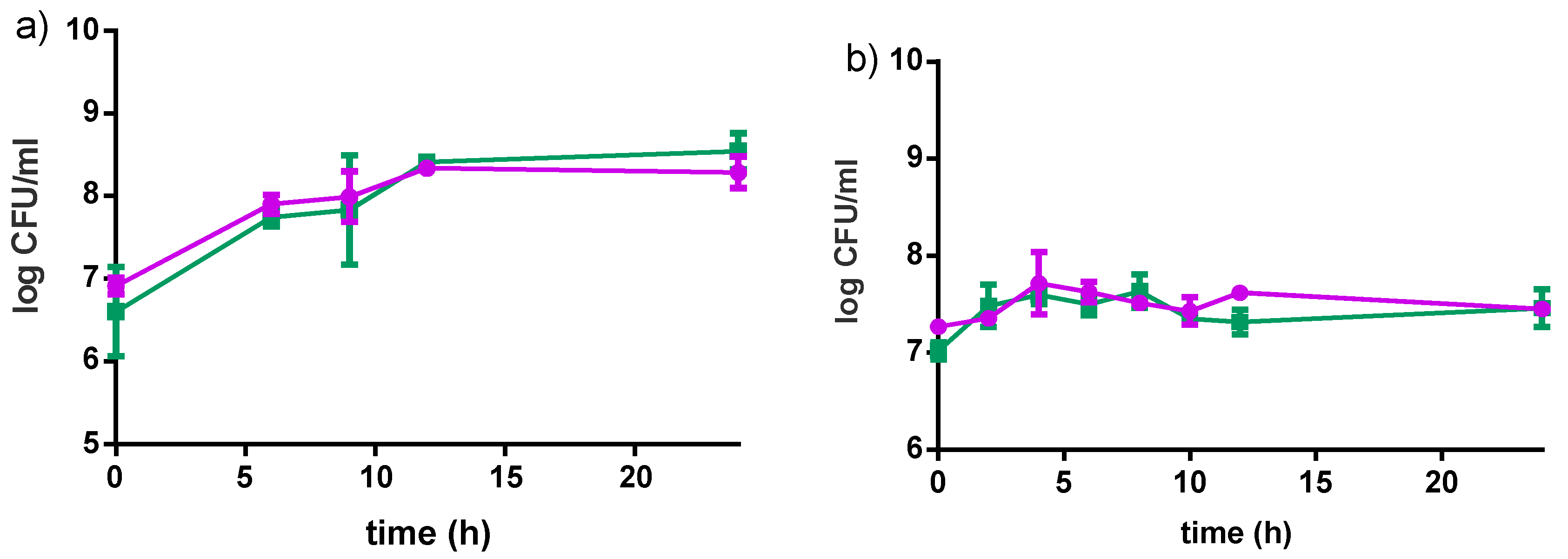
3.2.1. Bacterial Growth in Millet
3.2.2. Acidification Profile
3.2.3. Organic Acids and Carbohydrate Analysis
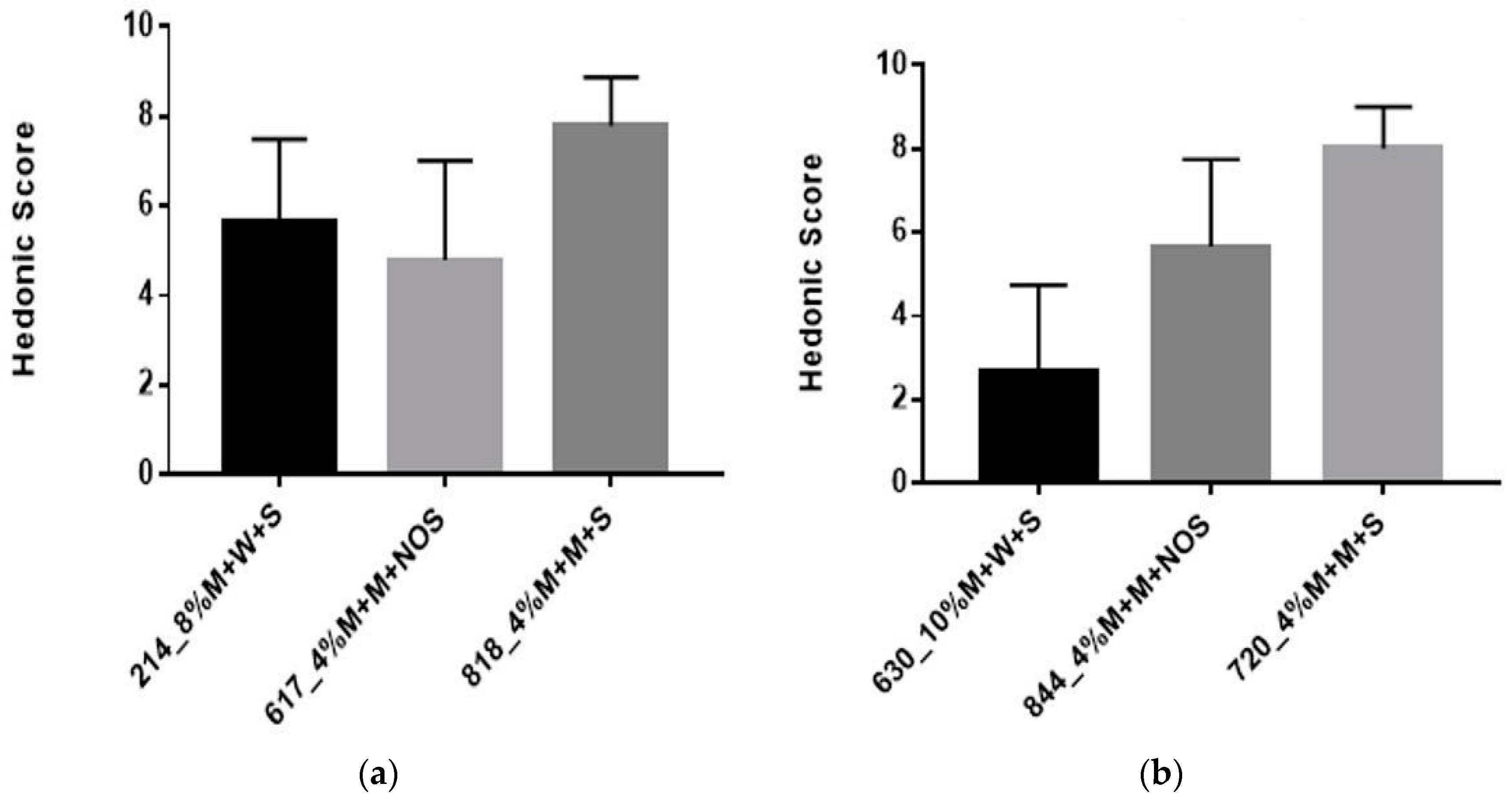
3.2.4. Sensory Evaluation
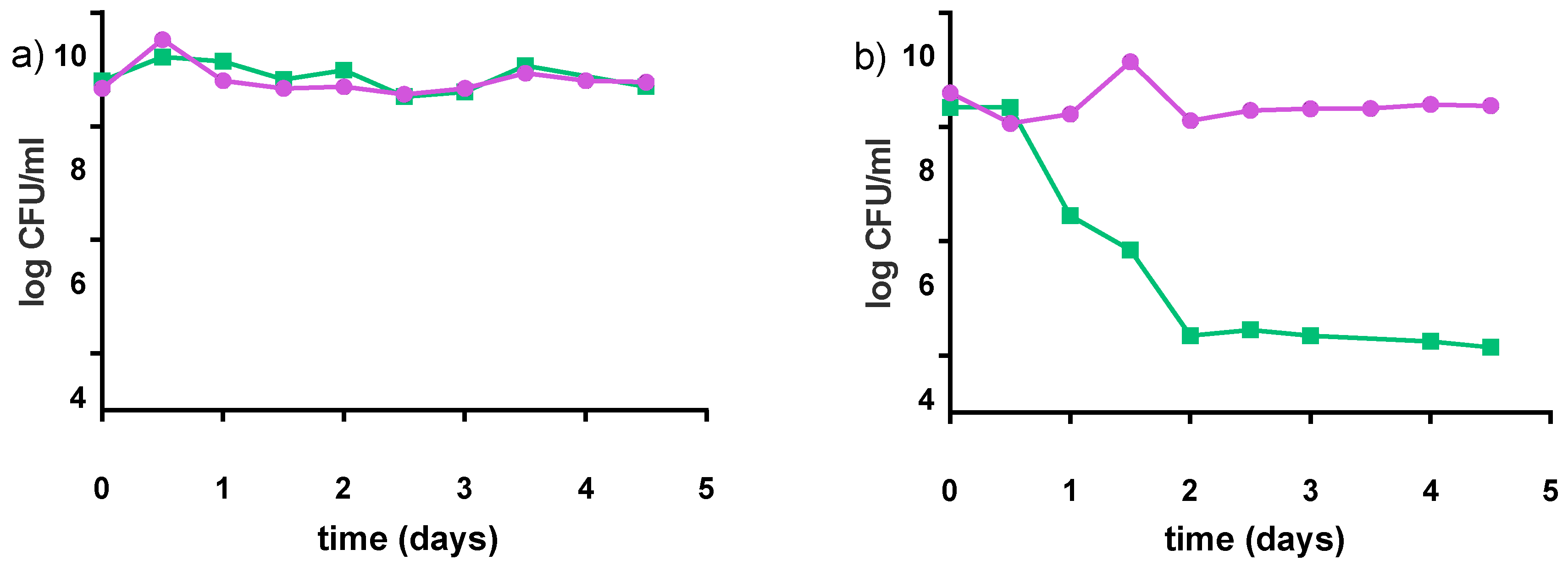
3.2.5. Stability at Non-Optimal Conditions
4. Discussion
4.1. Fermentation Ability of Fiti Sachet Bacteria
4.2. Product Development
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Organization for Economic Co-Operation and Development (OECD)/Food and Agriculture Organization (FAO). OECD-FAO Agricultural Outlook 2011–2020; OECD Publishing and FAO: Rome, Italy, 2011. [Google Scholar]
- Tou, E.H.; Mouquet-Rivier, C.; Picq, C.; Traorè, A.S.; Trèche, S.; Guyot, J.P. Improving the nutritional quality of ben-saalga, a traditional fermented millet-based gruel, by co-fermenting millet with ground nut and modifying the processing method. LWT 2007, 40, 1561–1569. [Google Scholar] [CrossRef]
- UN Inter-Agency Group for Child Mortality Estimation. Levels & Trends in Child Mortality Report; United Nations Children’s Fund: New York, NY, USA, 2013. [Google Scholar]
- Mosley, H.W.; Chen, L.C. An analytical framework for the study of child survival in developing countries. Popul. Dev. Rev. 2003, 81, 140–145. [Google Scholar] [CrossRef]
- Black, R.E.; Morris, S.S.; Bryce, J. Where and why are 10 million children dying each year? Lancet 2003, 361, 2226–2234. [Google Scholar] [CrossRef]
- Nannan, N.; Norman, R.; Hendricks, M.; Dhansay, M.A.; Bradshaw, D. Estimating the burden of disease attributable to childhood and maternal under nutrition in South Africa in 2000. S. Afr. Med. J. 2007, 97, 733–739. [Google Scholar] [PubMed]
- ACC/SCN Commission on the Nutrition Challenges of the 21th Century. Ending malnutrition by 2020: An agenda for change in the Millennium. Food Nutr. Bull. Suppl. 2000, 21, 88. [Google Scholar]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented foods: Patented approaches and formulations for nutritional supplementation and health promotion. Recent Pat. Food Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Nout, M.J.R.; Motarjemi, Y. Assessment of fermentation as a household technology for improving food safety: A joint FAO/WHO workshop. Food Contr. 1997, 8, 221–226. [Google Scholar] [CrossRef]
- Oyewole, O.B. Lactic fermented foods in Africa and their benefits. Food Contr. 1997, 8, 289–297. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Classification of fermented foods: Worldwide review of household fermentation techniques. Food Contr. 1997, 8, 311–317. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016, 8, 29630. [Google Scholar] [CrossRef] [PubMed]
- Charalampopulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Fedorak, R.; Kaufmann, P.; Karakan, T.; Kim, N.; et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Schnurer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations and World Health Organization Working Group: Geneva, Switzerland, 2002. [Google Scholar]
- Gull, A.; Prasad, K.; Kumar, P. Evaluation of functional, antinutritional, pasting and microstructural properties of Millet flours. J. Food Meas. Charact. 2016, 10, 96–102. [Google Scholar] [CrossRef]
- Chavan, J.K.; Kadam, S.S. Nutritional improvement of cereals by fermentation. Crit. Rev. Food Sci. Nutr. 1989, 28, 349–400. [Google Scholar] [CrossRef] [PubMed]
- Chavan, U.D.; Chavan, J.K.; Kadam, S.S. Effect of fermentation on soluble proteins and in vitro protein digestibility of sorghum, green gram and sorghum-green gram blends. J. Food Sci. 1988, 53, 1574–1575. [Google Scholar] [CrossRef]
- Lorri, W.; Svanberg, U. Lactic-fermented cereal gruels with improved in vitro protein digestibility. Int. J. Food Sci. Nutr. 1993, 44, 29–36. [Google Scholar] [CrossRef]
- Hamad, A.M.; Fields, M.L. Evaluation of the protein quality and available lysine of germinated and fermented cereals. J. Food Sci. 1979, 44, 456–459. [Google Scholar] [CrossRef]
- Sanni, A.I.; Onilude, A.A.; Ibidabpo, O.T. Biochemical composition of infant weaning food fabricated from fermented blends of cereals and soybean. Food Chem. 1999, 65, 35–39. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Sorghum and Millets in Human Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Reid, G. The potential role of probiotic yogurt for people living with HIV/AIDS. Gut Microbes 2010, 1, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Kort, R.; Westerik, N.; Serrano, L.M.; Douillard, F.P.; Gottstein, W.; Mukisa, I.M.; Tuijn, C.J.; Basten, L.; Hafkamp, B.; Meijer, C.; et al. A novel consortium of Lactobacillus rhamnosus and Streptococcus thermophilus for increased access to functional fermented foods. Microb. Cell Factories 2015, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Settachaimongkon, S.; Nout, M.J.R.; Antunes Fernandes, E.C.; Hettinga, K.A.; Vervoort, J.M.; van Hooijdonk, T.C.M.; Zwietering, M.H.; Smid, E.J.; van Valenberg, H.J. Influence of different proteolytic strains of Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. bulgaricus on the metabolite profile of set yoghurt. Int. J. Food Microbiol. 2014, 177, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; McMillan, A.; Angelini, M.; Gloor, G.B.; Sumarah, M.; Burton, J.P.; Reid, G. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome 2014, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.E. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J. Am. Soc. Mass Spectrom. 1999, 10, 770–781. [Google Scholar] [CrossRef]
- Styczynski, M.P.; Moxley, J.F.; Tong, L.V.; Walther, J.L.; Jensen, K.L.; Stephanopoulos, G.N. Systematic identification of conserved metabolites in GC/MS data for metabolomics and biomarker discovery. Anal. Chem. 2007, 79, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Haug, W.; Lantzsch, H.-J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Dietterich, L.H.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Fernando, N.; Fitzgerald, G.; Hasegawa, T.; et al. Impacts of elevated atmospheric CO2 on nutrient content of important food crops. Sci. Data 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Singh, V.; Simic, A.; Fang, Z. Effects of genotype and growth temperature on the contents of tannin, phytate and in vitro iron availability of sorghum grains. PLoS ONE 2016, 11, e0148712. [Google Scholar] [CrossRef] [PubMed]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria in milk and water-based cereal puddings. Int. Dairy J. 2004, 14, 957–965. [Google Scholar] [CrossRef]
- ISO/IDF. ISO/TS11869-IDF/RM150:2012. Fermented Milks Determination of Titratable Acidity Potentiometric Method, International Organisation for Standardisation: Geneva, Switzerland, 2012. [Google Scholar]
- Bodyfelt, F.W.; Tobias, J.; Trout, G.M. Sensory Evaluation of Dairy Products; NY7 VanRostrand Reinhold: New York, NY, USA, 1988; pp. 22–31. [Google Scholar]
- Hekmat, S.; Reid, G. Sensory properties of probiotic yogurt is comparable to standard yogurt. Nutr. Res. 2006, 26, 163–166. [Google Scholar] [CrossRef]
- Douillard, F.P.; Ribbera, A.; Kant, R.; Pietila, T.E.; Järvinen, M.; Messing, M.; Randazzo, C.L.; Paulin, L.; Laine, P.; Caggia, C.; et al. Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet. 2013, 9, e1003683. [Google Scholar] [CrossRef] [PubMed]
- Mercenier, A. Molecular genetics of Streptococcus thermophilus. FEMS Microbiol. Rev. 1990, 87, 61–77. [Google Scholar] [CrossRef]
- Poolman, B. Energy transduction in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, P.T.C. Catabolite Control of Sugar Metabolism in Streptococcus thermophilus. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2002. [Google Scholar]
- Hutkins, R.; Morris, H.A.; McKay, L. Galactokinase activity in Streptococcus thermophilus. Appl. Environ. Microbiol. 1985, 50, 777–780. [Google Scholar] [PubMed]
- Vaughan, E.E.; van den Bogaard, P.T.C.; Catzedu, P.; Kuipers, O.O.; de Vos, W.M. Activation of silent gal genes in the lac-gal regulation of Streptococcus thermophilus. J. Bacteriol. 2001, 183, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Salmeròn, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria, in maize porridge with added malted barley. Int. J. Food Microbiol. 2004, 91, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; van Sinderen, D.; Ventura, M. Genome analysis of food grade lactic acid-producing bacteria: From basics to applications. Curr. Genomics 2008, 9, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Wieser, H. Chemistry of cereal grains. In Handbook on Sourdough Biotechnology; Gobbetti, M., Ganzle, M., Eds.; Springer: New York, NY, USA, 2013; pp. 11–45. [Google Scholar]
- Hammes, W.P.; Vogel, R.F. The genus Lactobacillus. In The Genera of Lactic Acid Bacteria, 1st ed.; Wood, B.J.B., Holzapfel, W.H.N., Eds.; Chapman & Hall: Frimley Surrey, UK, 1995; Volume 2, pp. 19–54. [Google Scholar]
- Onyango, C.; Okoth, M.W.; Mbugua, S.K. Effect of drying lactic fermented uji (an East African sour porridge) on some carboxylic acids. J. Food Sci. Agric. 2000, 80, 1854–1858. [Google Scholar] [CrossRef]
- Gobbetti, M.; Corsetti, A. Lactobacillus sanfransiscod a key sourdough lactic acid bacterium: A review. Food Microbiol. 1997, 14, 175–187. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, S.K. Probiotic potential of Lactobacillus plantarum LD1 isolated from batter of DOSA, a South Indian fermented food. Probiotics Antimicrob. Proteins 2014, 6, 3–81. [Google Scholar] [CrossRef] [PubMed]
- Ghebrehiwot, H.M.; Shimelis, H.A.; Kirkman, K.P.; Laing, M.D.; Mabhaudhi, T. Nutritional and sensory evaluation of Injera prepared from tef and Eragrostis curvula (Schrad.) nees. Flours with sorghum blends. Front. Plant Sci. 2016, 7, 1059. [Google Scholar] [CrossRef] [PubMed]

| Carbon Source | L. rhamnosus GR-1 (µmax) | S. thermophilus C106 (µmax) |
|---|---|---|
| Dextrose | 0.4954 | 0.2669 |
| Fructose | 0.4045 | 0.1136 |
| Galactose | 0.4445 | 0.4251 |
| Lactose | 0.2311 | 0.8770 |
| Maltose | 0.0607 | 0.0959 |
| Melibiose | 0.0330 | 0.1397 |
| Raffinose | 0.0909 | 0.1348 |
| Sucrose | 0.0678 | 1.7046 |
| No C-source | 0.1029 | 0.0948 |
| Recipe | T = 0 h | T = 0 h | T = 9 h | T = 9 h | T = 12 h | T = 12 h |
|---|---|---|---|---|---|---|
| L. GR-1 | S. therm. | L. GR-1 | S. therm. | L. GR-1 | S. therm. | |
| Milk-Based | ||||||
| 4% millet, no added sugar | 9.33 E + 06 | 5.67 E + 06 | 4.00 E + 08 | 2.00 E + 09 | 6.70 E + 08 | 2.70 E + 09 |
| 4% millet, 5% sucrose | 6.50 E + 06 | 5.33 E + 06 | 2.50 E + 08 | 1.50 E + 09 | 9.00 E + 08 | 1.67 E + 09 |
| 4% millet, 5% honey | 2.25 E + 06 | 2.00 E + 06 | 2.10 E + 08 | 9.70 E + 08 | 3.50 E + 08 | 1.40 E + 09 |
| Flour, 3% sucrose | 3.67 E + 06 | 6.33 E + 06 | - | - | 7.67 E + 09 | 3.67 E + 10 |
| Flour, 50% water + 50% milk | 3.67 E + 06 | 6.00 E + 06 | - | - | 8.33 E + 09 | 4.67 E + 10 |
| Water-Based | ||||||
| 8% millet, 5% sucrose | 7.67 E + 06 | 4.67 E + 06 | - | - | 9.33 E + 07 | 3.30 E + 07 |
| 8% millet, no added sugar | 1.22 E + 07 | 7.88 E + 06 | 2.19 E + 08 | 2.52 E + 08 | 2.29 E + 08 | 3.62 E + 08 |
| Compound | T = 0 h (µg/mL) | T = 12 h (µg/mL) |
|---|---|---|
| d-Glucose | 986.2 | 52.6 |
| Sucrose | 847.8 | 341.9 |
| d-Mannose | 667.4 | Not detected |
| Lactic Acid | 99.0 | 806.8 |
| Acetic Acid | 2.3 | 3.2 |
| 1,2-Butandiol | 2.1 | 3.2 |
| Sample Code | Sample Composition |
|---|---|
| 214 | 8% millet in water, 60 min pre-treatment, 5% sucrose |
| 630 | 10% millet in water, 60 min pre-treatment, 3% sucrose |
| 818/720 | 4% millet in milk, 60 min pre-treatment, 5% sucrose, 12 h fermentation |
| 617/844 | 4% millet in milk, 60 min pre-treatment, 12 h fermentation |
| M4 | 4% millet in milk, 30 min pre-treatment, 5% sucrose, 12 h fermentation |
| M5 | 4% millet in milk, 60 min pre-treatment, 5% sucrose, 9 h fermentation |
| Panel Test A | Panel Test B | |
|---|---|---|
| % yogurt consumed/week | 89.5 | 67 |
| % porridge consumed/week | 47.4 | 67 |
| % milk based porridge consumed/week | 36.8 | 100 |
| % water based porridge consumed/week | 42.1 | 33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, E.; White, J.; Seney, S.; Hekmat, S.; McDowell, T.; Sumarah, M.; Reid, G. A Novel Millet-Based Probiotic Fermented Food for the Developing World. Nutrients 2017, 9, 529. https://doi.org/10.3390/nu9050529
Di Stefano E, White J, Seney S, Hekmat S, McDowell T, Sumarah M, Reid G. A Novel Millet-Based Probiotic Fermented Food for the Developing World. Nutrients. 2017; 9(5):529. https://doi.org/10.3390/nu9050529
Chicago/Turabian StyleDi Stefano, Elisa, Jessica White, Shannon Seney, Sharareh Hekmat, Tim McDowell, Mark Sumarah, and Gregor Reid. 2017. "A Novel Millet-Based Probiotic Fermented Food for the Developing World" Nutrients 9, no. 5: 529. https://doi.org/10.3390/nu9050529





