Body Composition Analysis Allows the Prediction of Urinary Creatinine Excretion and of Renal Function in Chronic Kidney Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.2.1. Body Composition Analysis: Measurement of Body Cell Mass
2.2.2. Measurement and Prediction of Renal Function: 24-h Urinary Creatinine Excretion, Creatinine Clearance, Glomerular Filtration Rate
2.3. Statistical Analysis
3. Results
3.1. Anthropometric, Clinical and Laboratory Data: Body Composition, and Urinary Creatinine Excretion
3.2. Predicted versus Measured Urinary Creatinine Excretion
3.3. Predicted versus Measured Creatinine Clearance
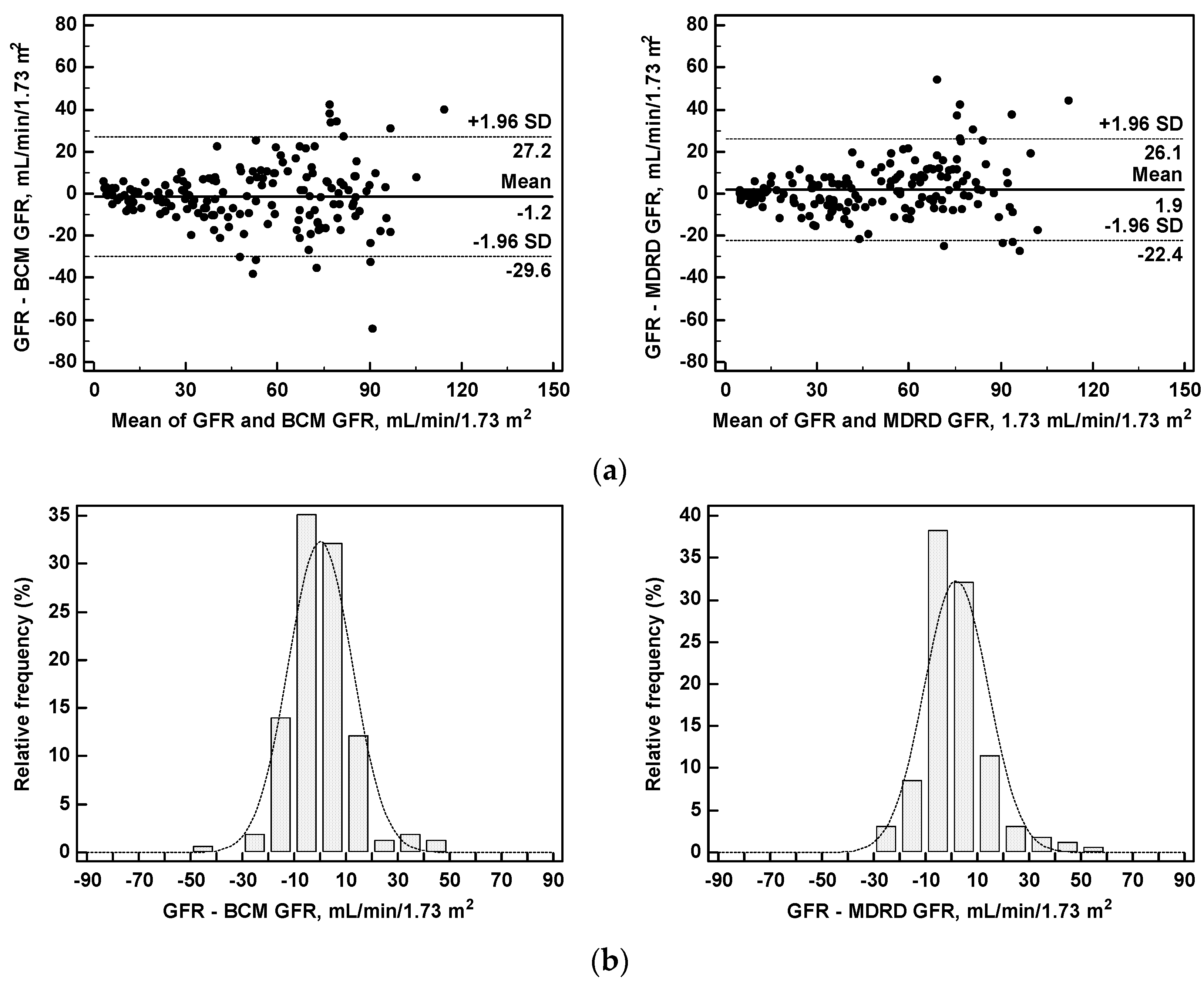
3.4. Predicted versus Measured Glomerular Filtration Rate
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Prigent, A. Monitoring renal function and limitations of renal function tests. Semin. Nucl. Med. 2007, 38, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R. Time to scrap creatinine clearance? Br. Med. J. 1986, 293, 1119–1120. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Ransil, B.J.; Harmatz, J.S.; Smith, T.W.; Duhme, D.W.; Koch-Weser, J. Variability of 24-hour urinary creatinine excretion by normal subjects. J. Clin. Pharmacol. 1976, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Lowrie, E.G.; Wilmore, D.W.; Gonzales, J.; Lew, N.L.; Ling, J.; Leboff, M.S.; Gottlieb, M.N.; Huang, W.; Zebrowski, B.; et al. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J. Am. Soc. Nephrol. 1995, 6, 75–81. [Google Scholar] [PubMed]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [PubMed]
- Cooper, B.A.; Aslani, A.; Ryan, M.; Zhu, F.J.-P.; Ibels, L.S.; Allen, B.J.; Pollock, C.A. Comparing different methods of assessing body composition in end-stage renal failure. Kidney Int. 2000, 58, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C.; Consani, C.; Ardini, M.; Bernabini, G.; Caprio, F.; Grassi, G.; Lucchesi, A.; Nerucci, B. Estimate of body water compartments and of body composition in maintenance hemodialysis patients: Comparison of single and multifrequency bioimpedance analysis. J. Ren. Nutr. 2005, 15, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Forbes, G.B.; Bruining, G.J. Urinary creatinine excretion and lean body mass. Am. J. Clin. Nutr. 1976, 29, 1359–1366. [Google Scholar] [PubMed]
- Lukaski, H.C. Methods for the assessment of human body composition: Traditional and new. Am. J. Clin. Nutr. 1987, 46, 537–556. [Google Scholar] [PubMed]
- Donadio, C.; Lucchesi, A.; Tramonti, G.; Bianchi, C. Creatinine clearance predicted from body cell mass is a good indicator of renal function. Kidney Int. 1997, 52, S166–S168. [Google Scholar]
- Donadio, C.; Lucchesi, A.; Tramonti, G.; Bianchi, C. Prediction of creatinine clearance from body composition analysis and plasma creatinine. Ren. Fail. 1998, 20, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C.; Consani, C.; Ardini, M.; Caprio, F.; Grassi, G.; Lucchesi, A. Prediction of glomerular filtration rate from body cell mass and plasma creatinine. Curr. Drug Discov. Technol. 2004, 1, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Donadio, C.; Tramonti, G. Noninvasive methods for the measurement of renal function. Nephron 1981, 28, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Bonadio, M.; Donadio, C.; Tramonti, G.; Figus, S. Measurement of glomerular filtration rate in man using DTPA-Tc99m. Nephron 1979, 24, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Meng, X.L.; Rosenthal, R.; Rubin, D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992, 111, 172–175. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991; pp. 336–357. [Google Scholar]
- Lalonde, R.L.; Pao, D. Correlation coefficient versus prediction error in assessing the accuracy of digoxin dosing methods. Clin. Pharm. 1984, 3, 178–183. [Google Scholar] [PubMed]
- Tape, T.G. Interpreting Diagnostic Tests. University of Nebraska Medical Center. Available online: http://gim.unmc.edu/dxtests/roc3.htm (accessed on 15 March 2016).
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachexia Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.H.; Hak, L.J.; Koch, G.G.; Wargin, W.A.; Chi, S.L.; Mattocks, A.M. Influence of range of renal function and liver disease on predictability of creatinine clearance. Clin. Pharmacol. Ther. 1981, 29, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Rolin, H.A., III; Hall, P.M.; Wei, R. Inaccuracy of estimated creatinine clearance for prediction of iothalamate glomerular filtration rate. Am. J. Kidney Dis. 1984, 4, 48–54. [Google Scholar] [CrossRef]
- Hossain, M.A.; Attia, A.; Shoker, A. Measurement error in estimated GFR slopes across transplant chronic kidney disease stages. Am. J. Nephrol. 2010, 31, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Botev, R.; Mallié, J.P.; Wetzels, J.F.; Couchoud, C.; Schück, O. The clinician and estimation of glomerular filtration rate by creatinine-based formulas: Current limitations and quo vadis. Clin. J. Am. Soc. Nephrol. 2011, 6, 937–950. [Google Scholar] [CrossRef] [PubMed]
- El-Minshawy, O.; El-Bassuoni, E. Validity of current equations to estimate glomerular filtration rate in kidney transplant recipients. Transplant. Proc. 2013, 45, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Gaspari, F.; Remuzzi, G. Assessing Renal Function by GFR Prediction Equations in Kidney Transplantation. Am. J. Transplant. 2005, 5, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Collier, V.U.; Walser, M. Creatinine metabolism in chronic renal failure. Clin. Sci. 1980, 58, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C.; Kanaki, A.; Donadio, E.; Tognotti, D. Assessment of nutritional status and risk of death in maintenance haemodialysis patients. Healthmed 2010, 4, 210–215. [Google Scholar]
- Flury, S.; Trachsler, J.; Schwarz, A.; Ambühl, P.M. Quantification of excretory renal function and urinary protein excretion by determination of body cell mass using bioimpedance analysis. BMC Nephrol. 2015, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C.; Ardini, M.; Bernabini, G.; Caprio, F.; Consani, C.; Grassi, G. Prediction of glomerular filtration rate in overweight and obese chronic kidney disease patients. In 12th Meeting in Cardionephrology; Timio, M., Wizemann, V., Venanzi, S., Eds.; Editoriale Bios: Cosenza, Italy, 2005; pp. 61–64. [Google Scholar]





| Parameters | All Patients n = 165 | Women n = 72 | Men n = 93 | Significance | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Median | IQR 25–75 | Median | IQR 25–75 | Median | IQR 25–75 | p | |
| Age, years | 17–81 | 57.0 | 46–65 | 58.5 | 53–65 | 53.0 | 40.8–65.3 | 0.0142 |
| Height, cm | 143–188 | 1620 | 154–172 | 154 | 151–159 | 170 | 165–176 | <0.0001 |
| Body weight, kg | 44.4–120.3 | 69.8 | 61.3–78.1 | 62.0 | 57.3–71.4 | 74.9 | 68.0–83.1 | <0.0001 |
| Body surface, m2 | 1.34–2.38 | 1.77 | 1.59–1.88 | 1.59 | 1.55–1.71 | 1.84 | 1.78–1.96 | <0.0001 |
| Body mass index, kg/m2 | 18.4–38.0 | 26.3 | 24.0–29.2 | 26.1 | 24.0–29.8 | 26.3 | 23.9–29.2 | 0.620 |
| Serum creatinine, mg/dL | 0.57–14.4 | 1.29 | 1.05–2.11 | 1.19 | 0.95–1.67 | 1.48 | 1.10–3.18 | <0.0006 |
| 24-h UCr, mg | 444–2102 | 1077 | 842–1337 | 883 | 738–1017 | 1270 | 1083–1506 | <0.0001 |
| Body cell mass, kg | 12.2–45.2 | 24.4 | 18.6–28.7 | 18.7 | 17.1–22.1 | 28.4 | 25.3–32.5 | <0.0001 |
| Fat mass, kg | 3.2–61.2 | 19.7 | 15.5–23.2 | 21.4 | 17.0–26.7 | 17.8 | 13.6–22.2 | 0.0003 |
| Extra-cellular water, kg | 8.7–46.3 | 15.5 | 13.2–17.4 | 12.9 | 11.7–14.9 | 16.6 | 15.3–19.1 | <0.0001 |
| 24-h UCr/Body weight, mg/kg | 8.0–26.3 | 15.8 | 12.8–18.0 | 13.9 | 11.4–15.8 | 17.3 | 15.1–19.8 | <0.0001 |
| 24-h UCr/ Body cell mass, mg/kg | 23.7–70.7 | 45.8 | 39.2–52.3 | 44.7 | 39.1–51.9 | 46.4 | 41.5–52.4 | 0.40 |
| Independent Variables | Coefficient | Standard Error | Rpartial | t | Probability | VIF |
|---|---|---|---|---|---|---|
| Constant | 522 | |||||
| BCM, kg | 14.99 | 4.50 | 0.256 | 3.332 | 0.0011 | 4.582 |
| Age, years | −7.56 | 1.16 | −0.459 | −6.513 | <0.0001 | 1.472 |
| Serum creatinine, mg/dL | −31.76 | 6.73 | −0.351 | −4.719 | <0.0001 | 1.086 |
| Body weight, kg | 8.54 | 1.73 | 0.365 | 4.934 | <0.0001 | 2.575 |
| Gender, male = 1, female = 0 | 169.91 | 39.41 | 0.324 | 4.311 | <0.0001 | 2.019 |
| Parameters | All Patients n = 165 | Women n = 72 | Men n = 93 | Statistical Significance | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Median | IQR 25–75 | Median | IQR 25–75 | Median | IQR 25–75 | p | |
| Measured UCr, mg | 444–2102 | 1077 | 842–1337 | 858 | 713–1020 | 1261 | 1085–1504 | <0.0001 |
| MR-BCM-UCr, mg | 520–1883 | 1073 | 869–1328 | 853 | 754–985 | 1305 | 1139–1450 | <0.0001 |
| MPE, mg | 175 | 163 | 184 | |||||
| Measured CCr, mL/min/1.73 m2 | 5.4–109.6 | 53.1 | 30.9–76.8 | 53.3 | 38.5–73.8 | 52.7 | 24.0–83.6 | 0.839 |
| MR-BCM CCr, mL/min/1.73 m2 | 5.1–107.0 | 54.4 | 30.3–75.4 | 55.0 | 36.6–72.6 | 55.0 | 36.6–72.6 | 0.903 |
| MPE, mL/min/1.73 m2 | 8.88 | 11.2 | 6.6 | |||||
| C&G CCr, mL/min/1.73 m2 | 7.0–118.5 | 53.4 | 30.4–78.0 | 53.7 | 38.1–73.5 | 50.9 | 25.0–83.0 | 0.427 |
| MPE, mL/min/1.73 m2 | 9.72 | 12.2 | 7.3 | |||||
| GFR, mL/min/1.73 m2 | 3.5–134.4 | 51.2 | 25.2–73.7 | 52.7 | 33.1–69.4 | 39.4 | 20.1–74.9 | 0.422 |
| BCM GFR, mL/min/1.73 m2 | 0.2–107.6 | 51.4 | 29.2–71.5 | 52.2 | 34.2–66.5 | 46.9 | 22.5–74.3 | 0.9790 |
| MPE, mL/min/1.73 m2 | 12.4 | 13.4 | 11.6 | |||||
| MDRD GFR, mL/min/1.73 m2 | 4.1–111.0 | 47.7 | 27.2–66.5 | 47.9 | 31.4–61.2 | 46.9 | 19.6–70.1 | 0.627 |
| MPE, mL/min/1.73 m2 | 12.5 | 14.4 | 10.9 | |||||
| BCM GFR, AUC | Cut-Off | Sensitivity Specificity | MDRD GFR, AUC | Cut-Off | Sensitivity Specificity | p | |
|---|---|---|---|---|---|---|---|
| GFR <90 mL/min/1.73 m2 | 0.858 | 55.5 | 59.5 100 | 0.832 | 55.6 | 62.8 91.7 | 0.175 |
| GFR <60 mL/min/1.73 m2 | 0.947 | 55.5 | 87.8 92.5 | 0.961 | 52.4 | 87.8 92.5 | 0.184 |
| GFR <45 mL/min/1.73 m2 | 0.981 | 51.1 | 94.9 91.9 | 0.984 | 45.9 | 94.9 93.0 | 0.630 |
| GFR <30 mL/min/1.73 m2 | 0.987 | 37.5 | 97.9 92.3 | 0.982 | 33.1 | 94.8 93.2 | 0.232 |
| GFR <15 mL/min/1.73 m2 | 0.998 | 21.4 | 100 97.1 | 0.995 | 15.5 | 96.3 98.6 | 0.120 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadio, C. Body Composition Analysis Allows the Prediction of Urinary Creatinine Excretion and of Renal Function in Chronic Kidney Disease Patients. Nutrients 2017, 9, 553. https://doi.org/10.3390/nu9060553
Donadio C. Body Composition Analysis Allows the Prediction of Urinary Creatinine Excretion and of Renal Function in Chronic Kidney Disease Patients. Nutrients. 2017; 9(6):553. https://doi.org/10.3390/nu9060553
Chicago/Turabian StyleDonadio, Carlo. 2017. "Body Composition Analysis Allows the Prediction of Urinary Creatinine Excretion and of Renal Function in Chronic Kidney Disease Patients" Nutrients 9, no. 6: 553. https://doi.org/10.3390/nu9060553





