Omega 3 Polyunsaturated Fatty Acids Improve Endothelial Dysfunction in Chronic Renal Failure: Role of eNOS Activation and of Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vivo Uremic Model and Procedures
2.3. Biochemical Parameters
2.4. Analysis of Vascular Reactivity
2.5. Western Blot
2.6. 3-Nitrotyrosine
2.7. Statistical Analysis
3. Results
3.1. Animal Characteristics and Phenotype
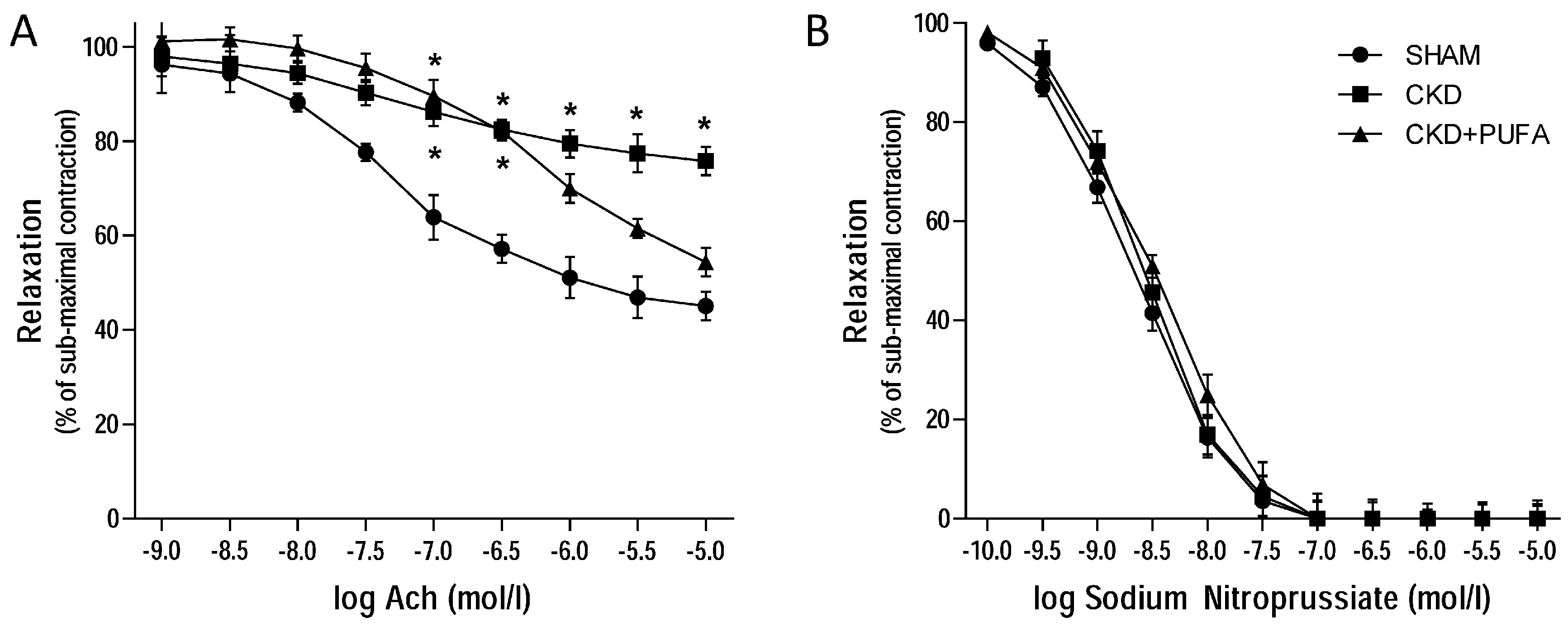
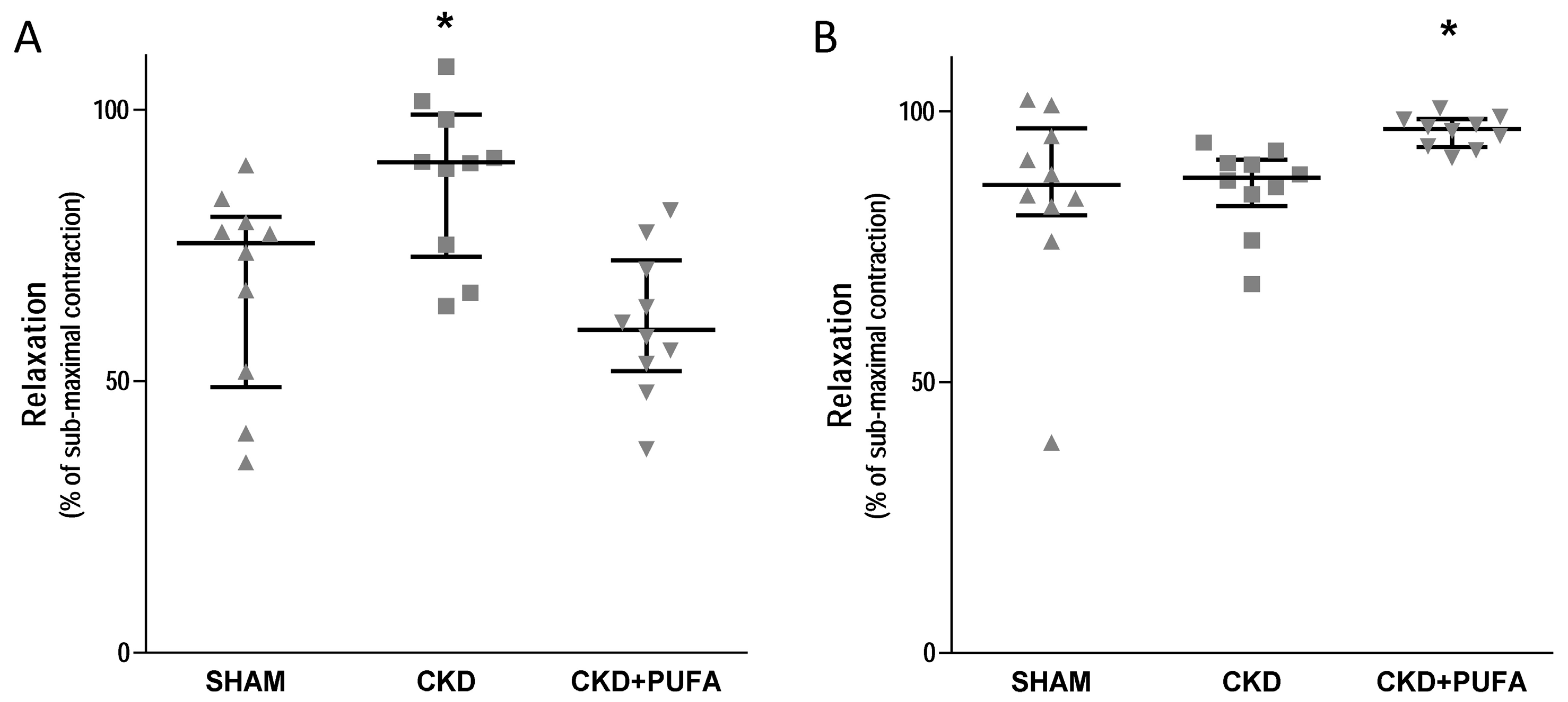
3.2. Vascular Reactivity
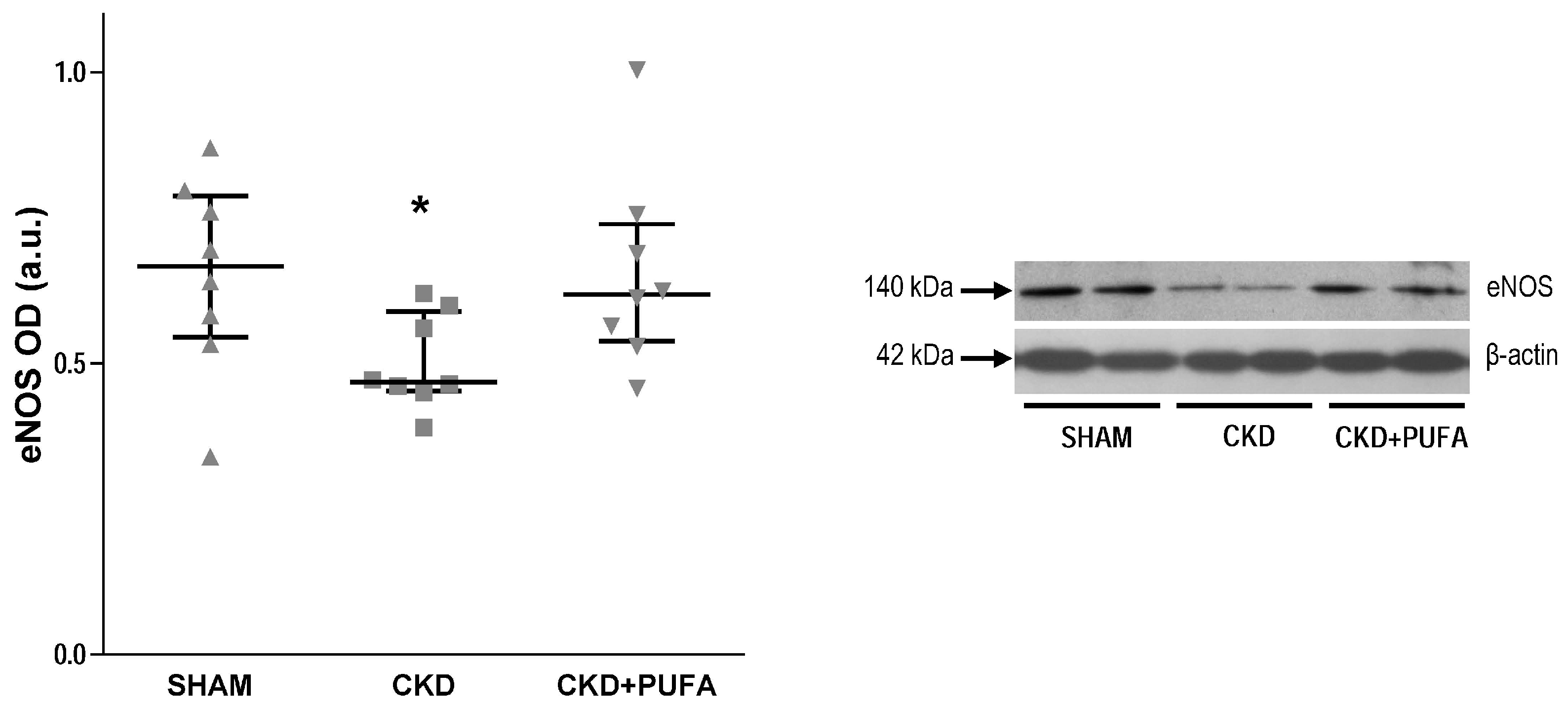
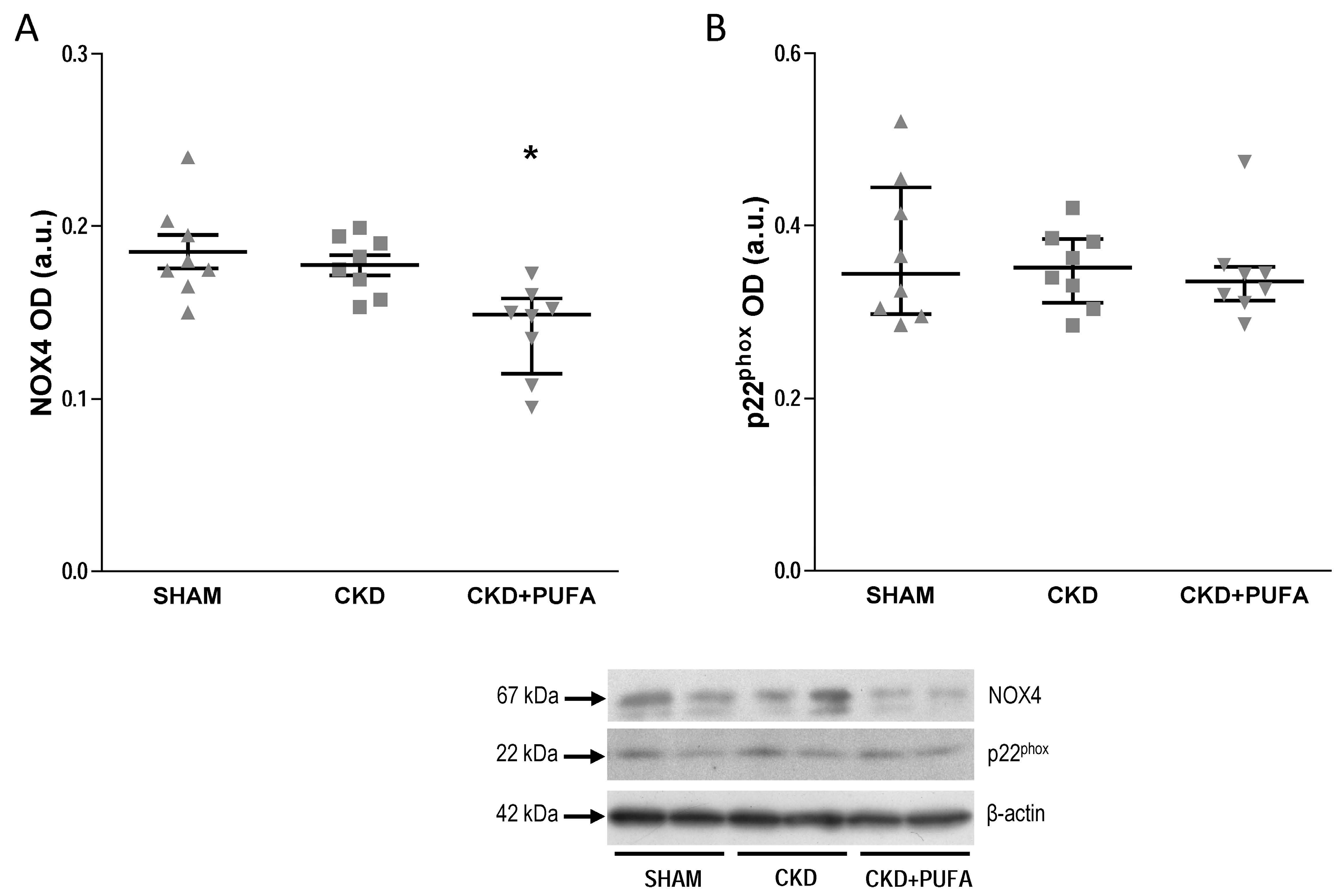
3.3. Aortic eNOS, NADPH Oxidase Subunits NOX4 and p22phox Expression
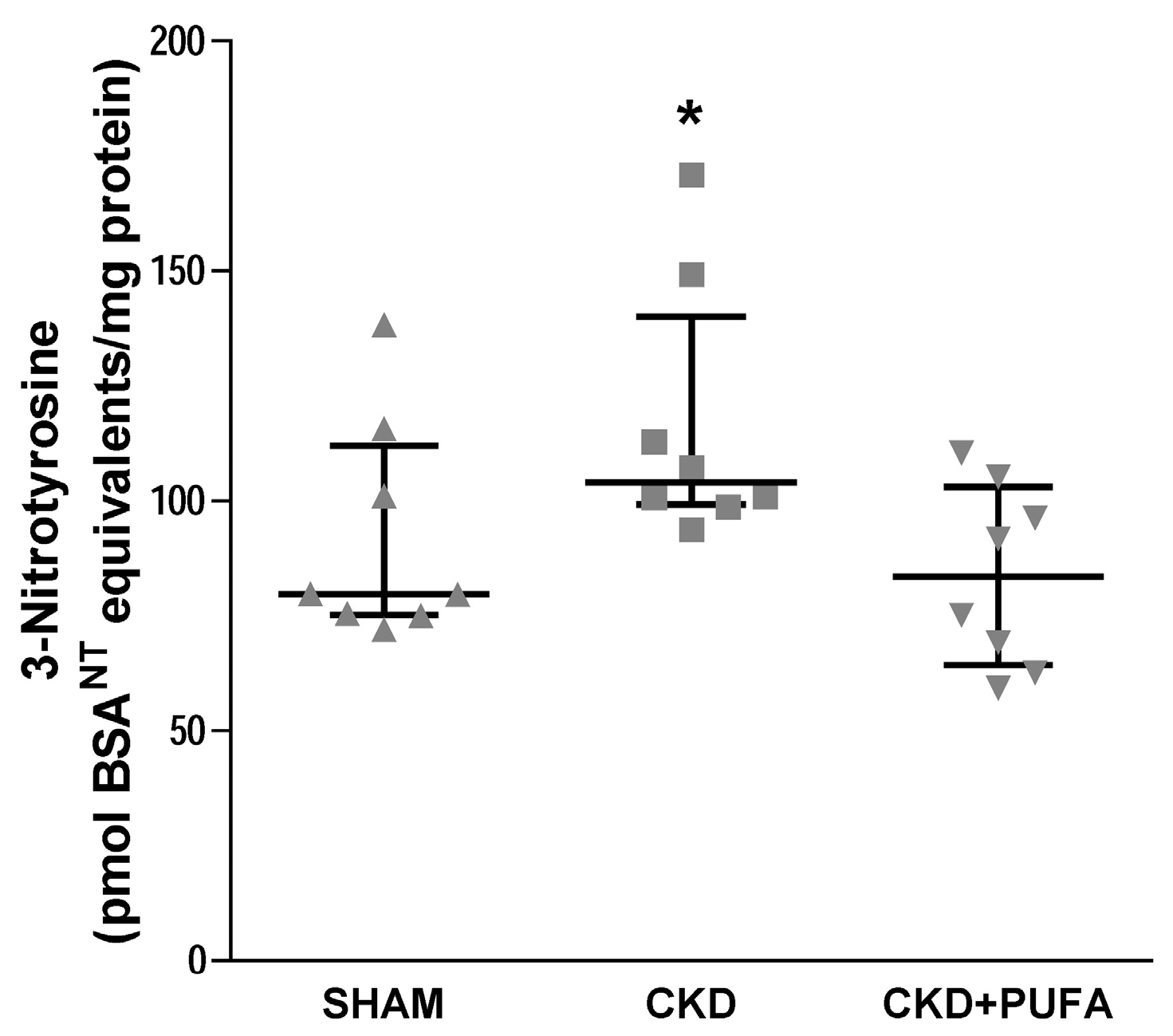
3.4. Aortic 3-Nitrotyrosine Expression
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balakumar, P.; Taneja, G. Fish oil and vascular endothelial protection: Bench to bedside. Free Rad. Biol. Med. 2012, 53, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, X.; Wang, L.; Lu, X.; Huang, J.; Cao, J.; Li, H.; Gu, D. Effect of omega-3 fatty acids supplementation on endothelial function: A meta-analysis of randomized controlled trials. Atherosclerosis 2012, 221, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Stehle, P. Impact of n-3 fatty acids on endothelial function: Results from human interventions studies. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, I.; Ayer, A.; Johnson, AD.; Ganz, P.; Mills, C.; Donovan, C.; Scherzer, R.; Shah, S.J.; Peralta, C.A.; Dubin, R.F. Sex differences in vascular dysfunction and cardiovascular outcomes: The cardiac, endothelial function, and arterial stiffness in ESRD (CERES) study. Hemodial. Int. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, L.; Mao, X.D.; Peng, W. Improvement of Huangqi decoction on endothelial dysfunction in 5/6 nephrectomized rats. Cell. Physiol. Biochem. 2016, 40, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Spradley, F.T.; White, J.J.; Paulson, W.D.; Pollock, D.M.; Pollock, J.S. Differential regulation of nitric oxide synthase function in aorta and tail artery from 5/6 nephrectomized rats. Physiol. Rep. 2013, 1, e00145. [Google Scholar] [CrossRef] [PubMed]
- Nesher, N.; Frolkis, I.; Schwartz, D.; Chernichovski, T.; Levi, S.; Pri-Paz, Y.; Chernin, G.; Shtabsky, A.; Ben-Gal, Y.; Paz, Y.; et al. L-arginine improves endothelial function, independently of arginine uptake, in aortas from chronic renal failure female rats. Am. J. Physiol. 2014, 306, F449–F456. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Giardinelli, A.; Sirolli, V.; Riganti, C.; Di Tomo, P.; Gazzano, E.; Di Silvestre, S.; Panknin, C.; Cortese-Krott, M.M.; Csonka, C.; et al. Nitric oxide synthetic pathway and cGMP levels are altered in red blood cells from end-stage renal disease patients. Mol. Cell. Biochem. 2016, 417, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Hasdan, G.; Benchetrit, S.; Rashid, G.; Green, J.; Bernheim, J.; Rathaus, M. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002, 61, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Chonchol, M.; Ikizler, T.A.; Farmer-Bailey, H.; Salas, N.; Chaudhry, R.; Wang, W.; Smits, G.; Tengesdal, I.; Dinarello, C.A.; et al. IL-1 Inhibition and Vascular Function in CKD. J. Am. Soc. Nephrol. 2017, 28, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hamm, L.L.; Mohler, E.R.; Hudaihed, A.; Arora, R.; Chen, C.S.; Liu, Y.; Browne, G.; Mills, K.T.; Kleinpeter, M.A.; et al. Interrelationship of Multiple Endothelial Dysfunction Biomarkers with Chronic Kidney Disease. PLoS ONE 2015, 10, e0132047. [Google Scholar]
- Sikorska-Wiśniewska, M.; Mika, A.; Śledziński, T.; Małgorzewicz, S.; Stepnowski, P.; Rutkowski, B.; Chmielewski, M. Disorders of serum omega-3 fatty acid composition in dialyzed patients, and their associations with fat mass. Ren. Fail. 2017, 39, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Kakiya, R.; Hayashi, T.; Tsujimoto, Y.; Sonoda, M.; Shima, H.; Mori, K.; Fukumoto, S.; Tahara, H.; Shioi, A.; et al. Serum n-3 and n-6 polyunsaturated fatty acid profile as an independent predictor of cardiovascular events in hemodialysis patients. Am. J. Kidney Dis. 2013, 62, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.M.; Shirley, D.G. Time course of the renal function response to partial nephrectomy: Measurements in conscious rats. Exp. Physiol. 2006, 92, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Semolic, A.; Ruozi, G.; Vinci, P.; Guarnieri, G.; Bortolotti, F.; Barbetta, D.; Zanetti, M.; Giacca, M.; Barazzoni, R. Unacylated ghrelin normalizes skeletal muscle oxidative stress and prevents muscle catabolism by enhancing tissue mitophagy in experimental chronic kidney disease. FASEB J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gortan Cappellari, G.; Burekovic, I.; Barazzoni, R.; Stebel, M.; Guarnieri, G. Caloric restriction improves endothelial dysfunction during vascular aging: Effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp. Gerontol. 2010, 45, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Barazzoni, R.; Cattin, L.; Muro, A.F.; Zanetti, M. Lack of fibronectin extra domain A alternative splicing exacerbates endothelial dysfunction in diabetes. Sci. Rep. 2016, 6, 37965. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Losurdo, P.; Mazzucco, S.; Panizon, E.; Jevnicar, M.; Macaluso, L.; Fabris, B.; Barazzoni, R.; Biolo, G.; Carretta, R.; et al. Treatment with n-3 polyunsaturated fatty acids reverses endothelial dysfunction and oxidative stress in experimental menopause. J. Nutr. Biochem. 2013, 24, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Svensson, M.; Povlsen, J.V.; Schmidt, E.B.; Aalkjær, C.; Christensen, J.H.; Ivarsen, P. Long chain n-3 polyunsaturated fatty acids and vascular function in patients with chronic kidney disease and healthy subjects: A cross-sectional and comparative study. BMC Nephrol. 2016, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.; Chattopadhyay, A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim. Biophys. Acta 2016, 1858, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fu, F.; Tie, R.; Liang, X.; Tian, F.; Xing, W.; Li, J.; Ji, L.; Xing, J.; Sun, X.; et al. Alpha-linolenic acid intake prevents endothelial dysfunction in high-fat diet-fed streptozotocin rats and underlying mechanisms. Vasa 2013, 42, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Casos, K.; Zaragozà, M.; Zarkovic, N.; Andrisic, L.; Portero-Otin, M.; Cacabelod, D.; Mitjavila, M.T. A fish-oil-rich diet reduces vascular oxidative stress in apoE(-/-) mice. Free Radic. Res. 2010, 44, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.M.; Booker, C.; Ellis, C.D.; Siew, E.D.; Graves, A.J.; Shintani, A.; Abumrad, N.N.; Himmerfalb, J.; Ikizler, T.A. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol. Dial. Transplant. 2015, 30, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Aihara, K.; Fukuda, D.; Takashima, A.; Hara, T.; Hotchi, J.; Ise, T.; Yamaguchi, K.; Tobiume, T.; Iwase, T.; et al. Effects of docosahexaenoic acid on the endothelial function in patients with coronary artery disease. J. Atheroscler. Thromb. 2015, 22, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Okano, K.; Tsuruta, Y.; Tsuruta, Y.; Tsuchiya, K.; Akiba, T.; Nitta, K. Eicosapentaenoic Acid (EPA) Decreases the All-Cause Mortality in Hemodialysis Patients. Intern. Med. 2015, 54, 3133–3137. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Schmidt, E.B.; Jorgensen, K.A.; Christensen, J.H.; on behalf the OPACH Study Groups. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo-controlled intervention trial. Clin. J. Am. Soc. Nephrol. 2006, 1, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Burke, V.; Mas, E.; Beilin, L.J.; Puddey, I.B.; Watts, G.F.; Irish, A.B.; Mori, T.A. n-3 fatty acids reduce plasma 20-hydroxyeicosatetraenoic acid and blood pressure in patients with chronic kidney disease. J. Hypertens. 2015, 33, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Annuk, M.; Zilmer, M.; Fellstrom, B. Endothelium-dependent vasodilation and oxidative stress in chronic renal failure: Impact on cardiovascular disease. Kidney Int. 2003, 84, S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Lee, CH.; Lee, S.D.; Ou, H.C.; Lai, S.C.; Cheng, Y.J. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int. J. Mol. Sci. 2014, 15, 10334–10349. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Orta, X.; Casós, K.; Sáiz, M.P.; Puig-Parellada, P.; Farriol, M.; Mitjavila, M.T. Upregulation of endothelial nitric oxide synthase in rat aorta after ingestion of fish oil-rich diet. Am. J. Physiol. 2004, 287, H567–H572. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.B.; Kwon, S.K.; Kwon, M.; Nagar, H.; Jeon, B.H.; Irani, K.; Yoon, S.H.; Kim, C.S. Docosahexaenoic acid improves vascular function via up-regulation of SIRT1 expression in endothelial cells. Biochem. Biophys. Res. Commun. 2013, 437, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, C.L.; Stice, J.P.; Hart, C.M.; Mbai, F.N.; Knowlton, A.A. Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Omura, M.; Kobayashi, S.; Mizukami, Y.; Mogami, K.; Todoroki-Ikeda, N.; Miyake, T.; Matsuzaki, M. Eicosapentaenoic acid (EPA) induces Ca(2+)-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001, 487, 361–366. [Google Scholar] [CrossRef]
- Zanetti, M.; Grillo, A.; Losurdo, P.; Panizon, E.; Mearelli, F.; Cattin, L.; Barazzoni, R.; Carretta, R. Omega-3 Polyunsaturated Fatty Acids: Structural and Functional Effects on the Vascular Wall. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.C.; Heagerty, A.M.; Bund, S.J.; Lawal, T.S.; Riemersma, R.A. Effect of dietary polyunsaturated fatty acids on contraction and relaxation of rat femoral resistance arteries. J. Cardiovasc. Pharmacol. 1994, 23, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Poulianiti, K.P.; Kaltsatou, A.; Mitrou, G.I.; Jamurtas, A.Z.; Koutedakis, Y.; Maridaki, M.; Stefanidis, I.; Sakkas, G.K.; Karatzaferi, C. Systemic redox imbalance in chronic kidney disease: A systematic review. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qin, Z.; Tang, J.; Han, P.; Xing, Q.; Wang, K.; Yu, J.; Zhou, G.; Tang, M.; Wang, W.; et al. Omega-3 polyunsaturated fatty acids ameliorates testicular ischemia-reperfusion injury through the induction of Nrf2 and inhibition of NF-κB in rats. Exp. Mol. Pathol. 2017, 24, 30282–30284. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.L.; McLennan, P.L.; Owen, A.J.; Theiss, M.L. Low dietary fish-oil threshold for myocardial membrane n-3 PUFA enrichment independent of n-6 PUFA intake in rats. J. Lipid Res. 2010, 51, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Wei, W.; Li, X. Effect of fish oil supplementation on fasting vascular endothelial function in humans: A meta-analysis of randomized controlled trials. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
| Measurement | SHAM | CKD | CKD + PUFA |
|---|---|---|---|
| N | 10 | 10 | 10 |
| Initial body weight (g) | 359 ± 7 | 362 ± 4 | 358 ± 6 |
| Final body weight (g) | 452 ± 7 | 420 ± 10 * | 412 ± 15 * |
| Average daily caloric intake (kcal/day) | 63.9 ± 1.9 | 62.0 ± 2.7 | 65.2 ± 2.2 |
| Urea (mg/dL) | 18.5 ± 3 | 30.3 ± 1 * | 30 ± 1 * |
| Creatinine (mg/dL) | 16.7 ± 1.2 | 26.8 ± 1.9 * | 29.6 ± 1.8 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanetti, M.; Gortan Cappellari, G.; Barbetta, D.; Semolic, A.; Barazzoni, R. Omega 3 Polyunsaturated Fatty Acids Improve Endothelial Dysfunction in Chronic Renal Failure: Role of eNOS Activation and of Oxidative Stress. Nutrients 2017, 9, 895. https://doi.org/10.3390/nu9080895
Zanetti M, Gortan Cappellari G, Barbetta D, Semolic A, Barazzoni R. Omega 3 Polyunsaturated Fatty Acids Improve Endothelial Dysfunction in Chronic Renal Failure: Role of eNOS Activation and of Oxidative Stress. Nutrients. 2017; 9(8):895. https://doi.org/10.3390/nu9080895
Chicago/Turabian StyleZanetti, Michela, Gianluca Gortan Cappellari, Davide Barbetta, Annamaria Semolic, and Rocco Barazzoni. 2017. "Omega 3 Polyunsaturated Fatty Acids Improve Endothelial Dysfunction in Chronic Renal Failure: Role of eNOS Activation and of Oxidative Stress" Nutrients 9, no. 8: 895. https://doi.org/10.3390/nu9080895





