Effects of β-Glucans Ingestion on Alveolar Bone Loss, Intestinal Morphology, Systemic Inflammatory Profile, and Pancreatic β-Cell Function in Rats with Periodontitis and Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diabetes Induction
2.3. Induction of Periodontal Disease
2.4. β-Glucan
2.5. β-Cell Function (HOMA-BF)
2.6. TNF-α e IL-10 Measurement
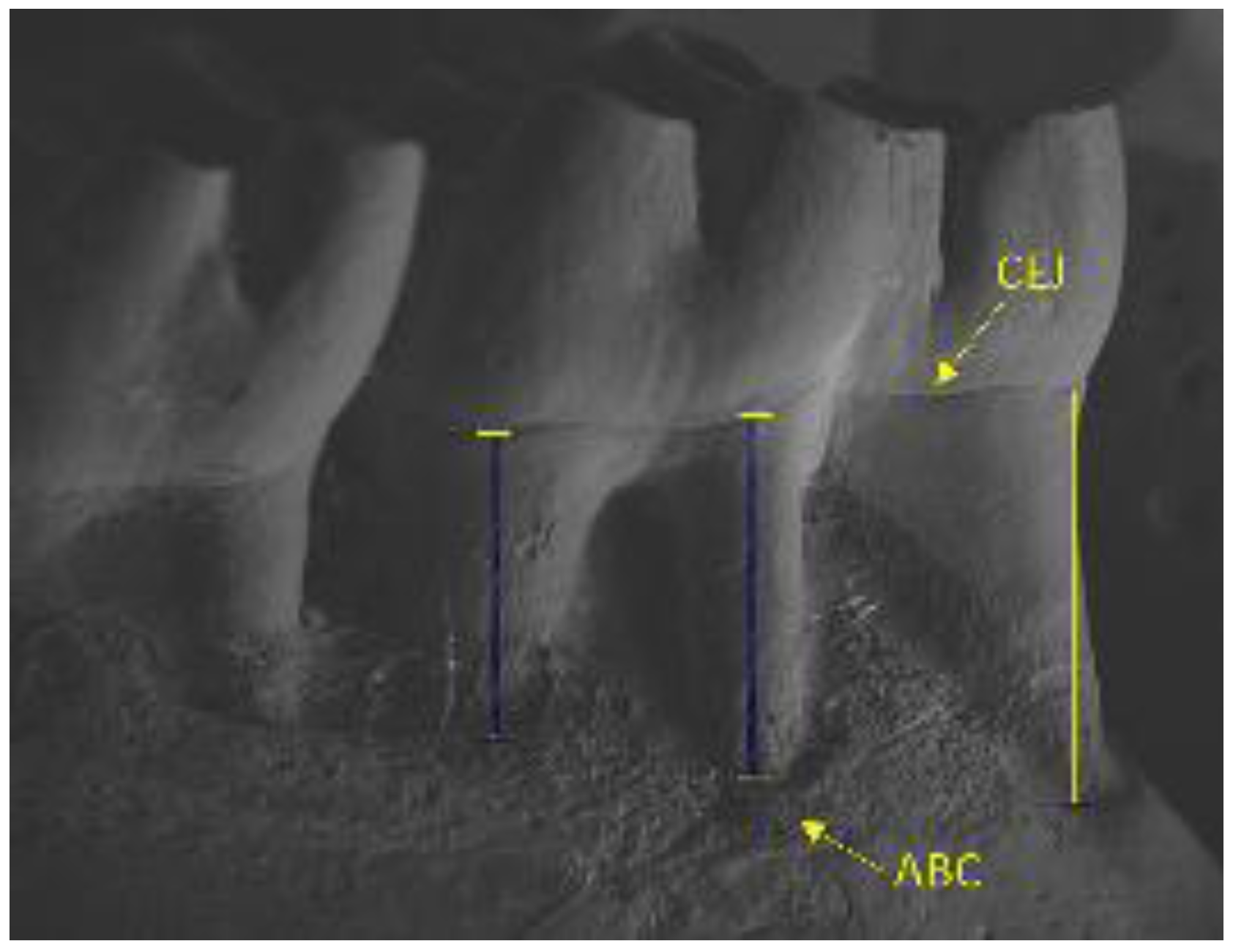
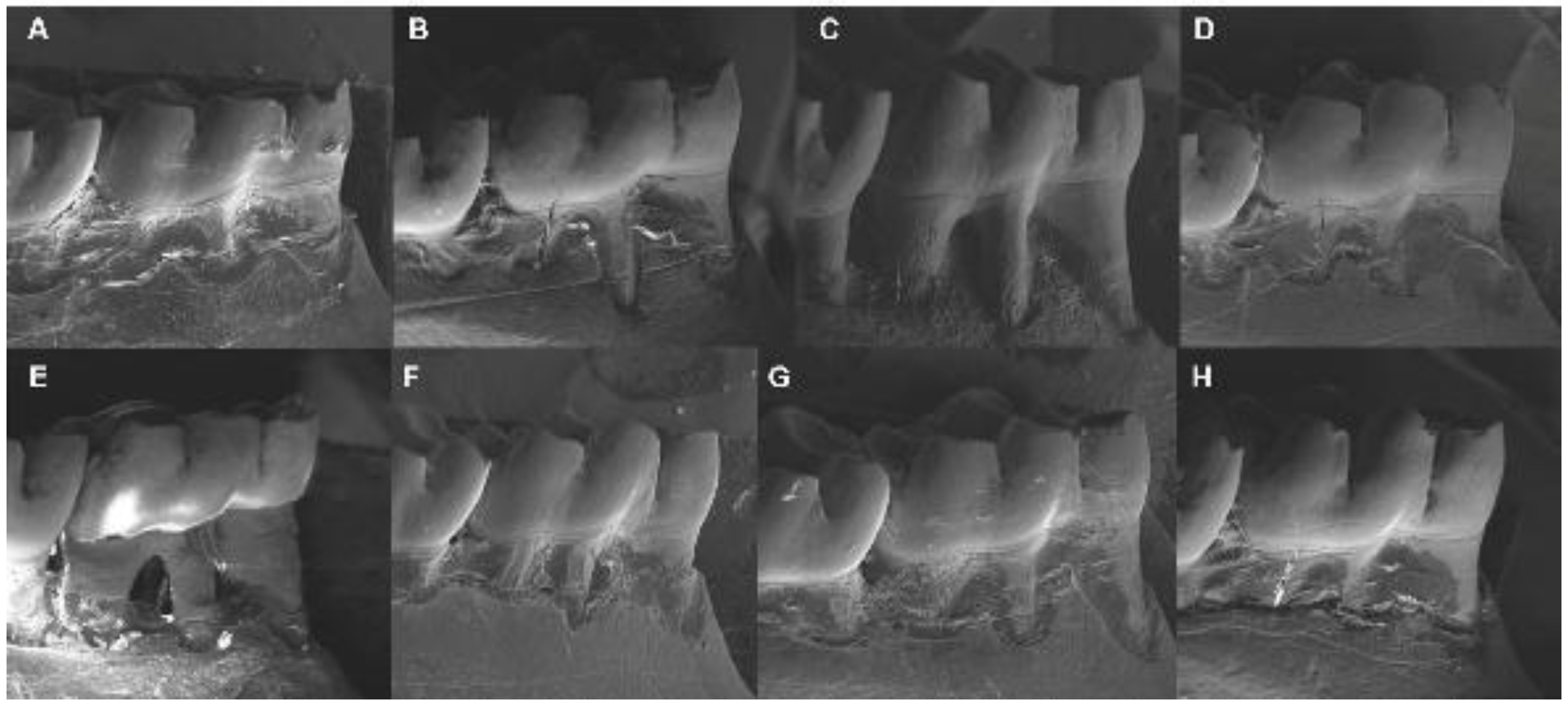
2.7. Alveolar Bone Loss by Scanning Electron Microscopy
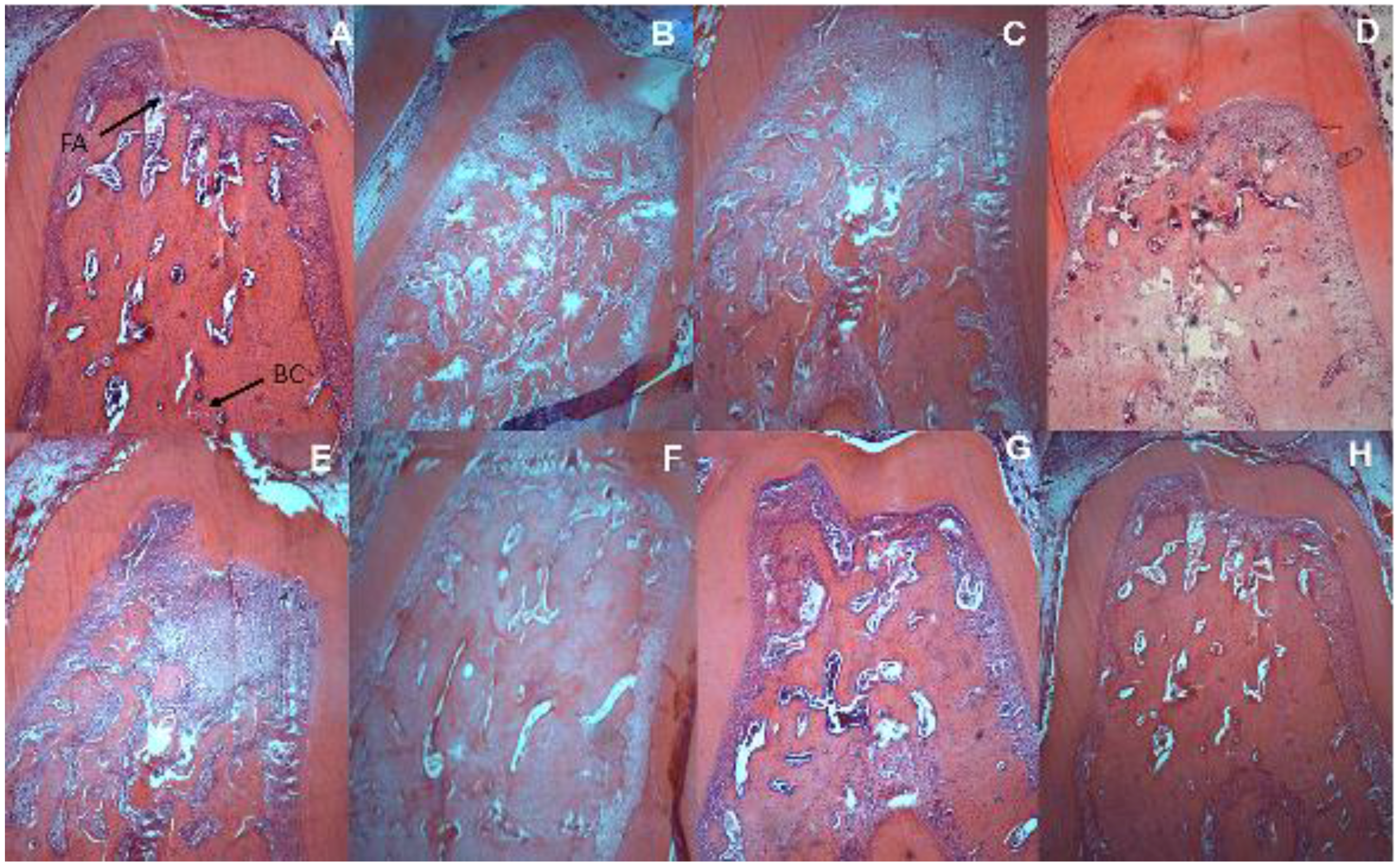
2.8. Histometric Analysis of Periodontal and Intestinal Tissue
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chapple, I.L.C.; Genco, R. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40 (Suppl. 1), S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Al-Khabbaz, A.K.; Al-Shammari, K.F. Diabetes mellitus and periodontal health: Dentists’ knowledge. Med. Princ. Pract. 2011, 20, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Effects of the aqueous extract of white tea (Camellia sinensis) in a streptozotocin-induced diabetes model of rats. Phytomedicine 2011, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Emrich, L.J.; Shlossman, M.; Genco, R.J. Periodontal Disease in Non-Insulin-Dependent Diabetes Mellitus. J. Periodontol. 1991, 62, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Mesia, R.; Gholami, F.; Huang, H.; Clare-Salzler, M.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Systemic inflammatory responses in patients with type 2 diabetes with chronic periodontitis. BMJ Open Diabetes Res. Care 2016, 4, e000260. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M. Glycemic Control and Alveolar Bone Loss Progression in Type 2 Diabetes. Ann. Periodontol. 1998, 3, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Yalda, B.; Collins, J.G.; Jones, B.H.; Smith, F.W.; Arnold, R.R.; Offenbacher, S. Inflammatory Mediator Response as a Potential Risk Marker for Periodontal Diseases in Insulin-Dependent Diabetes Mellitus Patients. J. Periodontol. 1997, 68, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.d.B.; Barroso, R.F.F.; Burbano, R.M.R.; Garcia, P.A.; Pinto, P.D.D.C.; Santos, N.P.C.D.; Santos, S.E.B.; Ribeiro-Dos-Santos, A.K.C. Effect of ancestry on interleukin-10 haplotypes in chronic periodontitis. Front. Biosci. (Elite Ed.) 2017, 9, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Loo, W.T.Y.; Fan, C.; Bai, L.; Yue, Y.; Dou, Y.; Wang, M.; Liang, H.; Cheung, M.N.B.; Chow, L.W.C.; Li, J.; et al. Gene polymorphism and protein of human pro- and anti-inflammatory cytokines in Chinese healthy subjects and chronic periodontitis patients. J. Transl. Med. 2012, 10 (Suppl. 1), S8. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.d.O.; Lobato, R.V.; Andrade, E.F.; de Macedo, C.G.; Napimoga, J.T.C.; Napimoga, M.H.; Messora, M.R.; Murata, R.M.; Pereira, L.J. β-Glucans (Saccharomyces cereviseae) Reduce Glucose Levels and Attenuate Alveolar Bone Loss in Diabetic Rats with Periodontal Disease. PLoS ONE 2015, 10, e0134742. [Google Scholar] [CrossRef] [PubMed]
- Santos Tunes, R.; Foss-Freitas, M.C.; Nogueira-Filho, G.d.R. Impact of periodontitis on the diabetes-related inflammatory status. J. Can. Dent. Assoc. 2010, 76, a35. [Google Scholar] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Verspreet, J.; Damen, B.; Broekaert, W.F.; Verbeke, K.; Delcour, J.A.; Courtin, C.M. A Critical Look at Prebiotics Within the Dietary Fiber Concept. Annu. Rev. Food Sci. Technol. 2016, 7, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Hendarto, H.; Inoguchi, T.; Maeda, Y.; Ikeda, N.; Zheng, J.; Takei, R.; Yokomizo, H.; Hirata, E.; Sonoda, N.; Takayanagi, R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012, 61, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Awuti, G.; Younusi, K.; Li, L.; Upur, H.; Ren, J. Epidemiological survey on the prevalence of periodontitis and diabetes mellitus in Uyghur adults from rural Hotan area in Xinjiang. Exp. Diabetes Res. 2012, 2012, 758921. [Google Scholar] [CrossRef] [PubMed]
- Lakschevitz, F.; Aboodi, G.; Tenenbaum, H.; Glogauer, M. Diabetes and periodontal diseases: interplay and links. Curr. Diabetes Rev. 2011, 7, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Offenbacher, S. Periodontal medicine: the emergence of a new branch of periodontology. Periodontology 2000, 23, 9–12. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Salini, J.; Vijay, V. Use of glimepiride and insulin sensitizers in the treatment of type 2 diabetes—a study in Indians. J. Assoc. Physicians India 2004, 52, 459–463. [Google Scholar] [PubMed]
- Kouidhi, S.; Berrhouma, R.; Rouissi, K.; Jarboui, S.; Clerget-Froidevaux, M.-S.; Seugnet, I.; Bchir, F.; Demeneix, B.; Guissouma, H.; Elgaaied, A.B. Human subcutaneous adipose tissue Glut 4 mRNA expression in obesity and type 2 diabetes. Acta Diabetol. 2013, 50, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J. The clinical potential of low-level C-peptide secretion. Expert Rev. Mol. Diagn. 2016, 16, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, K.; Jain, A.; Sharma, R.; Prashar, S.; Jain, R. Diabetes and periodontitis. J. Indian Soc. Periodontol. 2010, 14, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Luhm, J.; Langenkamp, U.; Hensel, J.; Frohn, C.; Brand, J.M.; Hennig, H.; Rink, L.; Koritke, P.; Wittkopf, N.; Williams, D.L.; et al. Beta-(1→3)-D-glucan modulates DNA binding of nuclear factors kappaB, AT and IL-6 leading to an anti-inflammatory shift of the IL-1beta/IL-1 receptor antagonist ratio. BMC Immunol. 2006, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, A.; Wang, Y.Y.; Morton, H.C.; Aasen, A.O.; Wang, J.E.; Johansen, F.-E. Oral and systemic administration of beta-glucan protects against lipopolysaccharide-induced shock and organ injury in rats. Clin. Exp. Immunol. 2007, 148, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Breivik, T.; Opstad, P.K.; Engstad, R.; Gundersen, G.; Gjermo, P.; Preus, H. Soluble beta-1,3/1,6-glucan from yeast inhibits experimental periodontal disease in Wistar rats. J. Clin. Periodontol. 2005, 32, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kang, S.J.; Kim, J.W.; Cho, H.R.; Moon, S.B.; Kim, K.Y.; Lee, H.S.; Han, C.H.; Ku, S.K.; Lee, Y.J. Effects of Polycan, a β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J. Periodontal Res. 2012, 47, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Dian, D.; Keshavarzian, A.; Fogg, L.; Fields, J.Z.; Farhadi, A. The role of oral hygiene in inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.Y.; Pratap, S.; Southerland, J.H.; Farmer-Dixon, C.M.; Lakshmyya, K.; Gangula, P.R. Role of oral and gut microbiome in nitric oxide-mediated colon motility. Nitric Oxide 2017. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; He, J.; Shen, Y.; Zhang, C.; Wang, J.; Chen, Y. New Frontiers in Genetics, Gut Microbiota, and Immunity: A Rosetta Stone for the Pathogenesis of Inflammatory Bowel Disease. BioMed Res. Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Adibmoradi, M.; Navidshad, B.; Seifdavati, J.; Royan, M. Effect of dietary garlic meal on histological structure of small intestine in broiler chickens. J. Poult. Sci. 2006, 43, 378–383. [Google Scholar] [CrossRef]
- Yang, H.; Liu, A.; Zhang, M.; Ibrahim, S.A.; Pang, Z.; Leng, X.; Ren, F. Oral administration of live Bifidobacterium substrains isolated from centenarians enhances intestinal function in mice. Curr. Microbiol. 2009, 59, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Domènech, A.; Pasquinelli, G.; De Giorgio, R.; Gori, A.; Bosch, F.; Pumarola, M.; Jiménez, M. Morphofunctional changes underlying intestinal dysmotility in diabetic RIP-I/hIFNβ transgenic mice. Int. J. Exp. Pathol. 2011, 92, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Sukhotnik, I.; Shamir, R.; Bashenko, Y.; Mogilner, J.G.; Chemodanov, E.; Shaoul, R.; Coran, A.G.; Shehadeh, N. Effect of oral insulin on diabetes-induced intestinal mucosal growth in rats. Dig. Dis. Sci. 2011, 56, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Schneeman, B.O. Dietary fiber and gastrointestinal function. Nutr. Rev. 1987, 45, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Battilana, P.; Ornstein, K.; Minehira, K.; Schwarz, J.M.; Acheson, K.; Schneiter, P.; Burri, J.; Jéquier, E.; Tappy, L. Mechanisms of action of beta-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. 2001, 55, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Cai, F.; Shen, R.; Liu, Y. Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta-glucan in streptozotocin-induced diabetic mice. Food Chem. 2011, 129, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.H.; Knop, F.K.; Frost, M.; Hallas, J.; Pottegård, A. Use of Antibiotics and Risk of Type 2 Diabetes: A Population-Based Case-Control Study. J. Clin. Endocrinol. Metab. 2015, 100, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Aw, W.; Fukuda, S. Understanding the role of the gut ecosystem in diabetes mellitus. J. Diabetes Investig. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; D’Aiuto, F.; Griffiths, G.; Patel, K.; Suvan, J.; Tonetti, M.S. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case–control study. J. Clin. Periodontol. 2007, 34, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Saxlin, T.; Suominen-Taipale, L.; Leiviskä, J.; Jula, A.; Knuuttila, M.; Ylöstalo, P. Role of serum cytokines tumour necrosis factor-α and interleukin-6 in the association between body weight and periodontal infection. J. Clin. Periodontol. 2009, 36, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Passoja, A.; Puijola, I.; Knuuttila, M.; Niemelä, O.; Karttunen, R.; Raunio, T.; Tervonen, T. Serum levels of interleukin-10 and tumour necrosis factor-α in chronic periodontitis. J. Clin. Periodontol. 2010, 37, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Scarel-Caminaga, R.M.; Trevilatto, P.C.; Souza, A.P.; Brito, R.B.; Camargo, L.E.A.; Line, S.R.P. Interleukin 10 gene promoter polymorphisms are associated with chronic periodontitis. J. Clin. Periodontol. 2004, 31, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Nanes, M.S. Tumor necrosis factor-alpha: Molecular and cellular mechanisms in skeletal pathology. Gene 2003, 321, 1–15. [Google Scholar] [CrossRef]
- Kikkert, R.; Laine, M.L.; Aarden, L.A.; van Winkelhoff, A.J. Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol. Immunol. 2007, 22, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Craandijk, J.; Hoek, F.J.; Dillen, P.M.E.W.; Velden, U. Van Der Elevation of Systemic Markers Related to Cardiovascular Diseases in the Peripheral Blood of Periodontitis Patients. J. Periodontol. 2000, 71, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Zhang, H.; Hu, B.; Wang, L.; Qian, H.; Qi, X. The anti-diabetic activity of oat β-d-glucan in streptozotocin–nicotinamide induced diabetic mice. Int. J. Biol. Macromol. 2016, 91, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.F.; Lobato, R.V.; de Araujo, T.V.; Zangeronimo, M.G.; de Sousa, R.V.; Pereira, L.J. Effect of beta-glucans in the control of blood glucose levels of diabetic patients: A systematic review. Nutr. Hosp. 2015, 31, 170–177. [Google Scholar]

| Experimental Groups |
|---|
| 1—Control |
| 2—Diabetes (Introduction of diabetes with streptozotocin) |
| 3—Periodontal disease (Induction of periodontal disease for 14 days) |
| 4—β-glucan (Treated since the first day of the experiment—28 days) |
| 5—Diabetes + Periodontal disease |
| 6—Diabetes + β-glucan |
| 7—β-glucan + Periodontal disease |
| 8—Diabetes + β-glucan + Periodontal disease |
| Diabetes | Periodontal Disease | β-Glucan | |
|---|---|---|---|
| − | + | ||
| − | − | 776 (175) Bb | 736 (183) Bb |
| − | + | 352 (147) Ba | 500 (199) Ba |
| + | − | 30 (4) Ay | 50 (11) Ax |
| + | + | 14 (1) Ay | 51 (4) Ax |
| Diabetes | Periodontal Disease | β-Glucan | |
|---|---|---|---|
| − | + | ||
| TNF-α | |||
| − | − | 2.61 (0.95) Aa | 2.81 (0.39) |
| − | + | 4.02 (0.64) Ab | 3.33 (0.92) |
| + | − | 4.29 (1.57) Ba | 3.79 (0.90) |
| + | + | 7.11 (1.51) Bbx | 3.05 (0.79) y |
| IL-10 | |||
| − | − | 24.62 (3.74) a | 20.69 (5.09) a |
| − | + | 35.56 (7.03) bx | 28.40 (4.98) by |
| + | − | 27.41 (2.70) a | 26.68 (3.51) |
| + | + | 37.45 (8.76) bx | 29.04 (5.86) y |
| Diabetes | Periodontal Disease | β-Glucan | |
|---|---|---|---|
| − | + | ||
| Scanning Electron Microscopy (mm) | |||
| − | − | 0.67 (0.11) Bax | 0.51 (0.05) ay |
| − | + | 1.00 (1.17) bx | 0.70 (0.17) by |
| + | − | 0.51 (0.12) Aa | 0.56 (0.08) a |
| + | + | 1.14 (0.05) bx | 0.78 (0.07) by |
| Histometric Analysis (mm2) | |||
| − | − | 1.36 (0.19) a | 1.39 (0.29) |
| − | + | 2.50 (0.41) bx | 1.58 (0.61) y |
| + | − | 1.26 (0.20) a | 1.14 (0.01) |
| + | + | 2.93 (0.21) bx | 1.25 (0.33) y |
| Diabetes | Periodontal Disease | β-Glucan | |
|---|---|---|---|
| − | + | ||
| Villus height (µm) | |||
| − | − | 396 (76) Ax | 574 (97) Ay |
| − | + | 409 (71) A | 508 (215) A |
| + | − | 700 (137) B | 772 (88) B |
| + | + | 667 (226) B | 767 (107) B |
| Crypt depth (µm) | |||
| − | − | 239 (38) | 252 (13) |
| − | + | 226 (43) x | 273 (19) y |
| + | − | 233 (36) | 251 (10) |
| + | + | 242 (63) x | 286 (34) y |
| Villus height/crypt depth | |||
| − | − | 1.65 (0.08) A | 2.29 (0.46) A |
| − | + | 1.87 (0.53) A | 1.86 (0.77) A |
| + | − | 3.06 (0.80) B | 3.09 (0.45) B |
| + | + | 2.76 (0.71) B | 2.68 (0.22) B |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De O. Silva, V.; Lobato, R.V.; Andrade, E.F.; Orlando, D.R.; Borges, B.D.B.; Zangeronimo, M.G.; De Sousa, R.V.; Pereira, L.J. Effects of β-Glucans Ingestion on Alveolar Bone Loss, Intestinal Morphology, Systemic Inflammatory Profile, and Pancreatic β-Cell Function in Rats with Periodontitis and Diabetes. Nutrients 2017, 9, 1016. https://doi.org/10.3390/nu9091016
De O. Silva V, Lobato RV, Andrade EF, Orlando DR, Borges BDB, Zangeronimo MG, De Sousa RV, Pereira LJ. Effects of β-Glucans Ingestion on Alveolar Bone Loss, Intestinal Morphology, Systemic Inflammatory Profile, and Pancreatic β-Cell Function in Rats with Periodontitis and Diabetes. Nutrients. 2017; 9(9):1016. https://doi.org/10.3390/nu9091016
Chicago/Turabian StyleDe O. Silva, Viviam, Raquel V. Lobato, Eric F. Andrade, Débora R. Orlando, Bruno D.B. Borges, Márcio G. Zangeronimo, Raimundo V. De Sousa, and Luciano J. Pereira. 2017. "Effects of β-Glucans Ingestion on Alveolar Bone Loss, Intestinal Morphology, Systemic Inflammatory Profile, and Pancreatic β-Cell Function in Rats with Periodontitis and Diabetes" Nutrients 9, no. 9: 1016. https://doi.org/10.3390/nu9091016





