Eicosapentaenoic Acid (EPA) Induced Macrophages Activation through GPR120-Mediated Raf-ERK1/2-IKKβ-NF-κB p65 Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Proliferation Assay
2.4. Morphologic Observations
2.5. Measurement of NO Production
2.6. Cytokine Assays
2.7. Nuclear Protein Extraction
2.8. Western Blot Analysis
2.9. Immunofluorescence Assay for NF-κB p65
2.10. Statistical Analysis
3. Result
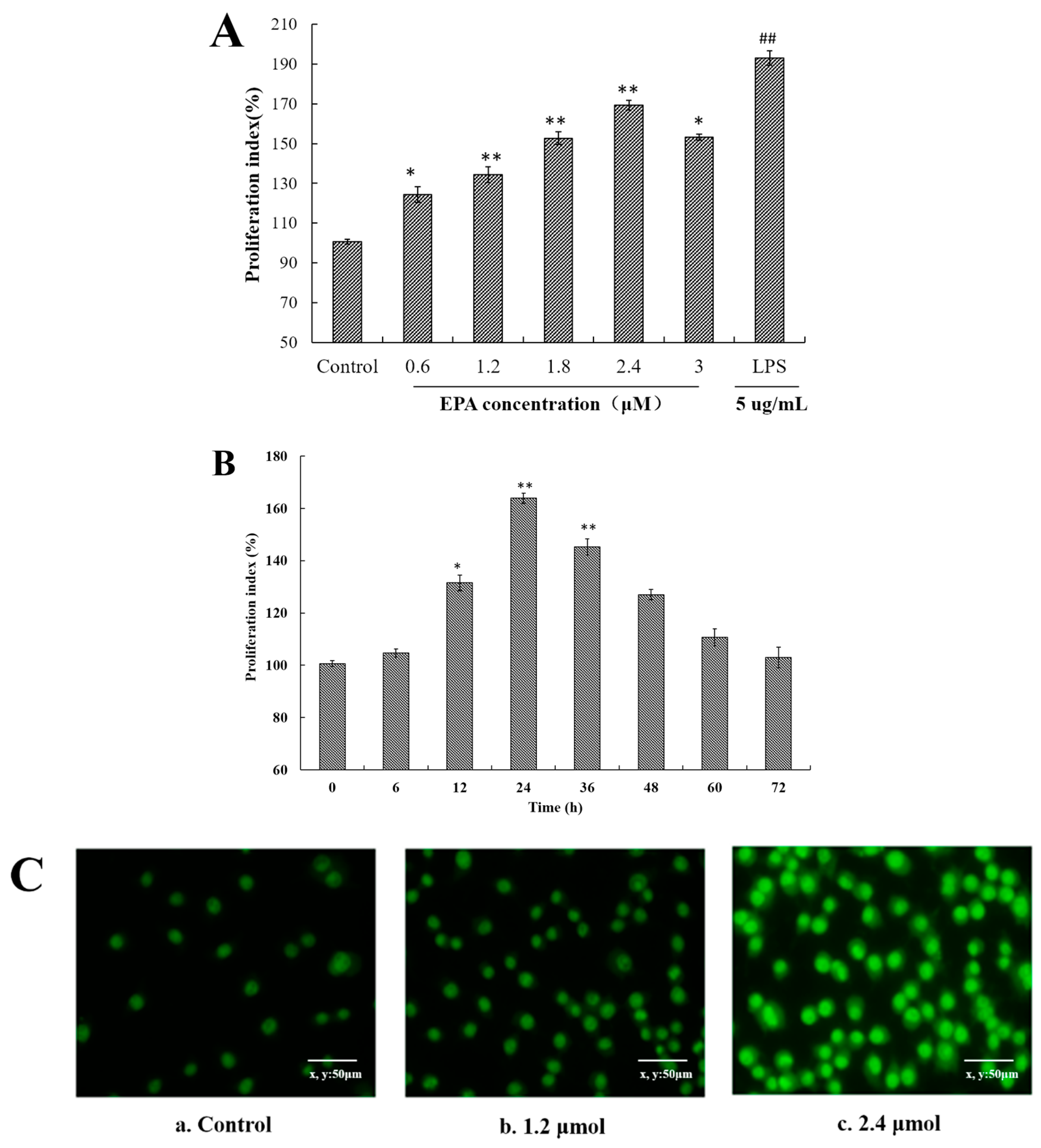
3.1. EPA Increased the Proliferation Index of RAW264.7 Cells
3.2. EPA Induced the Morphological Changes of RAW264.7 Cells
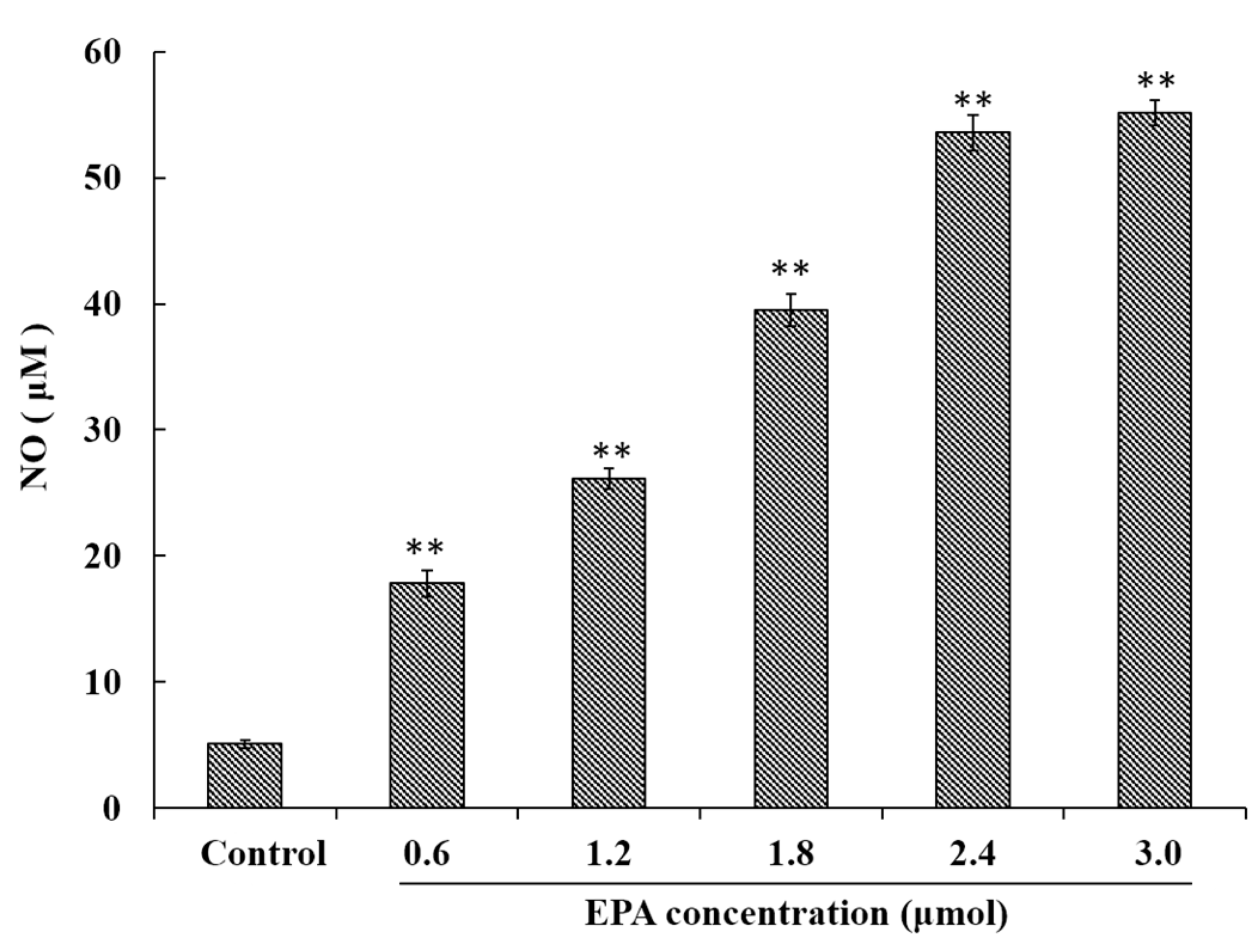
3.3. EPA Induced NO Production of RAW264.7 Cells
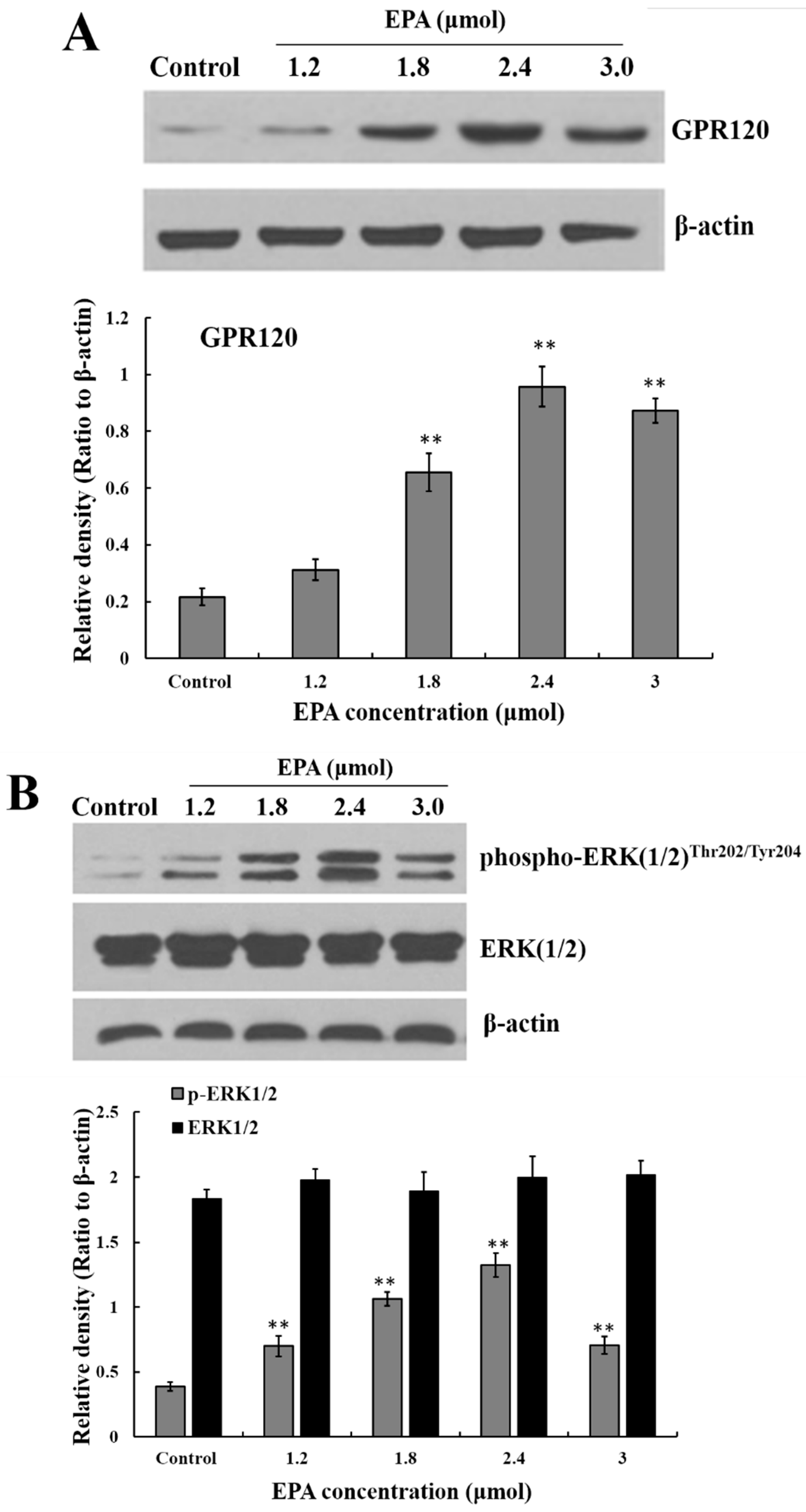
3.4. Effect of EPA on GPR120 Protein Expression
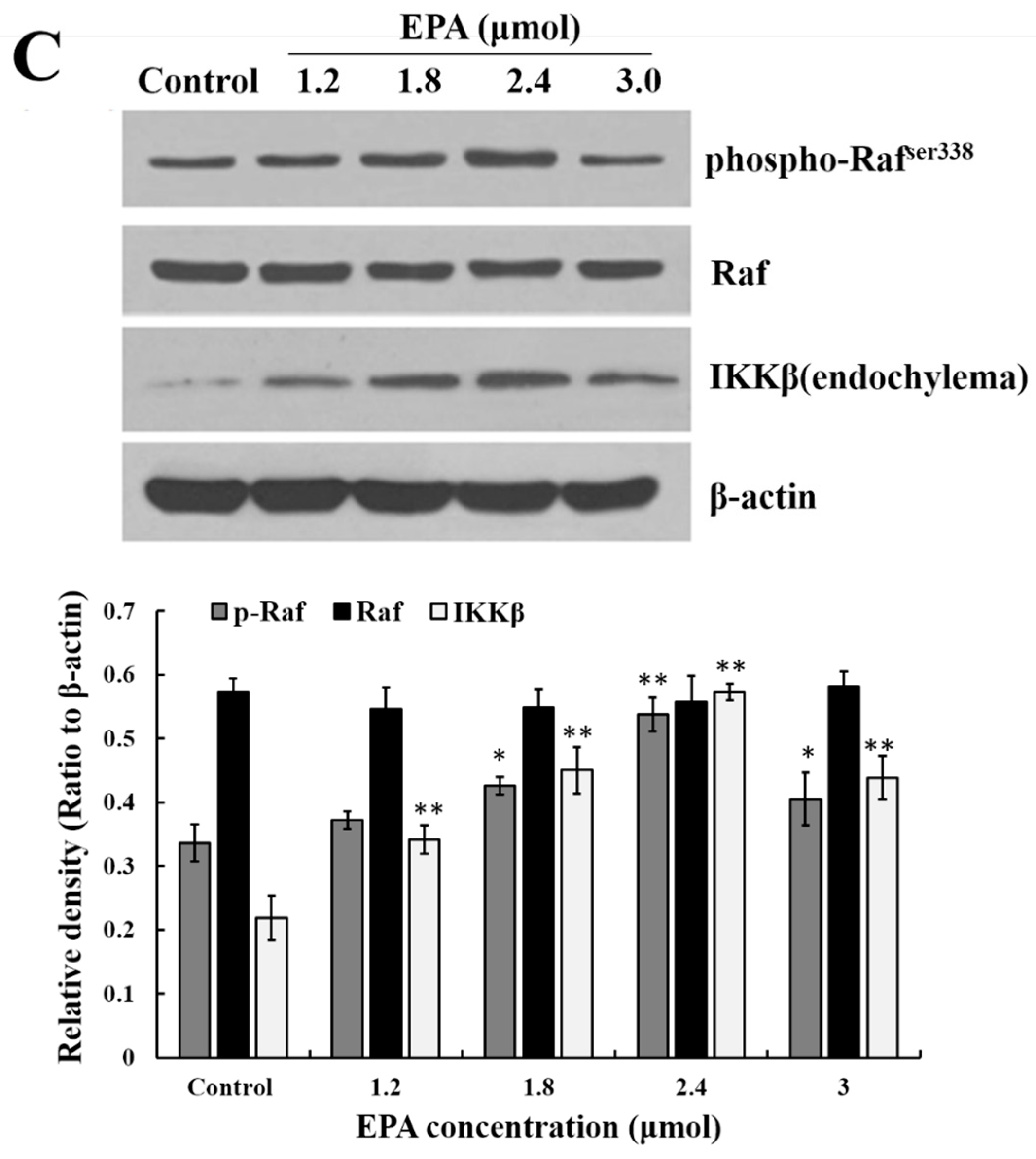
3.5. Measurement of ERK, Raf and IKKβ
3.6. Relationship between ERK1/2 and Raf, IKKβ
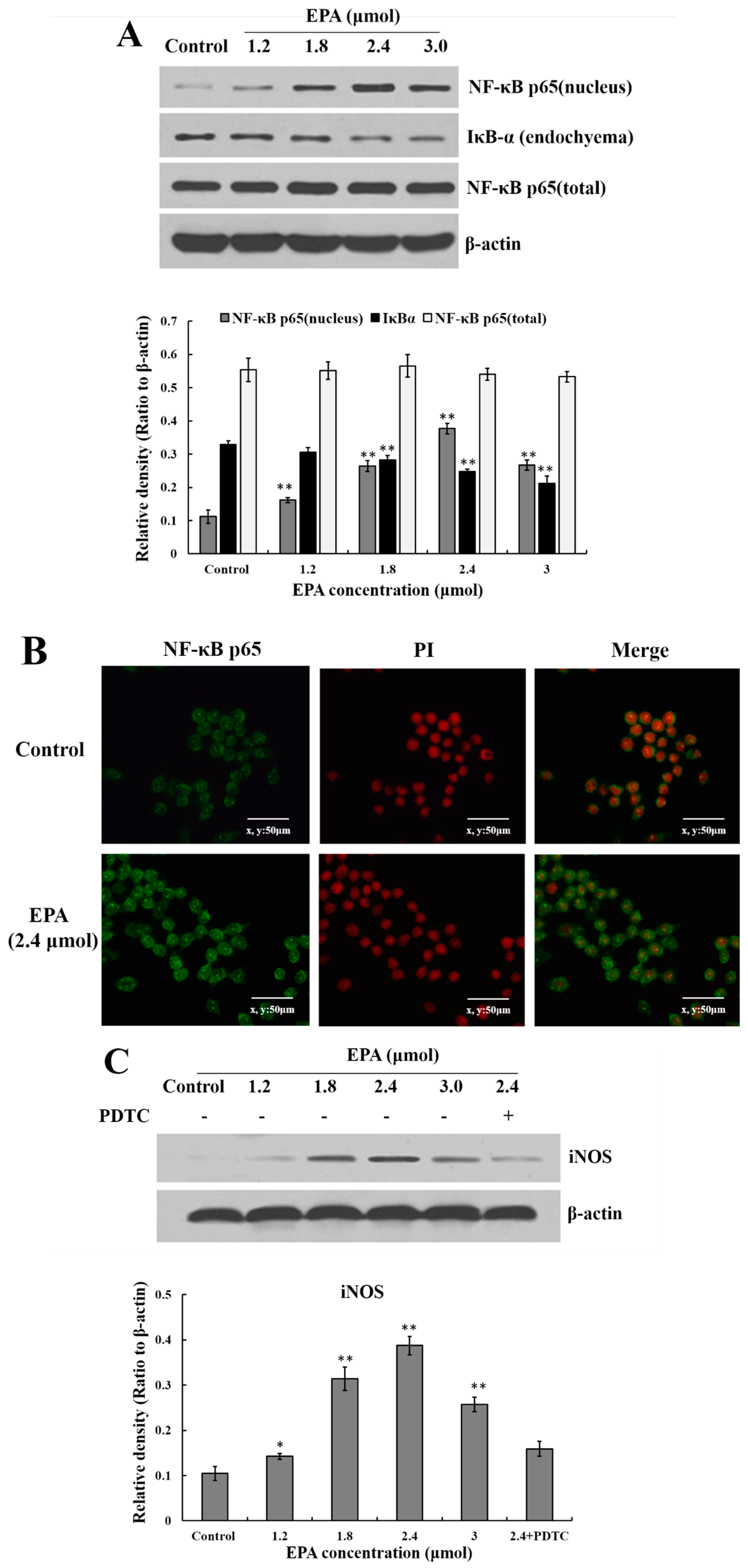
3.7. Effect of EPA on the Expression of NF-κB p65
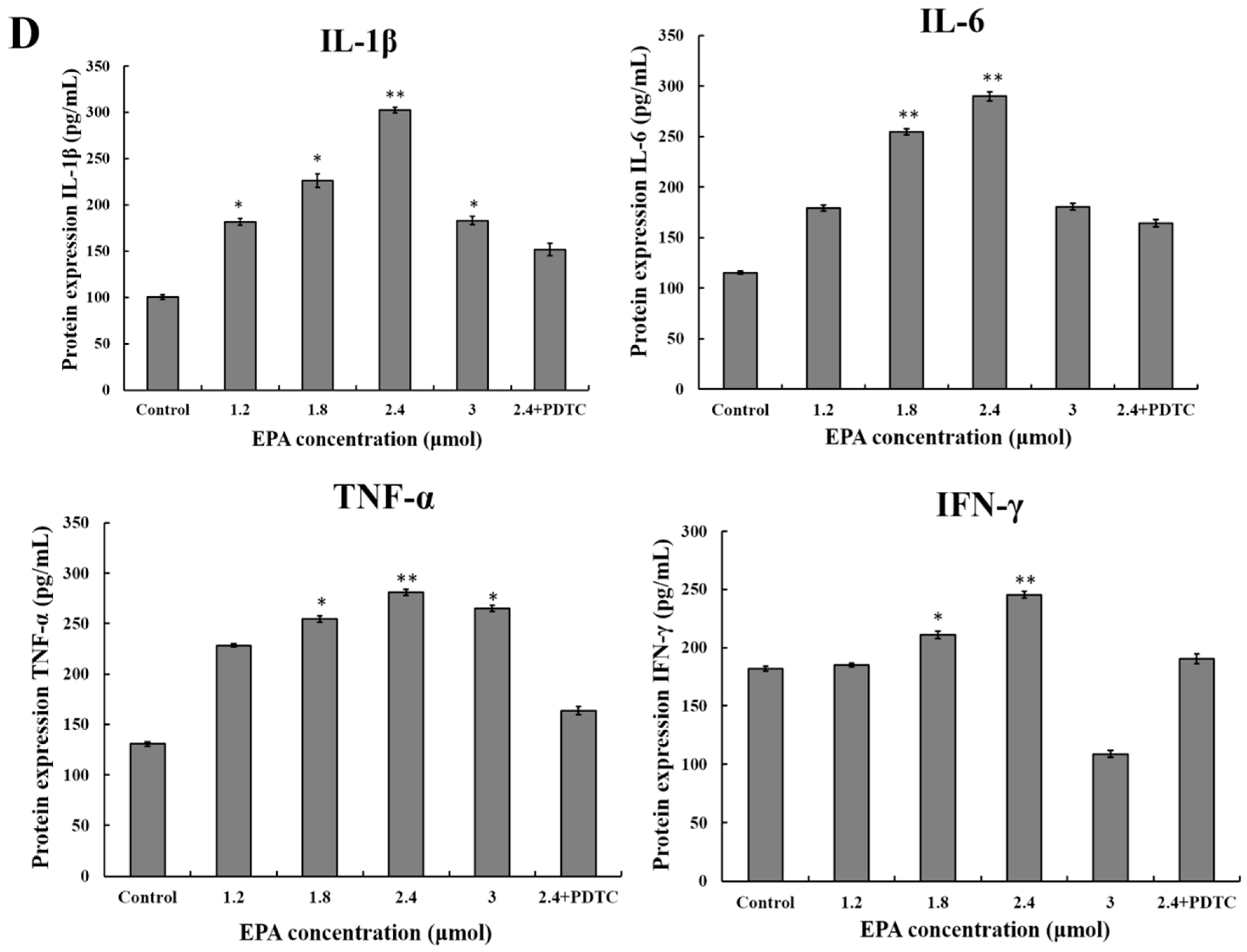
3.8. EPA Regulated the Protein Expression of iNOS and Cytokines
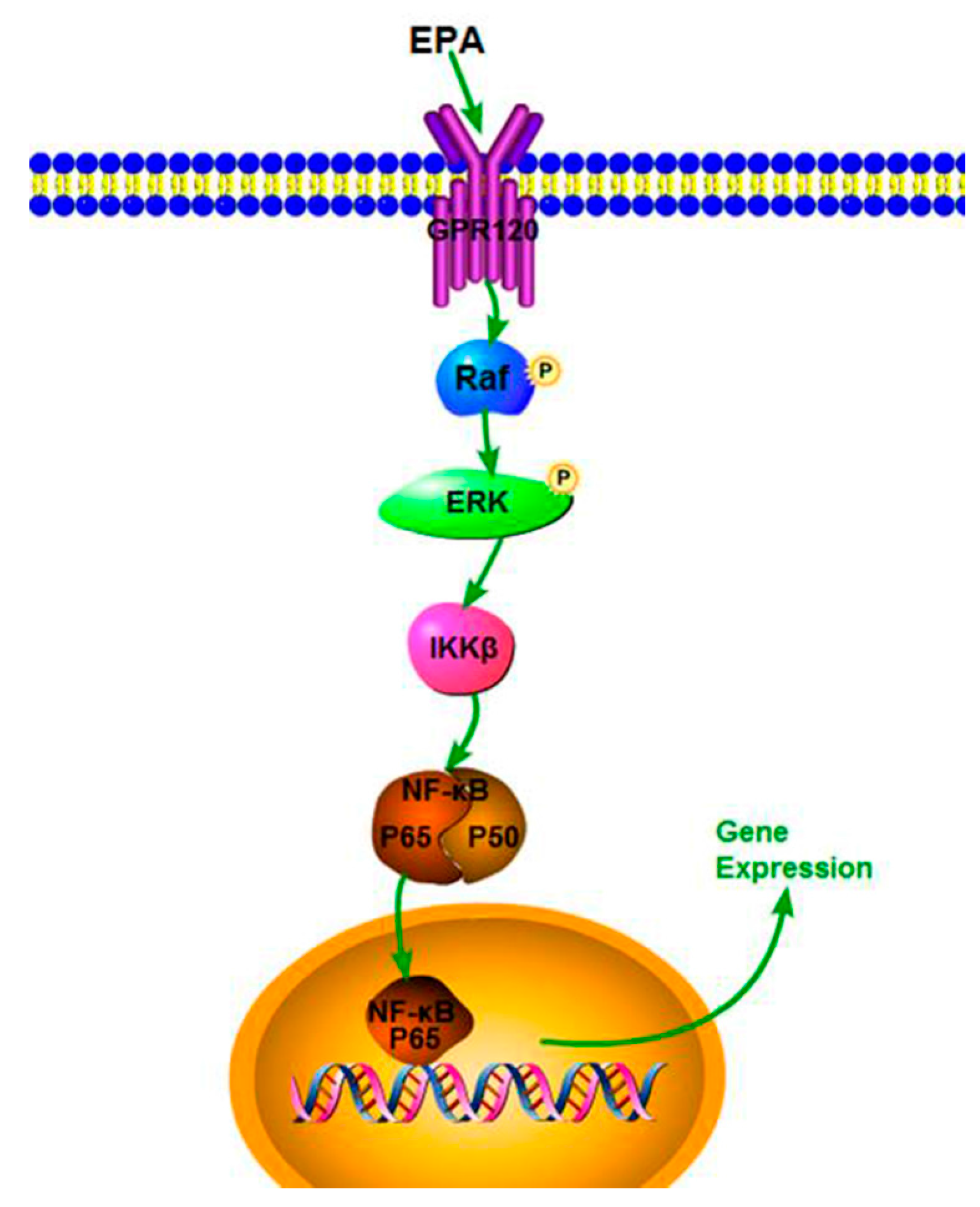
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calder, P.C. N-3 fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2004, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mayser, P.; Grimm, H.; Grimminger, F. N-3 fatty acids in psoriasis. Br. J. Nutr. 2007, 87, S77–S82. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of ω-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, B.; Ho, M.; Youssef, H.M.; Hill, A.; McMahon, H.; Hall, C.; Ogston, S.; Nuki, G.; Belch, J.J. Cod liver oil (n-3 fatty acids) as an non-steroidal anti-inflammatory drug sparing agent in rheumatoid arthritis. Rheumatology 2008, 47, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Woodman, R.J.; Mori, T.A.; Burke, V.; Puddey, I.B.; Barden, A.; Watts, G.F.; Beilin, L.J. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 2002, 166, 85–93. [Google Scholar] [CrossRef]
- Meade, C.; Mertin, J. Fatty acids and immunity. Adv. Lipid Res. 1978, 16, 127–165. [Google Scholar] [PubMed]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar] [PubMed]
- Laviano, A.; Rianda, S.; Molfino, A.; Fanelli, F.R. Omega-3 fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Long chain ω-3 fatty acids and cardiovascular disease: A systematic review. Br. J. Nutr. 2012, 107, S201–S213. [Google Scholar] [CrossRef] [PubMed]
- Maehre, H.; Jensen, I.-J.; Elvevoll, E.; Eilertsen, K.-E. Ω-3 fatty acids and cardiovascular diseases: Effects, mechanisms and dietary relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D.; Hughes, A.D.; Mayet, J.; Wright, A.R.; Kooner, J.; Chaturvedi, N.; Shore, A.C. Attenuation of microvascular function in those with cardiovascular disease is similar in patients of indian asian and european descent. BMC Cardiovasc. Disord. 2010, 10, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kew, S.; Mesa, M.D.; Tricon, S.; Buckley, R.; Minihane, A.M.; Yaqoob, P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am. Soc. Clin. Nutr. 2004, 79, 674–681. [Google Scholar]
- Gorjao, R.; Azevedo-Martins, A.K.; Rodrigues, H.G.; Abdulkader, F.; Arcisio-Miranda, M.; Procopio, J.; Curi, R. Comparative effects of dha and epa on cell function. Pharmacol. Ther. 2009, 122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.J.O.; Dalgleish, A.G. Chronic immune activation and inflammation as the cause of malignancy. Br. J. Cancer 2001, 85, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wan, F.; Wang, J.; Jin, Z.; Xu, X. Macrophage immunomodulatory activity of polysaccharides isolated from glycyrrhiza uralensis fish. Int. Immunopharmacol. 2008, 8, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Raschke, W.C.; Baird, S.; Ralph, P.; Nakoinz, I. Functional macrophage cell lines transformed by abelson leukemia virus. Cell 1978, 15, 261–267. [Google Scholar] [CrossRef]
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.-O.; Sosnovtsev, S.V.; Belliot, G.; Krug, A.; Mackenzie, J.M.; Green, K.Y.; Virgin, H.W., IV. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004, 2, e432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartley, J.W.; Evans, L.H.; Green, K.Y.; Naghashfar, Z.; Macias, A.R.; Zerfas, P.M.; Ward, J.M. Expression of infectious murine leukemia viruses by raw264.7 cells, a potential complication for studies with a widely used mouse macrophage cell line. Lab. Immunopathol. 2008, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through gpr40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through gpr120. Nat. Med. 2004, 11, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. Gpr120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Abies koreana essential oil inhibits drug-resistant skin pathogen growth and lps-induced inflammatory effects of murine macrophage. Lipids 2009, 44, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Huang, F.M.; Li, Y.C.; Chang, Y.C. Proinflammatory activation of macrophages by bisphenol a-glycidyl-methacrylate involved nfkappab activation via pi3k/akt pathway. Food Chem. Toxicol. 2012, 50, 4003–4009. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.T.; Chang, F.; Lehman, B.D.; Terrian, D.; Milella, M.; Tafuri, A.; et al. Roles of the raf/mek/erk pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.S.; Shapiro, P.; Ahn, N.G. Signal transduction through map kinase cascades. Adv. Cancer Res. 1998, 74, 49–139. [Google Scholar] [PubMed]
- Chen, T.G.; Chen, T.L.; Chang, H.C.; Tai, Y.T.; Cherng, Y.G.; Chang, Y.T.; Chen, R.M. Oxidized low-density lipoprotein induces apoptotic insults to mouse cerebral endothelial cells via a bax-mitochondria-caspase protease pathway. Toxicol. Appl. Pharmacol. 2007, 219, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, A.; Wang, C.; Mao, D.; Lu, M.; Cui, Y.; Jiao, R. Surfactin induces apoptosis in human breast cancer mcf7 cells through a ros/jnk-mediated mitochondrial/caspase pathway. Chemicobiol. Interact. 2010, 183, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-M.; Chen, T.-L.; Chiu, W.-T.; Chang, C.-C. Molecular mechanism of nitric oxide-induced osteoblast apoptosis. J. Orthop. Res. 2005, 23, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, A.H.; Jiao, R.Z.; Wang, C.L.; Mao, D.Z.; Yan, L.; Zeng, B. Surfactin induces apoptosis and g(2)/m arrest in human breast cancer mcf-7 cells through cell cycle factor regulation. Cell Biochem. Biophys. 2009, 55, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhou, M.; Wang, C.; Hou, L.; Zeng, B. Lectin purified from musca domestica pupa up-regulates no and inos production via tlr4/nf-kappab signaling pathway in macrophages. Int. Immunopharmacol. 2011, 11, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, F. Use of acridine orange staining for the detection of rotavirus rna in polyacrylamide gels. J. Virol. Methods 2003, 114, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. The role of the macrophage in immune regulation. Res. Immunol. 1998, 149, 685–688. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; Dunnen, J.D.; Litjens, M.; Hof, B.V.H.; Kooyk, Y.V. C-type lectin dc-sign modulates toll-like receptor signaling via raf1 kinase-dependent acetylation of transcription factor nf-κb. Immunity 2007, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Medvedev, A.E.; Thomas, K.E.; Cuesta, N.; Toshchakov, V.; Ren, T.; Cody, M.J.; Michalek, S.M.; Rice, N.R.; Vogel, S.N. Induction of in vitro reprogramming by toll-like receptor (tlr)2 and tlr4 agonists in murine macrophages: Effects of tlr “homotolerance” versus “heterotolerance” on nf-kappa b signaling pathway components. J. Immunol. 2003, 170, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Delhase, M.Y. The iκb kinase (ikk) and nf-κb: Key elements of proinflammatory signalling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Iκb kinases: Key regulators of the nf-κb pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Moses, A.; Fearon, K. Anti-catabolic effects of n-3 fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 1999, 2, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, L.; Qi, W.; Cheng, D.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Eicosapentaenoic acid (epa) induced apoptosis in hepg2 cells through ros-ca(2+)-jnk mitochondrial pathways. Biochem. Biophys. Res. Commun. 2015, 456, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot. Essent. Fatty Acids 2008, 79, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Nyquist, N.F.; Mosti, T.J.; Andersen, M.; Hstmark, A.T. Increased epa levels in serum phospholipids of humans after four weeks daily ingestion of one portion chicken fed linseed and rapeseed oil. Lipids Health Dis. 2012, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hino, M.; CNishizawa, T.K.; Yoshida, A.; Nakata, K.; Inagawa, H.; Hori, H.; Makino, K.; Terada, H.; Soma, G. Innate-immune therapy for lung carcinoma based on tissue-macrophage activation with lipopolysaccharide. Anticancer Res. 2005, 25, 3747–3754. [Google Scholar] [PubMed]
- Hess, D.; MJBendel, C.H.S. Escherichia coli and tnf-alpha modulate macrophage phagocytosis of candida glabrata. J. Surg. Res. 2009, 155, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Amit, I.; Yarden, Y. Regulation of mapks by growth factors and receptor tyrosine kinases. Biochim. Biophys. Acta 2007, 1773, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Wu, L.L.; Yu, G.Y. Tight junctions and paracellular fluid and ion transport in salivary glands. Chin. J. Dent. Res. 2013, 16, 13–46. [Google Scholar] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. Map kinase signaling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Goillot, E.; Raingeaud, J.; Ranger, A.; Tepper, R.I.; Davis, R.J.; Harlow, E.; Sanchez, I. Mitogen-activated protein kinase-mediated fas apoptotic signaling pathway. Proc. Natl. Acad. Sci. USA 1997, 94, 3302–3307. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. Lps induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-κb-a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Beinke, S.; Ley, S.C. Functions of nf-κb1 and nf-κb2 in immune cell biology. Biochem. J. 2004, 382, 393–409. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Song, S.; Niu, Y.; Meng, M.; Wang, C. Eicosapentaenoic Acid (EPA) Induced Macrophages Activation through GPR120-Mediated Raf-ERK1/2-IKKβ-NF-κB p65 Signaling Pathways. Nutrients 2017, 9, 937. https://doi.org/10.3390/nu9090937
Han L, Song S, Niu Y, Meng M, Wang C. Eicosapentaenoic Acid (EPA) Induced Macrophages Activation through GPR120-Mediated Raf-ERK1/2-IKKβ-NF-κB p65 Signaling Pathways. Nutrients. 2017; 9(9):937. https://doi.org/10.3390/nu9090937
Chicago/Turabian StyleHan, Lirong, Shumin Song, Yabing Niu, Meng Meng, and Chunling Wang. 2017. "Eicosapentaenoic Acid (EPA) Induced Macrophages Activation through GPR120-Mediated Raf-ERK1/2-IKKβ-NF-κB p65 Signaling Pathways" Nutrients 9, no. 9: 937. https://doi.org/10.3390/nu9090937



