Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives
Abstract
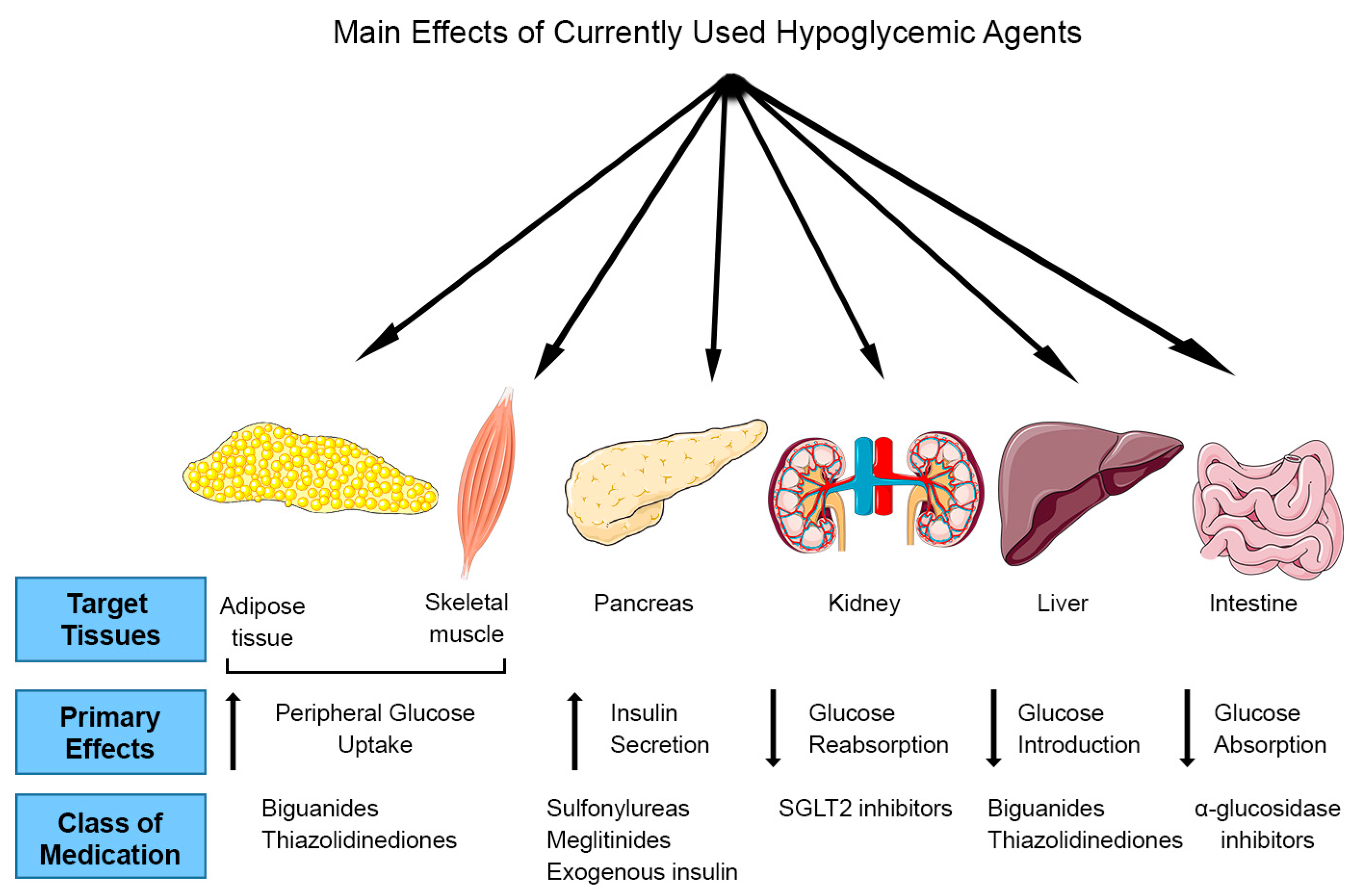
:1. Introduction
2. Phenolic Compounds of Rosemary
3. Evidence of Anti-Diabetic Effects of Rosemary Extract: In Vitro Studies (Cell Free Models)
4. Evidence of Anti-Diabetic Effects of Rosemary Extract: In Vitro Studies (Hepatocytes)
5. Evidence of Anti-Diabetic Effects of Rosemary Extract: In Vitro Studies (Adipocytes)
6. Evidence of Anti-Diabetic Effects of Rosemary Extract: In Vitro Studies (Skeletal Muscle Cells)
7. Evidence of Anti-Diabetic Effects of Rosemary Extract: In Vivo Animal Studies
8. Streptozotocin (STZ)-Induced Diabetes Model
9. Alloxan-Induced Diabetes Model
10. Genetically-Induced Diabetes Models
11. Diet-Induced Diabetes Model
12. Anti-Diabetic Effects of Rosemary Extract and Its Main Polyphenolic Constituents: In Vivo Human Studies
13. Conclusions
Acknowledgments
Author contribution
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate transaminase |
| CA | Carnosic acid |
| CAT | Catalase |
| C/EBPα | CCAAT-enhancer binding proteins, |
| COH | Carnosol |
| CRP | C-reactive protein |
| DDP-4 | Dipeptidyl peptide 4 |
| DGAT1 | Diacylglycerol acyltransferase 1 |
| ERK | Extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| FFA | Free fatty acids |
| FM | Fibromyalgia |
| FPG | Fasting plasma glucose |
| GL | Gastric lipase |
| GLP-1 | Glucagon-like peptide 1 |
| GPDH | Glycerol-3-phosphate dehydrogenase |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GU | Glucose uptake |
| HSL | Hormone sensitive lipase |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HbA1c | Glycated hemoglobin |
| IDF | International Diabetes Federation |
| ITT | Insulin tolerance test |
| LDL | Low-density-lipoprotein |
| MCP-1 | Monocyte chemoattract protein 1 |
| MDA | Malondialdehyde |
| MUFA | Monounsaturated fatty acids |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| OA | Osteoarthritis |
| OGTT | Oral glucose tolerance test |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PEPCK | Phosphoenolypyruvate carboxykinase |
| PL | Pancreatic lipase |
| PPAR | Peroxisome proliferator activated receptor, |
| RA | Rosmarinic acid |
| RE | Rosemary extract |
| ROS | Reactive oxygen species |
| SCD1 | Stearoyl coA desaturase 1 |
| SFA | Saturated fatty acids |
| SGLT1 | Sodium glucose cotransporters 1 |
| SGLT2 | Sodium-glucose cotransporter 2, |
| SOD | Superoxide dismutase |
| SREBP1 | Sterol regulatory element binding protein 1 |
| STZ | Streptozotocin |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNFα | Tumor necrosis factor α |
References
- Tripathy, D.; Chavez, A.O. Defects in insulin secretion and action in the pathogenesis of type 2 diabetes mellitus. Curr. Diabete Rep. 2010, 10, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Diabetes atlas: Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Zielinski, A.; Roach, A.H.; Jende, J.A.; Householder, A.M.; Cole, E.E.; Atway, S.A.; Amornyard, M.; Accursi, M.L.; Shieh, S.W.; et al. Pharmacologic treatment of type 2 diabetes oral medications. Ann. Pharmacother. 2015, 49, 540–556. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, S.; Kitaichi, K.; Hori, A.; Suwa, T.; Horikawa, Y.; Yamamoto, M.; Takeda, J.; Itoh, Y. The evaluation of risk factors associated with adverse drug reactions by metformin in type 2 diabetes mellitus. Biol. Pharm. Bull. 2012, 35, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hopper, I.; Skiba, M.; Krum, H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: Meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc. Ther. 2014, 32, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Forsmark, C.E. Incretins, Diabetes, pancreatitis and pancreatic cancer: What the GI specialist needs to know. Pancreatollogy 2016, 16, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Dailey, G.E. Empagliflozin: A new treatment option for patients with type 2 diabetes mellitus. Drugs Today 2015, 51, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research Drug Safety and Availability. FDA Drug Safety Communication: FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings about too Much Acid in the Blood and Serious Urinary Tract Infections. Available online: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm (accessed on 10 April 2016).
- Bent, S. Herbal medicine in the United States: Review of efficacy, safety, and regulation—Grand rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, F.; Herman, A.; Barlow, S.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, K. Use of rosemary extracts as a food additive-scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. EFSA J. 2008, 6, 721. [Google Scholar] [CrossRef]
- Schwarz, K. Phenolic Diterpenes from Rosemary and Sage; CRC: Boca Raton, FL, USA, 2002. [Google Scholar]
- González-Vallinas, M.; Reglero, G.; Ramírez de Molina, A. Rosemary (Rosmarinus officinalis L.) extract as a potential complementary agent in anticancer therapy. Nutr. Cancer 2015, 67, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, R.; Zhao, Y.; Yerke, A.; Sang, S. Preventive and protective properties of rosemary (Rosmarinus officinalis L.) in obesity and diabetes mellitus of metabolic disorders: A brief review. Curr. Opin. Food Sci. 2015, 2, 58–70. [Google Scholar] [CrossRef]
- Hassani, F.V.; Shirani, K.; Hosseinzadeh, H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: A review. Naunyn-Schmiedebergs Arch. Pharmacol. 2016, 389, 931–949. [Google Scholar] [CrossRef] [PubMed]
- De Raadt, P.; Wirtz, S.; Vos, E.; Verhagen, H. Short review of extracts of rosemary as a food additive. Eur. J. Nutr. Food Saf. 2015, 5, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Abrams, T.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; Kirkwood, C.; Giese, N.; Hoehn, K.; Iovin, R.; et al. An evidence-based systematic review of rosemary (Rosmarinus officinalis) by the natural standard research collaboration. J. Diet. Suppl. 2010, 7, 351–413. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Wang, M.; Wei, G.J.; Huang, T.C.; Huang, M.T. Chemistry and antioxidative factors in rosemary and sage. BioFactors 2000, 13, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124–130. [Google Scholar] [PubMed]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.-V.; Katsikoudi, A.; Kontogianni, V.G.; Kellici, T.F.; Iatrou, G.; Lamari, F.N.; Tzakos, A.G.; Margarity, M. Rosemary tea consumption results to anxiolytic- and anti-depressant-like behavior of adult male mice and inhibits all cerebral area and liver cholinesterase activity; phytochemical investigation and in silico studies. Chem. Biol. Interact. 2015, 237, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Genena, A.K.; Hense, H.; Smania Junior, A.; Machado de Souza, S. Rosemary (Rosmarinus officinalis)—A study of the composition, antioxidant and antimicrobial activities of extracts obtained with supercritical carbon dioxide. Cienc. Tecnol. Aliment. 2008, 28, 463–469. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Funke, I.; Melzig, M.F. Traditionally used plants in diabetes therapy: Phytotherapeutics as inhibitors of alpha-amylase activity. Rev. Bras. Farmacogn. 2006, 16, 1–5. [Google Scholar] [CrossRef]
- Koga, K.; Shibata, H.; Yoshino, K.; Nomoto, K. Effects of 50% ethanol extract from rosemary (Rosmarinus officinalis) on α-glucosidase inhibitory activity and the elevation of plasma glucose level in rats, and its active compound. J. Food Sci. 2006, 71, S507–S512. [Google Scholar] [CrossRef]
- McCue, P.P.; Shetty, K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar] [PubMed]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; de Mejia, E.G. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Almasri, I.; Al-Khalidi, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 2010, 4, 2235–2242. [Google Scholar] [CrossRef]
- Ibarra, A.; Cases, J.; Roller, M.; Chiralt-Boix, A.; Coussaert, A.; Ripoll, C. Carnosic-acid rich rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and improves cholesterol levels and glycaemia in mice on a high-fat diet. Br. J. Nutr. 2011, 106, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Kim, M.O.; Seo, J.H.; Kim, I.S.; Kim, N.Y.; Lee, S.H.; Park, J.; Kim, J.; Lee, H.S. Abietane diterpenoids of Rosmarinus officinalis and their diacylglycerol acyltransferase-inhibitory activity. Food Chem. 2012, 132, 1775–1780. [Google Scholar] [CrossRef]
- Yun, Y.S.; Noda, S.; Shigemori, G.; Kuriyama, R.; Takahashi, S.; Umemura, M.; Takahashi, Y.; Inoue, H. Phenolic diterpenes from rosemary suppress cAMP Responsiveness of gluconeogenic gene promoters. Phytother. Res. 2013, 27, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Moss-Pierce, T.; Ford, P.; Jiang, T. Rosemary (Rosmarinus officinalis L.) Extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in Hep G2 cells. J. Agric. Food Chem. 2013, 61, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Takikawa, Y.; Tabuchi, T.; Satoh, T.; Kosaka, K.; Suzuki, K. Carnosic acid (CA) prevents lipid accumulation in hepatocytes through the EGFR/MAPK pathway. J. Gastroenterol. 2012, 47, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, C.; Galvano, F.; Pierdomenico, L.; Speroni, E.; Guerra, M.C. Effects of rosmarinic acid against aflatoxin B1 and ochratoxin-a-induced cell damage in a human hepatoma cell line (Hep G2). J. Appl. Toxicol. 2004, 24, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tabuchi, T.; Tamaki, Y.; Kosaka, K.; Takikawa, Y.; Satoh, T. Carnosic acid and carnosol inhibit adipocyte differentiation in mouse 3T3-L1 cells through induction of phase 2 enzymes and activation of glutathione metabolism. Biochem. Biophys. Res. Commun. 2009, 382, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Gaya, M.; Repetto, V.; Toneatto, J.; Anesini, C.; Piwien-Pilipuk, G.; Moreno, S. Antiadipogenic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPAR pathways at the onset of the differentiation program. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3796–3806. [Google Scholar] [CrossRef] [PubMed]
- Babish, J.G.; Pacioretty, L.M.; Bland, J.S.; Minich, D.M.; Hu, J.; Tripp, M.L. Antidiabetic screening of commercial botanical products in 3T3-L1 adipocytes and db/db mice. J. Med. Food 2010, 13, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Sung, M.-K. Carnosic Acid Inhibits Lipid Accumulation in 3T3-L1 Adipocytes Through Attenuation of Fatty Acid Desaturation. J. Cancer Prev. 2015, 20, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Mun, S.T. Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Naimi, M.; Tsakiridis, T.; Stamatatos, T.C.; Alexandropoulos, D.I.; Tsiani, E. Increased skeletal muscle glucose uptake by rosemary extract through AMPK activation. Appl. Physiol. Nutr. Metab. 2015, 40, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Hundal, H.S. Carnosic acid stimulates glucose uptake in skeletal muscle cells via a PME-1/PP2A/PKB signalling axis. Cell. Signal. 2014, 26, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Naimi, M.; Vlavcheski, F.; Murphy, B.; Hudlicky, T.; Tsiani, E. Carnosic acid as a component of rosemary extract stimulates skeletal muscle cell glucose uptake via AMPK activation. Clin. Exp. Pharmacol. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Erenmemisoglu, A.; Saraymen, R.; Ustun, S. Effect of a Rosmarinus officinalis leave extract on plasma glucose levels in normoglycaemic and diabetic mice. Pharmazie 1997, 52, 645–646. [Google Scholar] [PubMed]
- Khalil, O.; Ramadan, K.; Danial, E.; Alnahdi, H.; Ayaz, N. Antidiabetic activity of Rosmarinus officinalis and its relationship with the antioxidant property. Afr. J. Pharm. Pharmacol. 2012, 6, 1031–1036. [Google Scholar]
- Al-Jamal, A.-R.; Alqadi, T. Effects of rosemary (Rosmarinus officinalis) on lipid profile of diabetic rats. Jordan J. Biol. Sci. 2011, 4, 199–203. [Google Scholar]
- Alnahdi, H.S. Effect of Rosmarinus officinalis extract on some cardiac enzymes of streptozotocin-induced diabetic rats. J. Health Sci. 2012, 2, 33–37. [Google Scholar] [CrossRef]
- Emam, M.A. Comparative evaluation of anti-diabetic activity of Rosmarinus officinailis L. and Chamomile recutita in streptozotocin induced diabetic rats. Agric. Biol. J. N. Am. 2012, 3, 247–252. [Google Scholar]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and hepatoprotective activity of Rosmarinus officinalis extract in diabetic rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.Z.A. Effect of Rosmarinus officinalis on lipid profile of streptozotocin-induced diabetic rats. Egypt. J. Hosp. Med. 2013, 53, 809–815. [Google Scholar] [CrossRef]
- Nazem, F.; Farhangi, N.; Neshat-Gharamaleki, M. Beneficial effects of endurance exercise with Rosmarinus officinalis labiatae leaves extract on blood antioxidant enzyme activities and lipid peroxidation in streptozotocin-induced diabetic rats. Can. J. Diabetes 2015, 39, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Runtuwene, J.; Cheng, K.-C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.R.; Inui, A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des. Dev. Ther. 2016, 10, 2193–2202. [Google Scholar] [CrossRef]
- Azevedo, M.F.; Lima, C.F.; Fernandes-Ferreira, M.; Almeida, M.J.; Wilson, J.M.; Pereira-Wilson, C. Rosmarinic acid, major phenolic constituent of Greek sage herbal tea, modulates rat intestinal SGLT1 levels with effects on blood glucose. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Header, E.; Elsawy, N.; El-boshy, M.; Basalamah, M.; Mubarak, M.; Hadda, T. POM analyses of constituents of Rosmarinus officinalis and their synergistic effect in experimental diabetic rats. J. Bioanal. Biomed. 2015, 7, 18–23. [Google Scholar] [CrossRef]
- Bakırel, T.; Bakırel, U.; Keleş, O.Ü.; Ülgen, S.G.; Yardibi, H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Alhader, A.; Hasan, Z.; Aqel, M. Hyperglycemic and insulin release inhibitory effects of Rosmarinus Officinalis. J. Ethnopharmacol. 1994, 43, 217–221. [Google Scholar] [CrossRef]
- Kensara, O.; ElSawy, N.; Altaf, F.; Header, E. Hypoglycemic and hepato-protective effects of Rosmarinus officinalis in experimental diabetic Rats. UQU Med. J. 2010, 1, 98–113. [Google Scholar]
- Tavafi, M.; Ahmadvand, H.; Khalatbari, A.; Tamjidipoor, A. Rosmarinic acid ameliorates diabetic nephropathy in uninephrectomized diabetic rats. Iran. J. Basic Med. Sci. 2011, 14, 275–283. [Google Scholar]
- Nusier, M.K.; Bataineh, H.N.; Daradkah, H.M. Adverse effects of rosemary (Rosmarinus officinalis L.) on reproductive function in adult male rats. Exp. Biol. Med. 2007, 232, 809–813. [Google Scholar]
- Wang, T.; Takikawa, Y.; Satoh, T.; Yoshioka, Y.; Kosaka, K.; Tatemichi, Y.; Suzuki, K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol. Res. 2011, 41, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.R.; Yáñez-Gascón, M.-J.; Villalba, R.G.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; de Gea, J.C.E.; García-Conesa, M.-T. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in zucker rats fed a rosemary extract rich in carnosic acid. PLoS ONE 2012, 7, e39773. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Norihisa, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Aprikian, O.; Monnard, I.; Moulin, J.; Membrez, M.; Beolor, J.-C.; Raab, T.; Mace, K.; Darimont, C. Rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta Med. 2010, 76, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Al Sheyab, F.M.; Abuharfeil, N.; Salloum, L.; Hani, R.B.; Awad, D.S. The effect of rosemary (Rosmarinus officinalis. L) plant extracts on the immune response and lipid profile in mice. J. Biol. Life Sci. 2011, 3. [Google Scholar] [CrossRef]
- Afonso, M.S.; de O Silva, A.M.; Carvalho, E.B.; Rivelli, D.P.; Barros, S.B.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic compounds from rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanithadevi, B.; Anuradha, C.V. Effect of rosmarinic acid on insulin sensitivity, glyoxalase system and oxidative events in liver of fructose-fed mice. Int. J. Diabetes Metab. 2008, 16, 35–44. [Google Scholar]
- Jung, D.Y.; Kim, E.Y.; Joo, S.Y.; Park, J.B.; Moon, C.; Kim, S.H.; Sim, E.Y.; Joh, J.W.; Kwon, C.H.; Kwon, G.Y.; et al. Prolonged survival of islet allografts in mice treated with rosmarinic acid and anti-CD154 antibody. Exp. Mol. Med. 2008, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Labban, L.; Mustafa, U.E.-S.; Ibrahim, Y.M. The Effects of Rosemary (Rosmarinus officinalis) Leaves Powder on Glucose Level, Lipid Profile and Lipid Perodoxation. Int. J. Clin. Med. 2014, 5, 297–304. [Google Scholar] [CrossRef]
- Sinkovic, A.; Suran, D.; Lokar, L.; Fliser, E.; Skerget, M.; Novak, Z.; Knez, Z. Rosemary extracts improve flow-mediated dilatation of the brachial artery and plasma PAI-1 activity in healthy young volunteers. Phytother. Res. PTR 2011, 25, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Lukaczer, D.; Darland, G.; Tripp, M.; Liska, D.; Lerman, R.H.; Schiltz, B.; Bland, J.S. A pilot trial evaluating Meta050, a proprietary combination of reduced iso-alpha acids, rosemary extract and oleanolic acid in patients with arthritis and fibromyalgia. Phytother. Res. PTR 2005, 19, 864–869. [Google Scholar] [CrossRef] [PubMed]
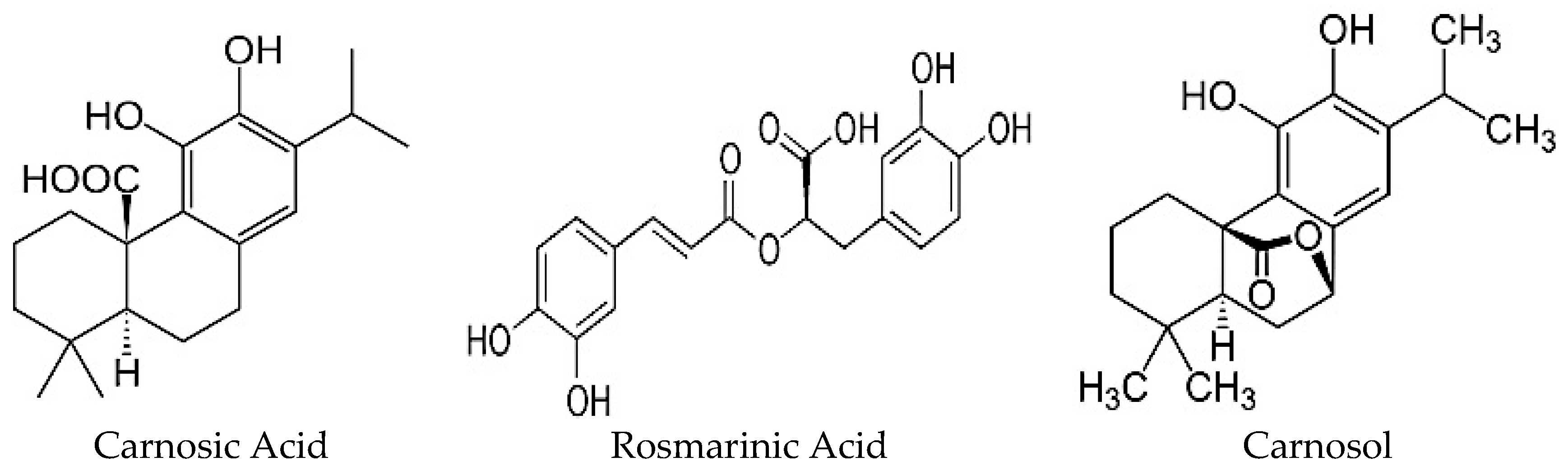
| Polyphenols | ||||||
|---|---|---|---|---|---|---|
| Phenolic Acids | Flavonoids | Phenolic Terpenes | ||||
| Hydroxycinnamic Acids | Hydroxybenzoic Acids | Hydroxyphenylacetic Acids | Flavones | Flavonols | Diterpenes | Triterprnres |
| Rosmarinic acid (C18H16O8) [18] | Vanillic acid (C8H18O4) [22] | Homovanillic acid (C9H10O4) [22] | Apigenin (C27H30O15) [18] | Rutin (C27H30O16) [18] | Carnosol (C20H26O4) [18,21,22] | Betulinic acid (C30H48O3) [23,24] |
| Chlorogenic acid (C16H18O9) [18,21,22] | Syringic acid (C9H10O5) [18,21,22] | p-Hydroxybenzoic acid (C7H6O3) [22] | Luteolin (C15H10O6) [18] | Kaempferol (C15H10O6) [18,21,22] | Carnosic acid (C20H28O4) [18,21,22] | Oleanolic acid (C30H48O3) [23,24] |
| o-,m-,p-Coumaric acid (C9H8O3) [18,21,22] | Caffeic acid (C9H7O4) [18,21,22] | Hispidulin (C16H12O6) [23,24] | Kaempferol-3-O-rutinoside (C27H30O15) [18,21,22] | Rosmanol (C20H26O5) [18,21,22] | Ursolic acid (C30H48O3) [23,24] | |
| m-Hydroxybenzoic acid (C7H6O3) [18,21,22] | Protocatechuic acid (C7H6O4) [18,21,22] | Genkwanin (C16H12O5) [23,24] | Naringenin-C-hexoside (C21H22O10) [21,22] | Epirosmanol (C20H26O5) [21,22] | ||
| Coumaroylqunic acid (C16H18O8) [21,22] | Dicaffeoylquinic acid (C25H24O12) [18,21,22] | Hesperetin (C28H34O15) [18] | Isorosmanol (C20H26O5) [18,21,22] | |||
| Ferulic acid (C16H20O9) [21,22] | Apigenin-7-O-glucoside (C21H20O10) [18] | Rosmaridiphenol (C20H28O3) [18] | ||||
| Quercetin (C15H10O7) [18] | Rosmadial (C20H24O5) [18] | |||||
| Isorhamnetin-3-O-hexoside (C22H22O12) [23,24] | Methoxycarnosol [23,24] | |||||
| Apigenin-acetylglucosidase [23,24] | Methoxycarnosate [23,24] | |||||
| Isohamnetin-lutelion [23,24] | ||||||
| Isorhamnetin-luteolin [23,24] | ||||||
| Cell-Free Model | Dose | Effects | Reference |
|---|---|---|---|
| α-glycosidase | RE 5.5–55 mg/mL | ↓ α-glycosidase activity (60%) | [28] |
| α-glycosidase | 50% ethanolic RE (IC50: 83–711 µg/mL) | ↓ α-glucosidase | [29] |
| Porcine Pancreatic α-amylase (PPAM) | RA 8.88 mM | ↓ PPAM activity (85%) | [30] |
| Dipeptidyl Peptidase IV (DPP-IV); Protein Tyrosine Phosphatase 1B (PTP1B) | Methanolic RE (IC50 = 6.5 ± 0.4 µM) RA (IC50 = 14.1 ± 1.7 µM) COH (IC50 > 100 µM) | ↓ DPP-IV activity (50%) RE (↓ PTP1B 40.9 ± 7.2%). | [31] |
| Porcine pancreatic lipase (PL); Hormone sensitive lipase (HSL) | Methanolic RE 6.3–200 µg/mL RE (IC50 = 13.8 for PL and 95.2 µg/mL for HSL) RA (IC50 = 125.2 for PL and 51.5 µg/mL for HSL) | ↓ PL and HSL activity RE (↓ PL: 36.8–95.1%) RE (↓ HSL: 2.4–64.9%) RE > RA | [33] |
| Human PL | Acetone RE 100 µg/mL rich in CA | ↓ human PL (70%) | [34] |
| Rat liver diacylglycerol acyltransferase (DGAT1) | COH (IC50 = 62.5 ± 2.1 µM) | ↓ DGAT1 activity (67.5–90.6%) | [35] |
| Cell/Model | Treatment | Effects | Reference |
|---|---|---|---|
| HepG2 hepatocytes | Methanolic RE 100 μg/mL | ↓ gluconeogenesis | [36] |
| HepG2 hepatocytes | Methanolic RE 0.4, 2, 10, 50 µg/mL | ↑ glucose consumption | [37] |
| ↑ glycolytic rate | |||
| ↓ glycogenesis comparable to metformin | |||
| ↑ β-oxidation | |||
| ↓ decreased fatty acid synthesis | |||
| ↔ cell viability | |||
| HepG2 hepatocytes | CA 10–20 µM | ↓ palmitate-induced lipid accumulation | [36] |
| ↔ cell viability | |||
| HepG2 hepatocytes | COH 20–40 µM | ↓ de novo formation of intracellular TG | [35] |
| ↔ cell viability | |||
| HepG2 hepatocytes | RA 25–50 µM | ↓ apoptosis | [39] |
| ↓ ROS production |
| Cell | Treatment | Effects | Reference |
|---|---|---|---|
| 3T3-L1 adipocytes | CA 3 µM, COH 3 µM | ↓ differentiation | [40] |
| ↑ intracellular GSH | |||
| 3T3-L1 adipocytes | Acetone RE 10–30 μg/mL CA 0.3–20 μM | Inhibited adipocyte differentiation ↔ cell viability | [41] |
| 3T3-L1 adipocytes | RE 50 µg/mL | ↑ intracellular lipid | [42] |
| ↑ glucose uptake | |||
| 3T3-L1 adipocytes | CA 0.1–10 µM | ↓ intracellular lipid accumulation | [43] |
| ↓ TG content (15.5–39.8%) | |||
| ↓ GPDH activity | |||
| ↔ cell viability | |||
| 3T3-L1 adipocytes pretreated with LPS | CA 0–20 µM | ↓ mRNA expression of TNFα, IL-6 and MCP-1 | [44] |
| ↓ TLR4 protein expression | |||
| ↓ Phospho-ERK levels | |||
| ↓ NF-κB activation |
| Cell/Model | Treatment | Effects | Reference |
|---|---|---|---|
| L6 myotubes | Methanolic RE 0.1–50 µg/mL | ↑ glucose uptake (GU) dose- and time-dependent Max stimulation: 5 µg/mL for 4 h comparable to insulin and metformin. ↔ cell viability | [45] |
| L6 myotubes | CA 1–50 µM | ↑ GU in a dose- and time-dependent manner Max stimulation: 20 µM for 6 h comparable to insulin and metformin ↔ cell viability | [46] |
| L6 myotubes | Methanolic CA 0.1–10 µM | ↑ GU dose- and time-dependent Max stimulation: 2 µM for 4 h comparable to insulin and metformin ↔ cell viability | [47] |
| Animal Model | Dose | Glucose | Other Measures | Reference |
|---|---|---|---|---|
| Streptozotocin (STZ)-Induced Diabetic Model | ||||
| STZ-induced diabetic Swiss albino mice | Ad libitum (10 g leaves of rosemary in 1 L boiling water) for 3 months | ↓ FPG in healthy and diabetic animals | ↔ creatinine, urea bilirubin, total albumin, alkaline phosphatase | [49] |
| STZ-induced diabetic male | aqueous and ethanolic RE 20 mg/kg/day | ↓ plasma glucose | ↓ α-glucosidase (AGc) | [29] |
| ddY mice | ||||
| Male Wistar rats | RA 577 µg/mL as drinking fluid for 14 days | ↓ FPG ↓ OGTT ↓ HOMA-IR indices ↑ serum insulin | ↓ hepatic glycogen content | [58] |
| STZ-induced diabetic male albino rats | aqueous RE, 200 mg/kg/day for 3 weeks | ↓ FPG | ↑ vitamin C | [50] |
| STZ-induced diabetic male albino rats | aqueous RE 4 g/kg/day for 4 weeks | ↓ FPG (20%) | ↓ TC, TG, LDL ↑ HDL | [51] |
| STZ-induced diabetic male albino rats | aqueous RE, 200 mg/kg/day 2 weeks prior and 3 weeks after STZ | ↓ FPG (36.9%) | ↓ TC, TG, LDL ↑HDL ↑ hemoglobin | [52] |
| STZ-induced diabetic male albino rats | aqueous RE, 200 mg/kg/day for 21 days | ↓ FPG | ↓ TC ↓ TG ↑ TAC | [53] |
| STZ-induced diabetic male albino rats | aqueous RE, 200 mg/kg/day 2 weeks prior and 3 weeks after STZ | ↓ FPG in both groups ↑ serum insulin ↑ C-peptide ↓ β-cell loss | ↑ total albumin | [54] |
| STZ-induced diabetic male Dawley rats | Dried rosemary leaves powder 5 g/100 g of diet | ↓ FPG (53.97%) ↓ HbA1c (24.56%) | ↓ TG (45.43%) ↓ TC (39.31%) ↓ LDL (33.89%) | [55] |
| Male albino Wistar rats | aqueous RE 200 mg/kg/day with/without moderate intensity exercise training for 8 weeks | ↓ FPG ↑ Serum CAT, SOD, GPx ↑ serum insulin ↓ MDA | [56] | |
| Sprague-Dawley male albino rats | aqueous RE 200 mg/kg/day for 6 weeks | ↓ FPG | ↑ Serum CAT, SOD, GSH ↓ MDA ↓ urea, uric acid and creatinine levels | [59] |
| Male Wistar rats | Intraperitoneal injection of 120, 160, 200 mg/kg RA for 7 days (acute) and 28 days (chronic) | ↓ FPG ↓ OGTT ↓ HOMA-IR indices ↑ ITT Normalized serum insulin | ↓ hepatic PEPCK expression/gluconeogenesis | [57] |
| Alloxan-Induced Diabetes Model | ||||
| Alloxan-induced diabetic rabbits | ethanol RE, 200 mg/kg for 6 h (acute); for 1 week (subacute) | ↓ FPG in healthy and diabetic rabbits ↑ plasma insulin | ↓ MDA ↑ SOD ↑ CAT | [60] |
| Alloxan-induced male diabetic rabbits | volatile RE, 25 mg/kg intramuscular injection for 30, 60 and 120 min | ↑ serum glucose | [61] | |
| ↓ serum insulin | ||||
| Alloxan-induced Sprague-Dawley male albino rats | 20% aqueous RE and 20% RE powdered food for 45 days | ↓ FPG | ↓ hepatocyte necrosis ↓ small hemorrhages ↓ hepatocyte degradation | [62] |
| Alloxan-induced Sprague-Dawley uninephrectomized rats | RA 100–200 mg/kg/day for 8 weeks | ↓ glomerulosclerosis ↓ creatinine and urea ↓ glomerular number ↓ serum MDA | [63] | |
| Male adult Sprague-Dawley rats | 70% aqueous RE, 250 and 500 mg/kg/day for 63 days | ↔ serum glucose | ↔ body weight TG, TC ↓ alanine aminotransferase (ALT) ↓ Aspartate Aminotransferase (AST) ↓ spermatogenesis ↓ testosterone ↓ sperm motility | [64] |
| Genetically-Induced Diabetes Models | ||||
| Male ob/ob mice | CA 17 mg/kg/day for 5 weeks | ↓ FPG (18%) ↓ OGTT glucose ↓ serum insulin (47%) | ↓ TC (24%) ↓ TG (60%) ↓ plasma FFA (13%) ↓ hepatic lipids ↓ ALT (64%) | [65] |
| Female Zucker lean (fa/+) and obese (fa/fa) rats | 0.5% w/w of aqueous RE enriched with CA for 64 days | ↔ plasma glucose ↓ insulin levels in lean animals | Inhibited gastric lipase activity in both lean (70%) and obese animals (80%) ↓ Serum total cholesterol, TG, LDL ↑ HDL | [66] |
| Diet-Induced Diabetes Models | ||||
| HFD-treated male C57BL/6J mice | aqueous RE, containing 20% CA 500 mg/kg/day for 16 weeks | ↓ FPG (72%) ↔ insulin | ↓ body weight ↑ fecal total lipid content (1–2 fold) ↓ fat mass ↓ TC (68%) ↔ TG | [34] |
| HFD- (olive oil) treated male ddY mice | CA 20 mg/kg for 14 days COH 200 mg/kg for 14 days | ↓ body weight (7%) ↑ epididymal fat ↓ pancreatic lipase (IC50 12 and 4.4 μg/mL for CA and COH respecively) | [67] | |
| HFD-treated male C57BL/6J mice | ethanolic RE 20 or 200 mg/kg/day for 50 days | ↔ FPG ↔ glucose tolerance ↔ insulin | ↓ body weight and fat mass (64% and 57%) ↓ Hepatic TG (39%) ↔ serum TG and TC ↑ fecal lipid excretion | [68] |
| Diet-induced HC female BALB/c mice | aqueous RE, 100 mg/kg/day for 36 days | ↓ TC, TG, LDL ↑ HD | [69] | |
| Diet-induced HC Wistar rats | aqueous RE, aqueous 70–140 mg/kg/day RE non-esterified phenolic 7–14 mg/kg/day of for 4 weeks | ↓ TC (39.8%) ↓ non-HDL (44.4%) | [70] | |
| Fructose-fed Swiss albino mice | RA 100 mg/kg/day for 60 days | ↓ FPG levels ↓ HbA1c ↓ OGTT glucose ↓ plasma insulin | ↑ diaphragm glucose utilization | [71] |
| Study Methodology | Treatment | Effect | Reference |
|---|---|---|---|
| 48 healthy individuals | Dry rosemary powder 2, 5 or 10 g/day, for 8 weeks | ↓ FPG ↓ TC, ↓ LDL, ↓ TG, ↑ HDL ↓ MDA, ↓ GR ↑ vitamin C ↑ β-carotene | [73] |
| 12 healthy, young volunteers | RE 77.7 mg | ↓ PAI-1 | [74] |
| COH 0.97 mg | |||
| CA 8.6 mg | |||
| RA 10.30 mg for 21 days | |||
| 72 patients with rheumatic disease including osteoarthritis (OA), rheumatoid arthritis, fibromyalgia (FM) | Meta050 compound (RE, oleanolic acid and reduced iso-alpha-acids) | ↓ CRP | [75] |
| 440 mg/day for 4 weeks 3 times per day | ↓ arthritis pain scores | ||
| 880 mg/day for 4 weeks 2 times per day | ↔ fibromyalgia scores |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. https://doi.org/10.3390/nu9090968
Naimi M, Vlavcheski F, Shamshoum H, Tsiani E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients. 2017; 9(9):968. https://doi.org/10.3390/nu9090968
Chicago/Turabian StyleNaimi, Madina, Filip Vlavcheski, Hesham Shamshoum, and Evangelia Tsiani. 2017. "Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives" Nutrients 9, no. 9: 968. https://doi.org/10.3390/nu9090968





