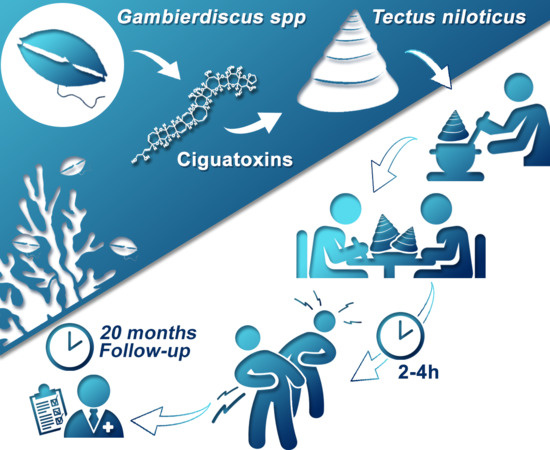
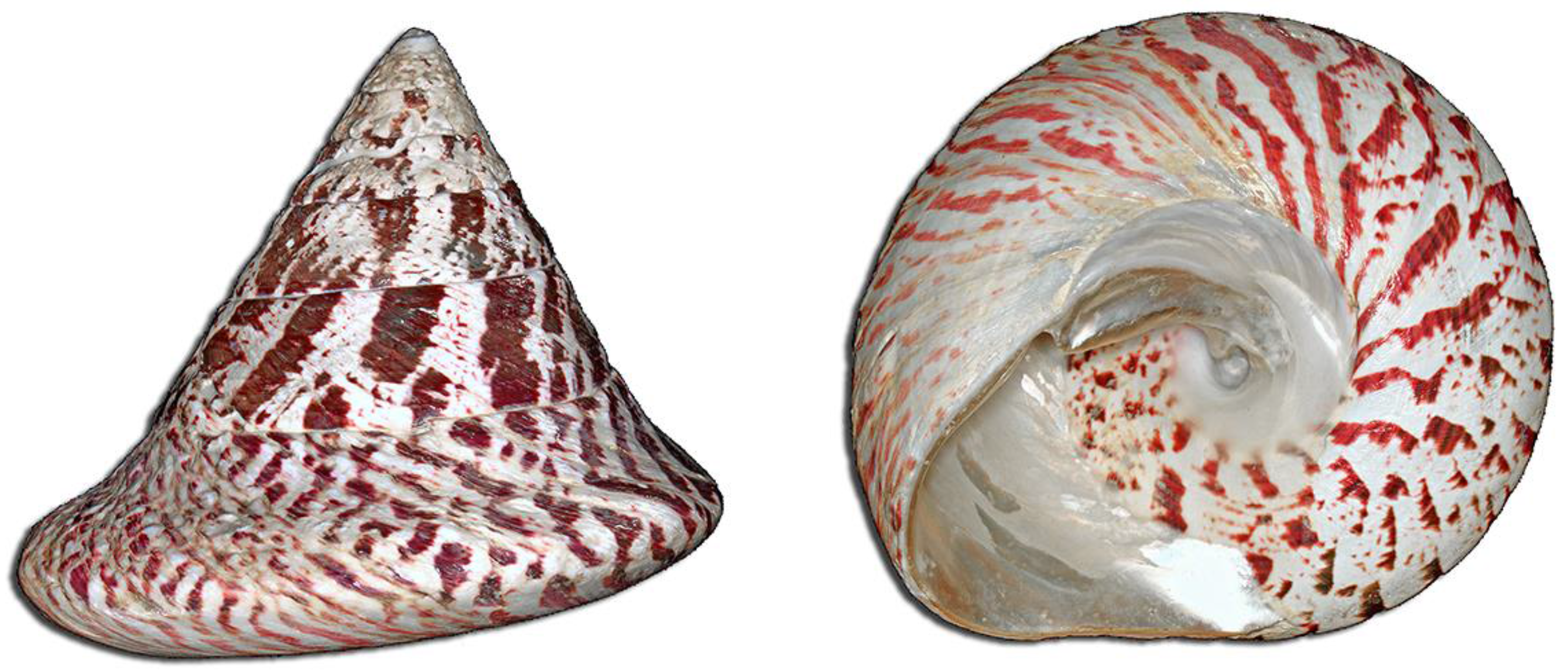
Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Clinical Characterization and Follow-Up of a Mass Poisoning Event in Nuku Hiva Island (French Polynesia)
Abstract
:1. Introduction
2. Results
2.1. Patients Description and Acute Clinical Manifestations
2.2. Six- and 20-Month Medical Follow-Up
2.2.1. Chronic Symptoms
2.2.2. Recurrence of Symptoms and Triggering Factors
2.2.3. Medical Management of Acute and Chronic Symptoms in Patients No. 1–8
2.3. Index Case Description (Patient No. 9)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Context
5.2. Acute Phase Description
5.3. Six and 20-Month Medical Follow-Up
5.4. Index Case Description (Patient No. 9)
5.5. CTX Extraction and Detection from Tectus niloticus Samples
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skinner, M.P.; Brewer, T.D.; Johnstone, R.; Fleming, L.E.; Lewis, R.J. Ciguatera fish poisoning in the pacific islands (1998 to 2008). PLoS Negl. Trop. Dis. 2011, 5, e1416. [Google Scholar] [CrossRef] [PubMed]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Gatti, C.; Oelher, E.; Legrand, A.M. Severe seafood poisoning in French Polynesia: A retrospective analysis of 129 medical files. Toxicon 2008, 51, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Bagnis, R.; Kuberski, T.; Laugier, S. Clinical observations on 3009 cases of ciguatera (fish poisoning) in the south pacific. Am. J. Trop. Med. Hyg. 1979, 28, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Feldman, R.L.; Nau, A.W.; Kibler, S.R.; Wayne Litaker, R. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon 2010, 56, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Quod, J.P.; Turquet, J. Ciguatera in reunion island (SW Indian Ocean): Epidemiology and clinical patterns. Toxicon 1996, 34, 779–785. [Google Scholar] [CrossRef]
- Boada, L.D.; Zumbado, M.; Luzardo, O.P.; Almeida-Gonzalez, M.; Plakas, S.M.; Granade, H.R.; Abraham, A.; Jester, E.L.; Dickey, R.W. Ciguatera fish poisoning on the west africa coast: An emerging risk in the canary islands (Spain). Toxicon 2010, 56, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y. Ciguatera fish poisoning in East Asia and Southeast Asia. Mar. Drugs 2015, 13, 3466–3478. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Sato, S.; Tawong, W.; Sakanari, H.; Uehara, K.; Shah, M.M.; Suda, S.; Yasumoto, T.; Taira, Y.; Yamaguchi, H.; et al. Genetic diversity and distribution of the ciguatera-causing dinoflagellate Gambierdiscus spp. (dinophyceae) in coastal areas of Japan. PLoS ONE 2013, 8, e60882. [Google Scholar] [CrossRef] [PubMed]
- Bentur, Y.; Spanier, E. Ciguatoxin-like substances in edible fish on the eastern Mediterranean. Clin. Toxicol. 2007, 45, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Perez, S.; Alfonso, A.; Vale, C.; Rodriguez, P.; Gouveia, N.N.; Gouveia, N.; Delgado, J.; Vale, P.; Hirama, M.; et al. First toxin profile of ciguateric fish in Madeira Arquipelago (Europe). Anal. Chem. 2010, 82, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Bavastrelli, M.; Bertucci, P.; Midulla, M.; Giardini, O.; Sanguigni, S. Ciguatera fish poisoning: An emerging syndrome in Italian travelers. J. Travel Med. 2001, 8, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Winter, F.D. Ciguatera poisoning: An unwelcome vacation experience. Proc. Bayl. Univ. Med. Cent. 2009, 22, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Mattei, C.; Vetter, I.; Eisenblatter, A.; Krock, B.; Ebbecke, M.; Desel, H.; Zimmermann, K. Ciguatera fish poisoning: A first epidemic in Germany highlights an increasing risk for European countries. Toxicon 2014, 91, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, E.; Juzans, P.; Legrand, A.M.; Molgo, J. Nodal swelling produced by ciguatoxin-induced selective activation of sodium channels in myelinated nerve fibers. Neuroscience 1996, 71, 1121–1131. [Google Scholar] [CrossRef]
- Hogg, R.C.; Lewis, R.J.; Adams, D.J. Ciguatoxin (CTX-1) modulates single tetrodotoxin-sensitive sodium channels in rat parasympathetic neurones. Neurosci. Lett. 1998, 252, 103–106. [Google Scholar] [CrossRef]
- Inserra, M.C.; Israel, M.R.; Caldwell, A.; Castro, J.; Deuis, J.R.; Harrington, A.M.; Keramidas, A.; Garcia-Caraballo, S.; Maddern, J.; Erickson, A.; et al. Multiple sodium channel isoforms mediate the pathological effects of pacific ciguatoxin-1. Sci. Rep. 2017, 7, 42810. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Liberona, J.L.; Molgo, J.; Jaimovich, E. Pacific ciguatoxin-1b effect over Na+ and K+ currents, inositol 1,4,5-triphosphate content and intracellular Ca2+ signals in cultured rat myotubes. Br. J. Pharmacol. 2002, 137, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Kumar-Roine, S.; Darius, H.T.; Chinain, M.; Laurent, D.; Pauillac, S. Pacific ciguatoxin 1b-induced modulation of inflammatory mediators in a murine macrophage cell line. Toxicon 2010, 56, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Bidard, J.N.; Vijverberg, H.P.; Frelin, C.; Chungue, E.; Legrand, A.M.; Bagnis, R.; Lazdunski, M. Ciguatoxin is a novel type of Na+ channel toxin. J. Biol. Chem. 1984, 259, 8353–8357. [Google Scholar] [PubMed]
- Zhang, X.; Cao, B.; Wang, J.; Liu, J.; Tung, V.O.; Lam, P.K.; Chan, L.L.; Li, Y. Neurotoxicity and reactive astrogliosis in the anterior cingulate cortex in acute ciguatera poisoning. Neuromol. Med. 2013, 15, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.K. A theoretical discourse on the pharmacology of toxic marine ingestions. Ann. Emerg. Med. 1987, 16, 1006–1015. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y. Severe bradycardia and prolonged hypotension in ciguatera. Singap. Med. J. 2013, 54, e120–e122. [Google Scholar] [CrossRef] [PubMed]
- Pearn, J. Neurology of ciguatera. J. Neurol. Neurosurg. Psychiatry 2001, 70, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Chateau-Degat, M.L.; Beuter, A.; Vauterin, G.; Nguyen, N.L.; Chinain, M.; Darius, T.; Legrand, A.M.; Chansin, R.; Dewailly, E. Neurologic signs of ciguatera disease: Evidence of their persistence. Am. J. Trop. Med. Hyg. 2007, 77, 1170–1175. [Google Scholar] [PubMed]
- Cameron, J.; Capra, M.F. The basis of the paradoxical disturbance of temperature perception in ciguatera poisoning. J. Toxicol. Clin. Toxicol. 1993, 31, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Chateau-Degat, M.L.; Huin-Blondey, M.O.; Chinain, M.; Darius, T.; Legrand, A.M.; Nguyen, N.L.; Laudon, F.; Chansin, R.; Dewailly, E. Prevalence of chronic symptoms of ciguatera disease in French Polynesian adults. Am. J. Trop. Med. Hyg. 2007, 77, 842–846. [Google Scholar] [PubMed]
- Vetter, I.; Touska, F.; Hess, A.; Hinsbey, R.; Sattler, S.; Lampert, A.; Sergejeva, M.; Sharov, A.; Collins, L.S.; Eberhardt, M.; et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 2012, 31, 3795–3808. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y. Characteristic features and contributory factors in fatal ciguatera fish poisoning--implications for prevention and public education. Am. J. Trop. Med. Hyg. 2016, 94, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Derian, A.; Khurana, S.; Rothenberg, J.; Plumlee, C. Intractable seizures and rehabilitation in ciguatera poisoning. Am. J. Phys. Med. Rehabil. 2017, 96, e89–e92. [Google Scholar] [CrossRef] [PubMed]
- Oehler, E.; Gatti, C.; Legrand, A.M.; Ghawche, F. [ciguatera and acute polyradiculoneuritis. Description of two cases in French Polynesia: Immunoallergic hypothesis?]. Med. Trop. 2009, 69, 75–77. [Google Scholar]
- Friedman, M.A.; Arena, P.; Levin, B.; Fleming, L.; Fernandez, M.; Weisman, R.; Bernstein, J.; Schrank, K.; Blythe, D.; Backer, L.; et al. Neuropsychological study of ciguatera fish poisoning: A longitudinal case-control study. Arch. Clin. Neuropsychol. 2007, 22, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Pearn, J. Chronic ciguatera: One organic cause of the chronic fatigue syndrome. J. Chronic Fatigue Syndr. 1996, 2, 29–34. [Google Scholar] [CrossRef]
- Baumann, F.; Bourrat, M.B.; Pauillac, S. Prevalence, symptoms and chronicity of ciguatera in New Caledonia: Results from an adult population survey conducted in Noumea during 2005. Toxicon 2010, 56, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Pearn, J.H. Chronic fatigue syndrome: Chronic ciguatera poisoning as a differential diagnosis. Med. J. Aust. 1997, 166, 309–310. [Google Scholar] [PubMed]
- Blythe, D.G.; De Sylva, D.P.; Fleming, L.E.; Ayyar, R.A.; Baden, D.G.; Shrank, K. Clinical experience with i.V. Mannitol in the treatment of ciguatera. Bull. Soc. Pathol. Exot. 1992, 85, 425–426. [Google Scholar] [PubMed]
- Glaziou, P.; Martin, P.M. Study of factors that influence the clinical response to ciguatera fish poisoning. Toxicon 1993, 31, 1151–1154. [Google Scholar] [CrossRef]
- Lewis, R.J. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef]
- Laurent, D.; Kerbrat, A.-S.; Darius, H.T.; Girard, E.; Golubic, S.; Benoit, E.; Sauviat, M.-P.; Chinain, M.; Molgo, J.; Pauillac, S. Are cyanobacteria involved in ciguatera fish poisoning-like outbreaks in New Caledonia? Harmful Algae 2008, 7, 827–838. [Google Scholar] [CrossRef]
- Villeneuve, A.; Laurent, D.; Chinain, M.; Gugger, M.; Humbert, J.F. Molecular characterization of the diversity and potential toxicity of cyanobacterial mats in two tropical lagoons in the south pacific ocean. J. Phycol. 2012, 48, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Roué, M.; Darius, H.T.; Picot, S.; Ung, A.; Viallon, J.; Gaertner-Mazouni, N.; Sibat, M.; Amzil, Z.; Chinain, M. Evidence of the bioaccumulation of ciguatoxins in giant clams (Tridacna maxima) exposed to Gambierdiscus spp. Cells. Harmful Algae 2016, 57, 78–87. [Google Scholar] [CrossRef]
- Pawlowiez, R.; Darius, H.T.; Cruchet, P.; Rossi, F.; Caillaud, A.; Laurent, D.; Chinain, M. Evaluation of seafood toxicity in the Australes Archipelago (French Polynesia) using the neuroblastoma cell-based assay. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Rodriguez, I.; Barreiro, A.; Kaufmann, M.; Isabel Neto, A.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. First report of ciguatoxins in two starfish species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins 2015, 7, 3740–3757. [Google Scholar] [CrossRef] [PubMed]
- Mak, Y.L.; Wai, T.C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.; Chan, L.L.; Lam, P.K. Pacific ciguatoxins in food web components of coral reef systems in the republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.D.; Baria, M.V.B.; dela Cruz, D.W. Effects of grazing by herbivorous gastropod (Trochus niloticus) on the survivorship of cultured coral spat. Zool. Stud. 2013, 52, 44. [Google Scholar] [CrossRef]
- Gillett, R. Pacific islands trochus introductions 1927–1998. SPC Trochus Inf. Bull. 2002, 9, 9–13. [Google Scholar]
- Bour, W. Un Mollusque Nacrier du Pacifique. Biologie, Écologie et Gestion Rationnelle d’un Mollusque Nacrier du Pacifique: Le Troca (Trochus niloticus L.) de Nouvelle Calédonie; Editions de l’ORSTOM, Collection Etudes et Thèses; Institut Francais de Recherche Scientifique pour le Développement en Coopération: Paris, France, 1992; p. 174. [Google Scholar]
- French Polynesia. Délibération relative à la protection de certaines espèces animales marines et d’eau douce du patrimoine naturel polynésien. In Journal Officiel de la Polynésie Française; Government of French Polynesia: Tahiti, French Polynesia, 1988. [Google Scholar]
- Angibaud, G.; Leveque, J.M.; Laurent, D.; Gaultier, C. [Neurological features after consumption of a variety of neo-caledonian shellfish]. Rev. Neurol. 2000, 156, 65–66. [Google Scholar] [PubMed]
- Darius, H.T.; Roue, M.; Sibat, M.; Viallon, J.; Gatti, C.M.I.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Tectus niloticus (Tegulidae, Gastropod) as a novel vector of ciguatera poisoning: Detection of pacific ciguatoxins in toxic samples from Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Roine, S.; Taiana Darius, H.; Matsui, M.; Fabre, N.; Haddad, M.; Chinain, M.; Pauillac, S.; Laurent, D. A review of traditional remedies of ciguatera fish poisoning in the pacific. Phytother. Res. 2011, 25, 947–958. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperaviciute, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; de Bakker, P.I.; et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D. Endoscopic evaluation of gastro-esophageal reflux disease. Yale J. Biol. Med. 1999, 72, 93–100. [Google Scholar] [PubMed]
- Birinyi-Strachan, L.C.; Gunning, S.J.; Lewis, R.J.; Nicholson, G.M. Block of voltage-gated potassium channels by pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol. Appl. Pharmacol. 2005, 204, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, C.; Molgo, J.; Benoit, E. Involvement of both sodium influx and potassium efflux in ciguatoxin-induced nodal swelling of frog myelinated axons. Neuropharmacology 2014, 85, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottein, M.Y.; Wang, Z.; Ramsdell, J.S. Toxicokinetics of the ciguatoxin P-CTX-1 in rats after intraperitoneal or oral administration. Toxicology 2011, 284, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lange, W.R.; Lipkin, K.M.; Yang, G.C. Can ciguatera be a sexually transmitted disease? J. Toxicol. Clin. Toxicol. 1989, 27, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bottein Dechraoui, M.Y.; Rezvani, A.H.; Gordon, C.J.; Levin, E.D.; Ramsdell, J.S. Repeat exposure to ciguatoxin leads to enhanced and sustained thermoregulatory, pain threshold and motor activity responses in mice: Relationship to blood ciguatoxin concentrations. Toxicology 2008, 246, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Stommel, E.W.; Parsonnet, J.; Jenkyn, L.R. Polymyositis after ciguatera toxin exposure. Arch. Neurol. 1991, 48, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.M. Ciguatoxins: Cyclic polyether modulators of voltage-gated ion channel function. Mar. Drugs 2006, 4, 88–118. [Google Scholar] [CrossRef]
- Shoemaker, R.C.; House, D.; Ryan, J.C. Defining the neurotoxin derived illness chronic ciguatera using markers of chronic systemic inflammatory disturbances: A case/control study. Neurotoxicol. Teratol. 2010, 32, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.C.; Ungaro, R.F.; Baker, H.V.; Moldawer, L.L.; Robertson, A.; Abbott, M.; Roberts, S.M.; Grattan, L.M.; Morris, J.G., Jr. Gene expression patterns in peripheral blood leukocytes in patients with recurrent ciguatera fish poisoning: Preliminary studies. Harmful Algae 2016, 57, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.C.; Wu, Q.; Shoemaker, R.C. Transcriptomic signatures in whole blood of patients who acquire a chronic inflammatory response syndrome (CIRS) following an exposure to the marine toxin ciguatoxin. BMC Med. Genom. 2015, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Schicchi, A.; Pasi, A.; Lonati, D.; Coccini, T.; Locatelli, C.A.; Martinetti, M. Ciguatoxin-induced chronic disease unmasks people carrying hla epitopes peculiar to celiac disease and rheumatoid arthritis. Clin. Toxicol. 2017, 55, 469. [Google Scholar]
- Lonati, D.; Martinetti, M.; Pasi, A.; Gatti, A.; Buonocore, M.; Locatelli, C.A. Clinical findings and genomic biomarkers in three cases of chronic ciguatera poisoning. Clin. Toxicol. 2014, 52, 395. [Google Scholar]
- Ciguatera-Online. Available online: www.ciguatera-online.com (accessed on 6 February 2014).
- Kumar-Roiné, S.; Matsui, M.; Pauillac, S.; Laurent, D. Ciguatera fish poisoning and other seafood intoxication syndromes: A revisit and a review of the existing treatments employed in ciguatera fish poisoning. S. Pac. J. Nat. Appl. Sci. 2010, 28, 1–26. [Google Scholar] [CrossRef]
- Berlin, R.M.; King, S.L.; Blythe, D.G. Symptomatic improvement of chronic fatigue with fluoxetine in ciguatera fish poisoning. Med. J. Aust. 1992, 157, 567. [Google Scholar] [PubMed]
- Palafox, N.A.; Jain, L.G.; Pinano, A.Z.; Gulick, T.M.; Williams, R.K.; Schatz, I.J. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA 1988, 259, 2740–2742. [Google Scholar] [CrossRef] [PubMed]
- Pearn, J.H.; Lewis, R.J.; Ruff, T.; Tait, M.; Quinn, J.; Murtha, W.; King, G.; Mallett, A.; Gillespie, N.C. Ciguatera and mannitol: Experience with a new treatment regimen. Med. J. Aust. 1989, 151, 77–80. [Google Scholar] [PubMed]
- Bagnis, R.; Spiegel, A.; Boutin, J.P.; Burucoa, C.; Nguyen, L.; Cartel, J.L.; Capdevielle, P.; Imbert, P.; Prigent, D.; Gras, C.; et al. [Evaluation of the efficacy of mannitol in the treatment of ciguatera in French Polynesia]. Med. Trop. 1992, 52, 67–73. [Google Scholar]
- Birinyi-Strachan, L.C.; Davies, M.J.; Lewis, R.J.; Nicholson, G.M. Neuroprotectant effects of iso-osmolar d-mannitol to prevent pacific ciguatoxin-1 induced alterations in neuronal excitability: A comparison with other osmotic agents and free radical scavengers. Neuropharmacology 2005, 49, 669–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnorf, H.; Taurarii, M.; Cundy, T. Ciguatera fish poisoning: A double-blind randomized trial of mannitol therapy. Neurology 2002, 58, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.E.; Hoffman, R.S. Is mannitol the treatment of choice for patients with ciguatera fish poisoning? Clin. Toxicol. 2017, 55, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.T.; Villar, L.A. Symptomatic improvement with amitriptyline in ciguatera fish poisoning. N. Engl. J. Med. 1986, 315, 65. [Google Scholar] [PubMed]
- Brett, J.; Murnion, B. Pregabalin to treat ciguatera fish poisoning. Clin. Toxicol. 2015, 53, 588. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.M.; Vasquez, P.A.; Perret, C.F. Treatment of ciguatera poisoning with gabapentin. N. Engl. J. Med. 2001, 344, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Jullian, V.; Pawlowiez, R.; Kumar-Roine, S.; Haddad, M.; Darius, H.T.; Gaertner-Mazouni, N.; Chinain, M.; Laurent, D. Protective effect of Heliotropium foertherianum (Boraginaceae) folk remedy and its active compound, rosmarinic acid, against a pacific ciguatoxin. J. Ethnopharmacol. 2012, 143, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef] [PubMed]
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | F | M | M | M | M | F | F | M | M |
| Age | 59 | 65 | 72 | 50 | 53 | 47 | 39 | 58 | 45 |
| Health condition before poisoning | E | G | G | E | E | E | E | E | E |
| Notable medical history | no | RC | HT | no | no | HT | no | no | no |
| Symptoms onset (hours) | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 2 |
| Gastrointestinal manifestations | |||||||||
| Nausea | +++ | ++ | + | +++ | + | +++ | +++ | ||
| Vomiting | +++ | +++ | +++ | ++ | +++ | +++ | |||
| Diarrhea | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Abdominal pain | +++ | ++ | ++ | +++ | +++ | ++ | |||
| Dysphagia/Hiccup | x | +++ | |||||||
| Esophagitis | +++ | ||||||||
| Gastrointestinal symptoms duration | 6 h | >1 m | 48 h | 2 w | 12 h | 48 h | 18 h | 18 h | >1 m |
| Cardiovascular manifestations | |||||||||
| Hypotension | +++ | +++ | ++ | +++ | +++ | ++ | ++ | ||
| Heart rhythm disorder | + | +++ | ++ | ||||||
| Bradycardia | + | ++ | ++ | ++ | ++ | +++ | |||
| Tachycardia | |||||||||
| Cardiovascular symptoms duration | 12 h | 24 h | 72 h | 24 h | 24 h | 24 h | |||
| Neurological manifestations | |||||||||
| Cold allodynia | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Tingling of extremities | +++ | +++ | x | +++ | +++ | +++ | +++ | +++ | |
| Touch disturbances | +++ | +++ | x | +++ | + | +++ | +++ | ||
| Itching | +++ | +++ | +++ | +++ | +++ | ++ | ++ | ||
| Dysgeusia | x | x | x | x | + | ++ | + | ||
| Burning sensation (throat, mouth) | x | x | x | x | + | +++ | ++ | + | |
| Dizziness | x | ++ | + | ||||||
| Vision disorder | x | x | x | x | + | ++ | |||
| Language disorder | x | + | ++ | ||||||
| Headache | x | ++ | |||||||
| Balance disorder | x | ++ | |||||||
| Anxiety | x | x | |||||||
| Others | |||||||||
| Asthenia | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Myalgia | +++ | +++ | x | x | ++ | +++ | ++ | ++ | |
| Urogenital disturbances, burning, pain | x | x | x | ++ | ++ | ++ | +++ | ||
| Arthralgia | + | +++ | ++ | x | ++ | ++ | |||
| Sleep disorder | +++ | +++ | |||||||
| Hypothermia | ++ | ++ | +++ | +++ | |||||
| Shivers | +++ | +++ | |||||||
| Feet swelling, burning, pain | x | x | x | x | x | ||||
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-Up (Month) | 6 | 20 | 6 | 20 | 6 | 20 | 6 | 20 | 6 | 20 | 6 | 20 | 6 | 20 | 6 | 20 | 6 | 20 |
| Gastrointestinal manifestations | ||||||||||||||||||
| Diarrhea | +++ | |||||||||||||||||
| Neurological manifestations | ||||||||||||||||||
| Tingling of extremities | +++ | +++ | +++ | +++ | ++ | + | + | +++ | ||||||||||
| Touch disturbance | +++ | +++ | + | +++ | ++ | + | ++ | |||||||||||
| Cold allodynia | +++ | +++ | ++ | + | + | +++ | ++ | ++ | ++ | |||||||||
| Itching | +++ | +++ | ++ | +++ | +++ | ++ | + | ++ | ||||||||||
| Balance disorder | + | |||||||||||||||||
| Dysgeusia | + | |||||||||||||||||
| Burning (throat, mouth) | +++ | + | ++ | + | ++ | |||||||||||||
| Urogenital disturbances, burning, pain | +++ | |||||||||||||||||
| Others | ||||||||||||||||||
| Asthenia | + | + | +++ | ++ | + | ++ | ||||||||||||
| Myalgia | + | +++ | ++ | |||||||||||||||
| Sleep disorder | +++ | + | ++ | + | +++ | |||||||||||||
| Irritability | +++ | + | + | |||||||||||||||
| Shivers | ++ | ++ | ||||||||||||||||
| Hypothermia | + | ++ | ++ | |||||||||||||||
| Feet swelling, burning, pain | x | +++ | + | |||||||||||||||
| Restless legs syndrome | + | |||||||||||||||||
| Factors | Patient No. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Alcohol | + | + | + | + | + | + | |||
| Fish | + | + | |||||||
| Beef | + | + | + | + | + | + | + | + | |
| Pork | + | + | |||||||
| Nuts | + | + | |||||||
| Food rich in protein | + | + | |||||||
| Cheese | + | ||||||||
| Salted biscuits | + | ||||||||
| Physical activity | + | + | |||||||
| Ambient temperature variation | + | + | + | ||||||
| Fatigue, lack of sleep | + | + | |||||||
| Rapid weight loss | + | + | + | + | |||||
| Wind, sun exposure | + | ||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, C.M.i.; Lonati, D.; Darius, H.T.; Zancan, A.; Roué, M.; Schicchi, A.; Locatelli, C.A.; Chinain, M. Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Clinical Characterization and Follow-Up of a Mass Poisoning Event in Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 102. https://doi.org/10.3390/toxins10030102
Gatti CMi, Lonati D, Darius HT, Zancan A, Roué M, Schicchi A, Locatelli CA, Chinain M. Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Clinical Characterization and Follow-Up of a Mass Poisoning Event in Nuku Hiva Island (French Polynesia). Toxins. 2018; 10(3):102. https://doi.org/10.3390/toxins10030102
Chicago/Turabian StyleGatti, Clémence Mahana iti, Davide Lonati, Hélène Taiana Darius, Arturo Zancan, Mélanie Roué, Azzurra Schicchi, Carlo Alessandro Locatelli, and Mireille Chinain. 2018. "Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Clinical Characterization and Follow-Up of a Mass Poisoning Event in Nuku Hiva Island (French Polynesia)" Toxins 10, no. 3: 102. https://doi.org/10.3390/toxins10030102