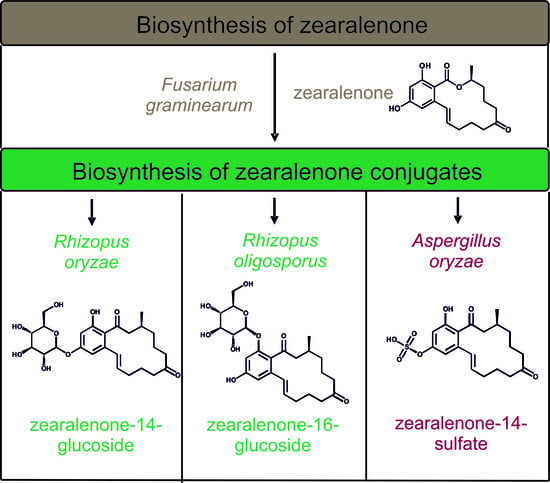
Biosynthesis and Characterization of Zearalenone-14-Sulfate, Zearalenone-14-Glucoside and Zearalenone-16-Glucoside Using Common Fungal Strains
Abstract
:1. Introduction
2. Results
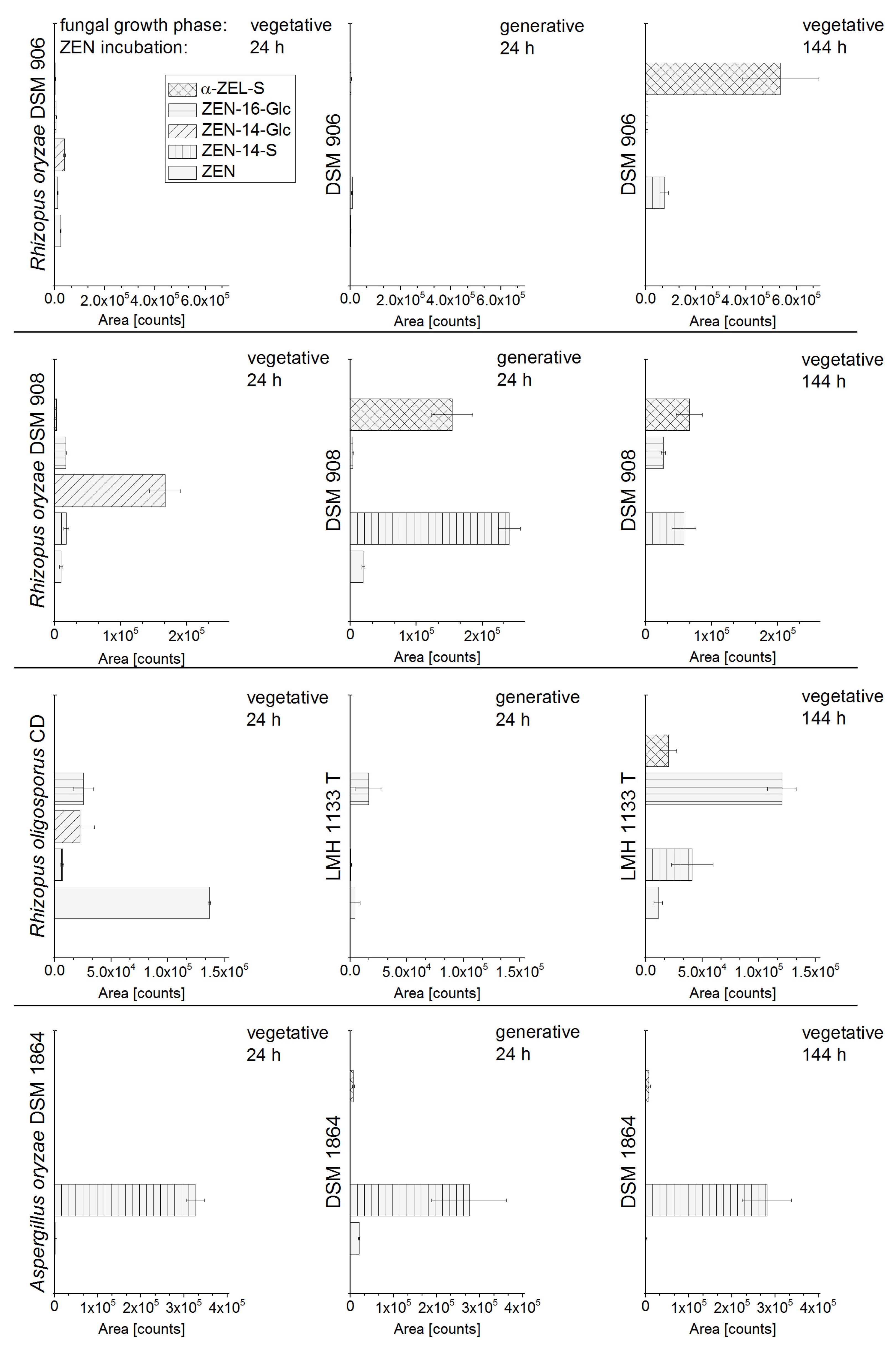
2.1. In Vitro Screening of Conjugate Producing Aspergillus and Rhizopus Species
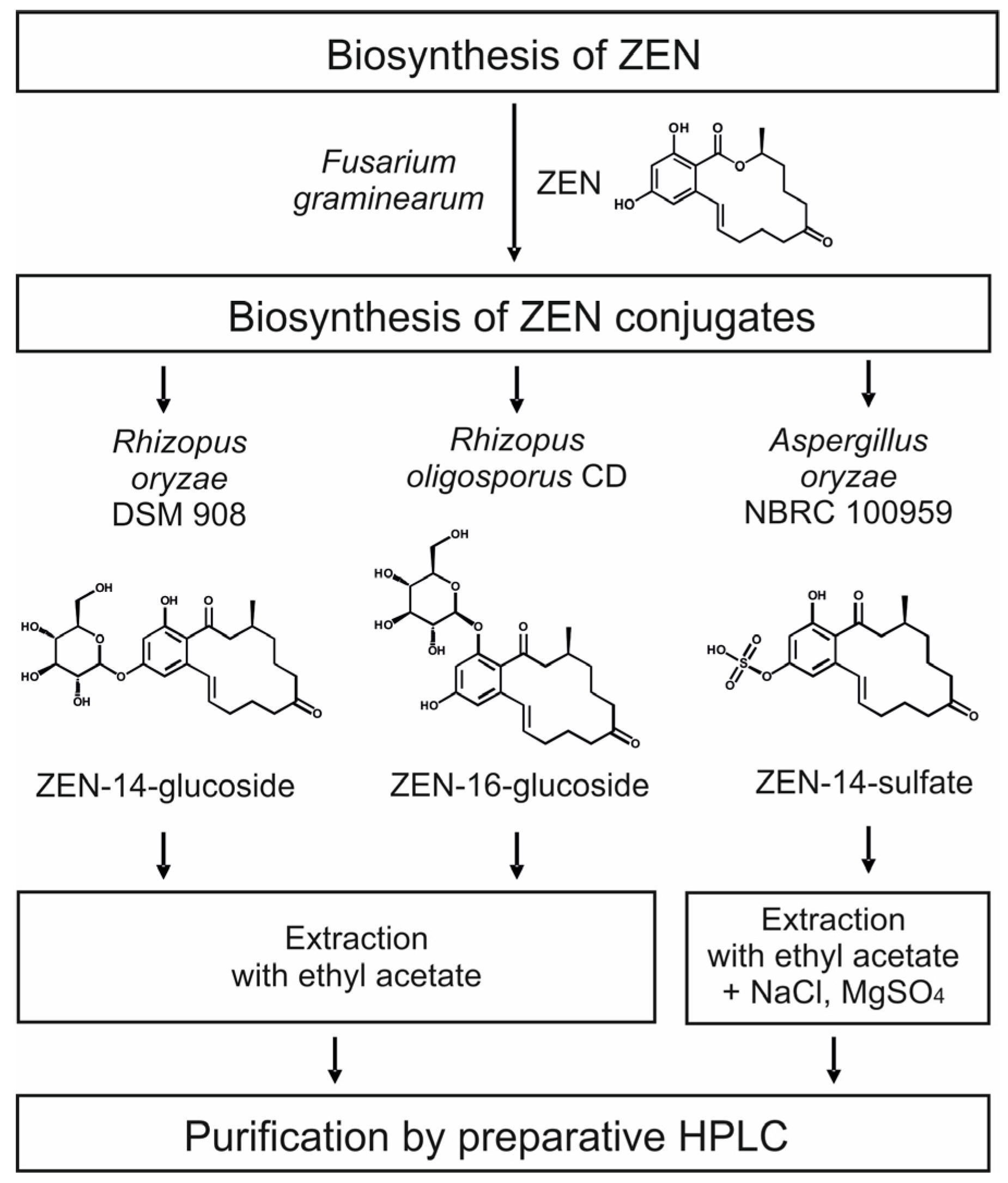
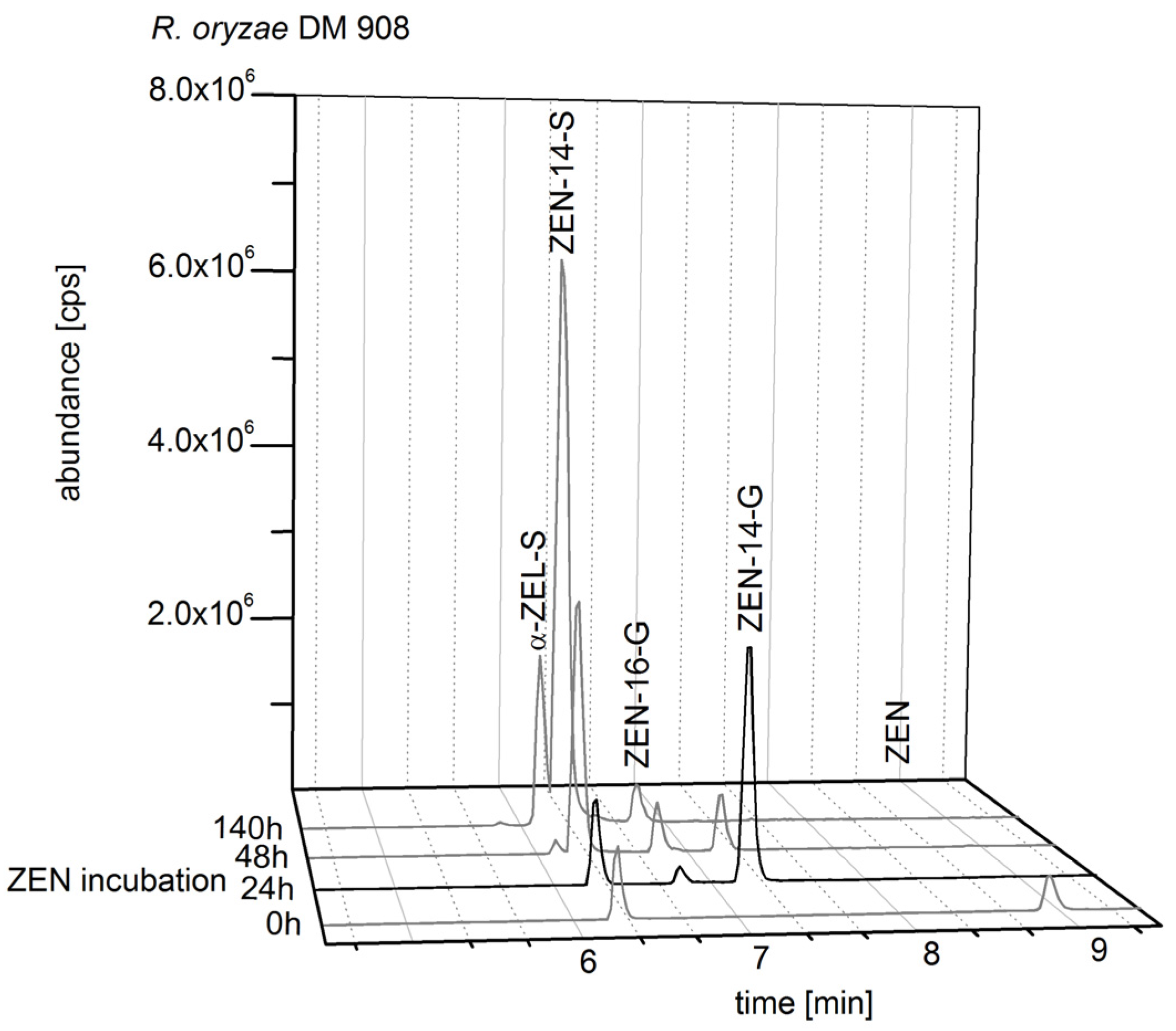
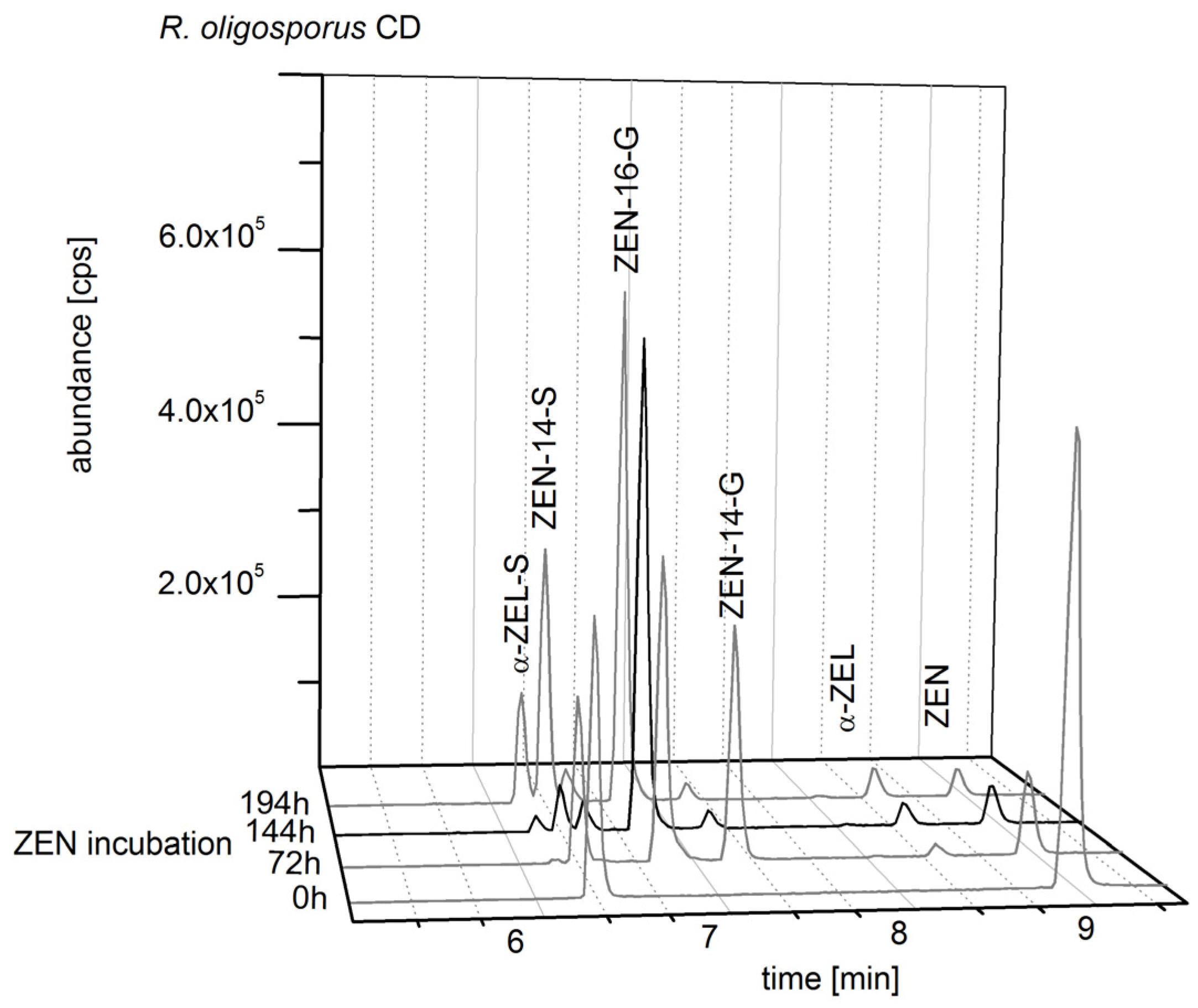
2.2. Consecutive Biosynthesis of ZEN Conjugates
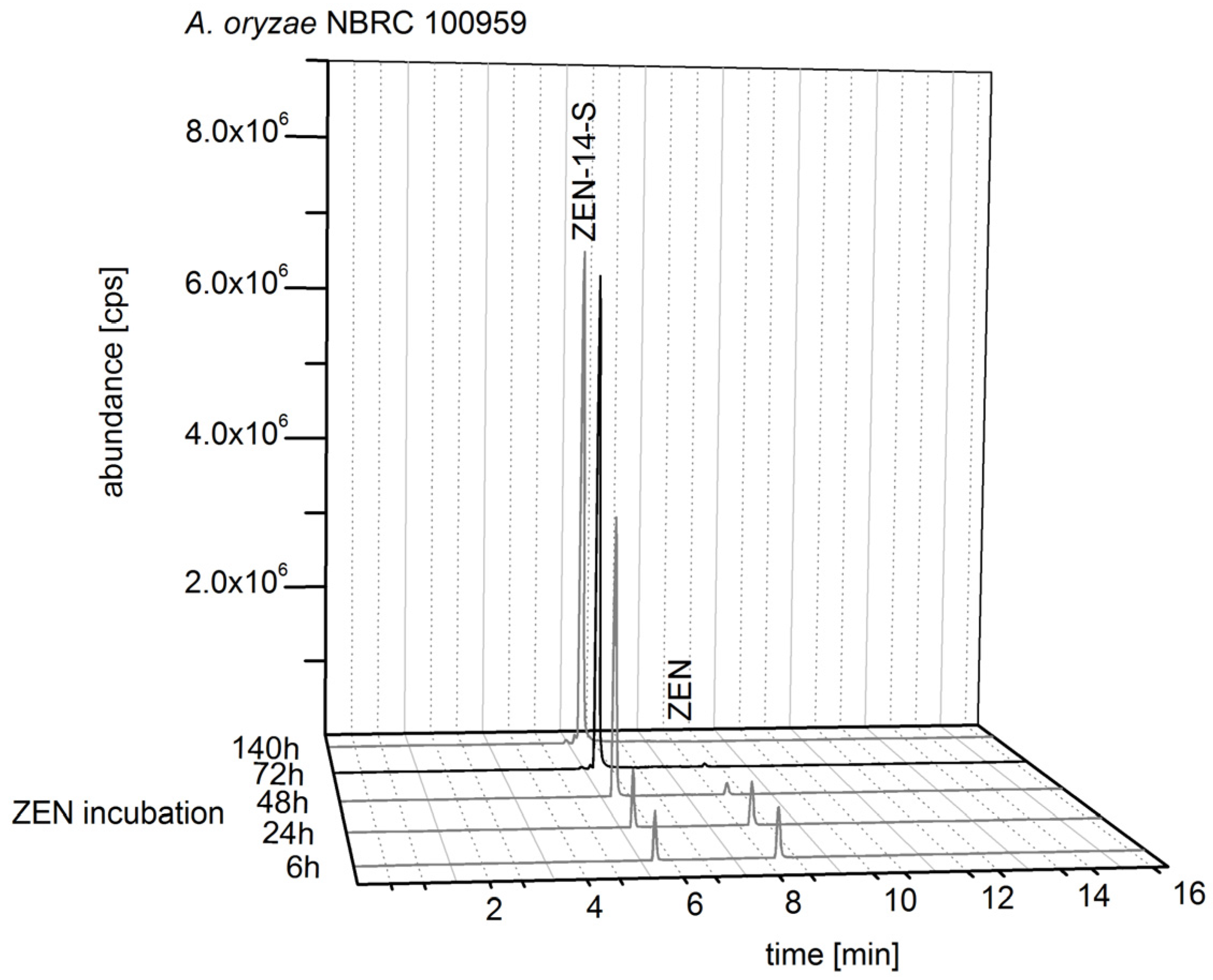
2.2.1. Biosynthesis of ZEN
2.2.2. Biosynthesis of ZEN Conjugates
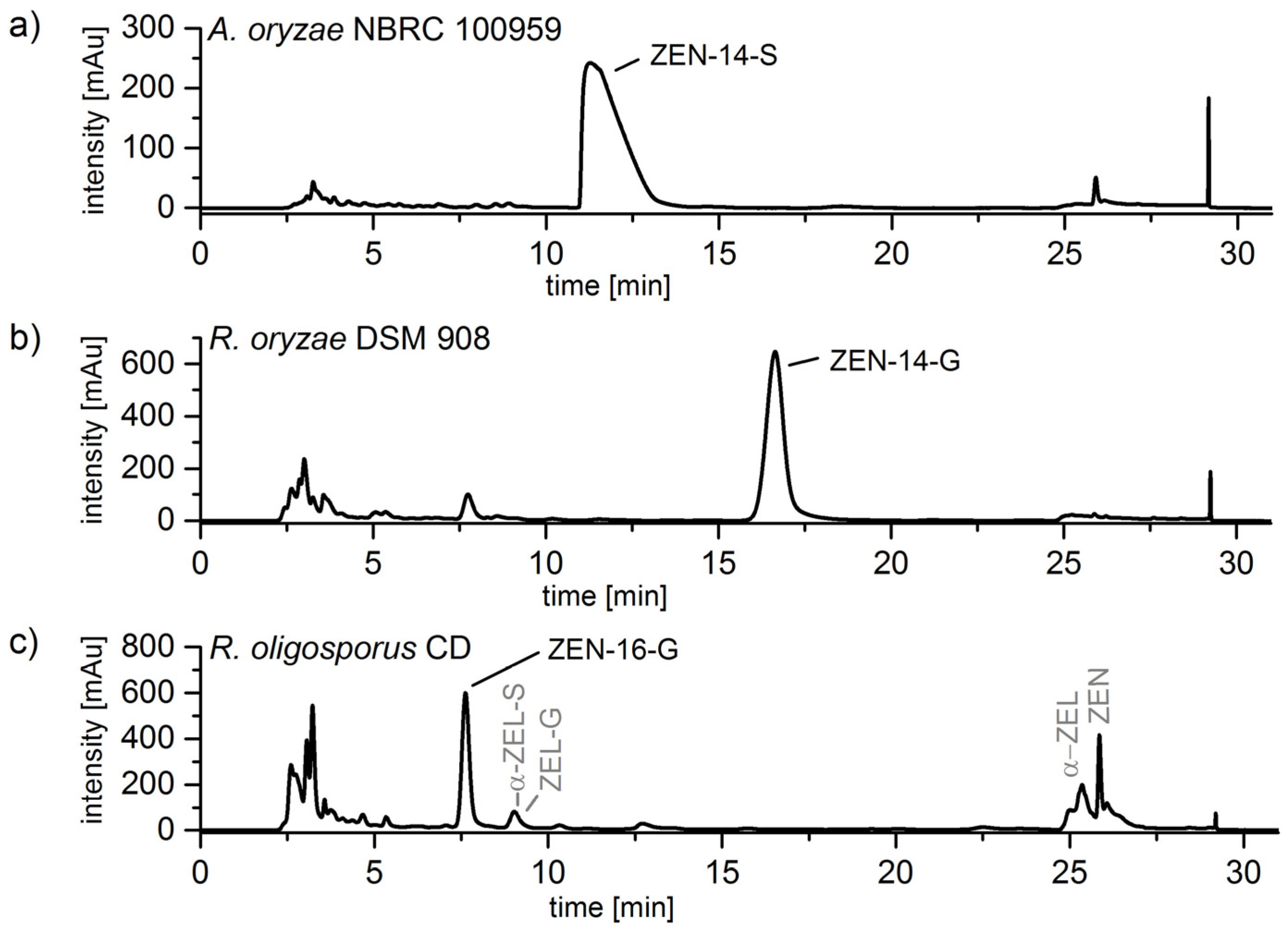
2.3. Cleanup by Liquid–Liquid-Extraction (LLE) and Preparative Chromatography
2.4. Structure Identification and Purity of ZEN-14-S, ZEN-14-G and ZEN-16-G
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Media
5.2. HPLC-MS/MS Analysis
5.3. In Vitro-Screening of Aspergillus and Rhizopus Species
5.4. Biosynthesis of ZEN
5.5. Biosynthesis of ZEN Conjugates
5.6. Liquid–Liquid-Extraction (LLE) and Preparartive Chromatography
5.7. NMR Analysis
5.8. Purity of ZEN-14-S, ZEN-14-G and ZEN-16-G
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caldwell, R.W.; Tuite, J.; Stob, M.; Baldwin, R. Zearalenone production by fusarium species. Appl. Microbiol. 1970, 20, 31–34. [Google Scholar] [PubMed]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by fusarium spp. Appl. Environ. Microbiol. 1991, 57, 146–150. [Google Scholar] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesaert, G.; De Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Vendl, O.; Crews, C.; MacDonald, S.; Krska, R.; Berthiller, F. Occurrence of free and conjugated fusarium mycotoxins in cereal-based food. Food Addit. Contam. Part A 2010, 27, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky Paris, M.P.; Schweiger, W.; Hametner, C.; Stueckler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-o-glucoside: A new masked mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Krenn, P.; Berthiller, F.; Schweiger, W.; Hametner, C.; Ludwig, R.; Adam, G.; Krska, R.; Schuhmacher, R. Production of zearalenone-4-glucoside, a-zearalenol-4-glucoside and ss-zearalenol-4-glucoside. Mycotoxin Res. 2007, 23, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Moeller, A.; Kunte, H.-J.; Koch, M.; Maul, R. Biotransformation of the mycotoxin zearalenone by fungi of the genera rhizopus and aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Andre, F.; Delaforge, M.; Lebrihi, A. Transformation of zearalenone to zearalenone-sulfate by aspergillus spp. World Mycotoxin J. 2010, 3, 183–191. [Google Scholar] [CrossRef]
- Kyprianou, M. Commission regulation (ec) no 1126/2007 of 28 september 2007 amending regulation (ec) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L 255, 14–17. [Google Scholar]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, P.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.C.; Churchwell, M.I.; Doerge, D.R. Metabolism and pharmacokinetics of zearalenone following oral and intravenous administration in juvenile female pigs. Food Chem. Toxicol. 2017, 106, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.; Kommer, A.; Dempe, J.S.; Hildebrand, A.A.; Metzler, M. Absorption and metabolism of the mycotoxin zearalenone and the growth promotor zeranol in caco-2 cells in vitro. Mol. Nutr. Food Res. 2011, 55, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Massart, F.; Saggese, G. Oestrogenic mycotoxin exposures and precocious pubertal development. Int. J. Androl. 2010, 33, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sáenz de Rodriguez, C.A.; Bongiovanni, A.M.; Conde de Borrego, L. An epidemic of precocious development in puerto rican children. J. Pediatr. 1985, 107, 393–396. [Google Scholar] [CrossRef]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Gorniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayed, Y.; Ayed-Boussema, I.; Ouanes, Z.; Bacha, H. In vitro and in vivo induction of chromosomeaberrations by alpha- and beta-zearalenols: Comparison with zearalenone. Mutat. Res. 2011, 726, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, L.; Wu, D.; Liu, F. Anti-apoptotic action of zearalenone in MCF-7 cells. Ecotoxicol. Environ. Saf. 2005, 62, 441–446. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on the risk for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar] [CrossRef]
- Mikula, H.; Sohr, B.; Skrinjar, P.; Weber, J.; Hametner, C.; Berthiller, F.; Krska, R.; Adam, G.; Froehlich, J. Sulfation of beta-resorcylic acid esters-first synthesis of zearalenone-14-sulfate. Tetrahedron Lett. 2013, 54, 3290–3293. [Google Scholar] [CrossRef]
- Mikula, H.; Weber, J.; Svatunek, D.; Skrinjar, P.; Adam, G.; Krska, R.; Hametner, C.; Froehlich, J. Synthesis of zearalenone-16-beta,d-glucoside and zearalenone-16-sulfate: A tale of protecting resorcylic acid lactones for regiocontrolled conjugation. Beilstein J. Org. Chem. 2014, 10, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Varga, E.; Lupi, F.; Malachova, A.; Hametner, C.; Berthiller, F.; Adam, G. Synthesis of mono- and di-glucosides of zearalenone and alpha-/beta-zearalenol by recombinant barley glucosyltransferase hvugt14077. Toxins 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Hering, L.; Bisping, B.; Rehm, H.J. Patterns and formation of fatty acids at tempe fermentation by several strains of Rhizopus sp. Eur. J. Lipid Sci. Technol. 1991, 93, 303–308. [Google Scholar]
- Dubos, T.; Pasquali, M.; Pogoda, F.; Hoffmann, L.; Beyer, M. Evidence for natural resistance towards trifloxystrobin in Fusarium graminearum. Eur. J. Plant Pathol. 2011, 130, 239–248. [Google Scholar] [CrossRef]
| Species | Designation | Source |
|---|---|---|
| Rhizopus oryzae | DSM 906 | DSMZ * |
| Rhizopus oryzae | DSM 907 | DSMZ * |
| Rhizopus oryzae | DSM 908 | DSMZ * |
| Rhizopus stolonifer | DSM 855 | DSMZ * |
| Rhizopus microsporus var. chinensis | DSM 1834 | DSMZ * |
| Rhizopus oligosporus | CD (LMH 1133 T) | Hering et al. [25] |
| Aspergillus oryzae | DSM 1864 | DSMZ * |
| Aspergillus oryzae | NBRC 100959 | Nite Biological Resource Center (Tokio, Japan) |
| Fungal Strain | Isolate | Isolated by |
|---|---|---|
| F. graminearum F1 | 37 | Gossmann, HU Berlin 1994 |
| F. graminearum F2 | 09-53b | Pogoda, Luxemburg 2009 |
| F. graminearum F3 | MUCL 11946 | Kinnard, Belgien 1969 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzekowski, A.; Drewitz, T.; Keller, J.; Pfeifer, D.; Kunte, H.-J.; Koch, M.; Rohn, S.; Maul, R. Biosynthesis and Characterization of Zearalenone-14-Sulfate, Zearalenone-14-Glucoside and Zearalenone-16-Glucoside Using Common Fungal Strains. Toxins 2018, 10, 104. https://doi.org/10.3390/toxins10030104
Borzekowski A, Drewitz T, Keller J, Pfeifer D, Kunte H-J, Koch M, Rohn S, Maul R. Biosynthesis and Characterization of Zearalenone-14-Sulfate, Zearalenone-14-Glucoside and Zearalenone-16-Glucoside Using Common Fungal Strains. Toxins. 2018; 10(3):104. https://doi.org/10.3390/toxins10030104
Chicago/Turabian StyleBorzekowski, Antje, Tatjana Drewitz, Julia Keller, Dietmar Pfeifer, Hans-Jörg Kunte, Matthias Koch, Sascha Rohn, and Ronald Maul. 2018. "Biosynthesis and Characterization of Zearalenone-14-Sulfate, Zearalenone-14-Glucoside and Zearalenone-16-Glucoside Using Common Fungal Strains" Toxins 10, no. 3: 104. https://doi.org/10.3390/toxins10030104
APA StyleBorzekowski, A., Drewitz, T., Keller, J., Pfeifer, D., Kunte, H. -J., Koch, M., Rohn, S., & Maul, R. (2018). Biosynthesis and Characterization of Zearalenone-14-Sulfate, Zearalenone-14-Glucoside and Zearalenone-16-Glucoside Using Common Fungal Strains. Toxins, 10(3), 104. https://doi.org/10.3390/toxins10030104