Carboxyl-Functionalized, Europium Nanoparticle-Based Fluorescent Immunochromatographic Assay for Sensitive Detection of Citrinin in Monascus Fermented Food
Abstract
:1. Introduction
2. Results
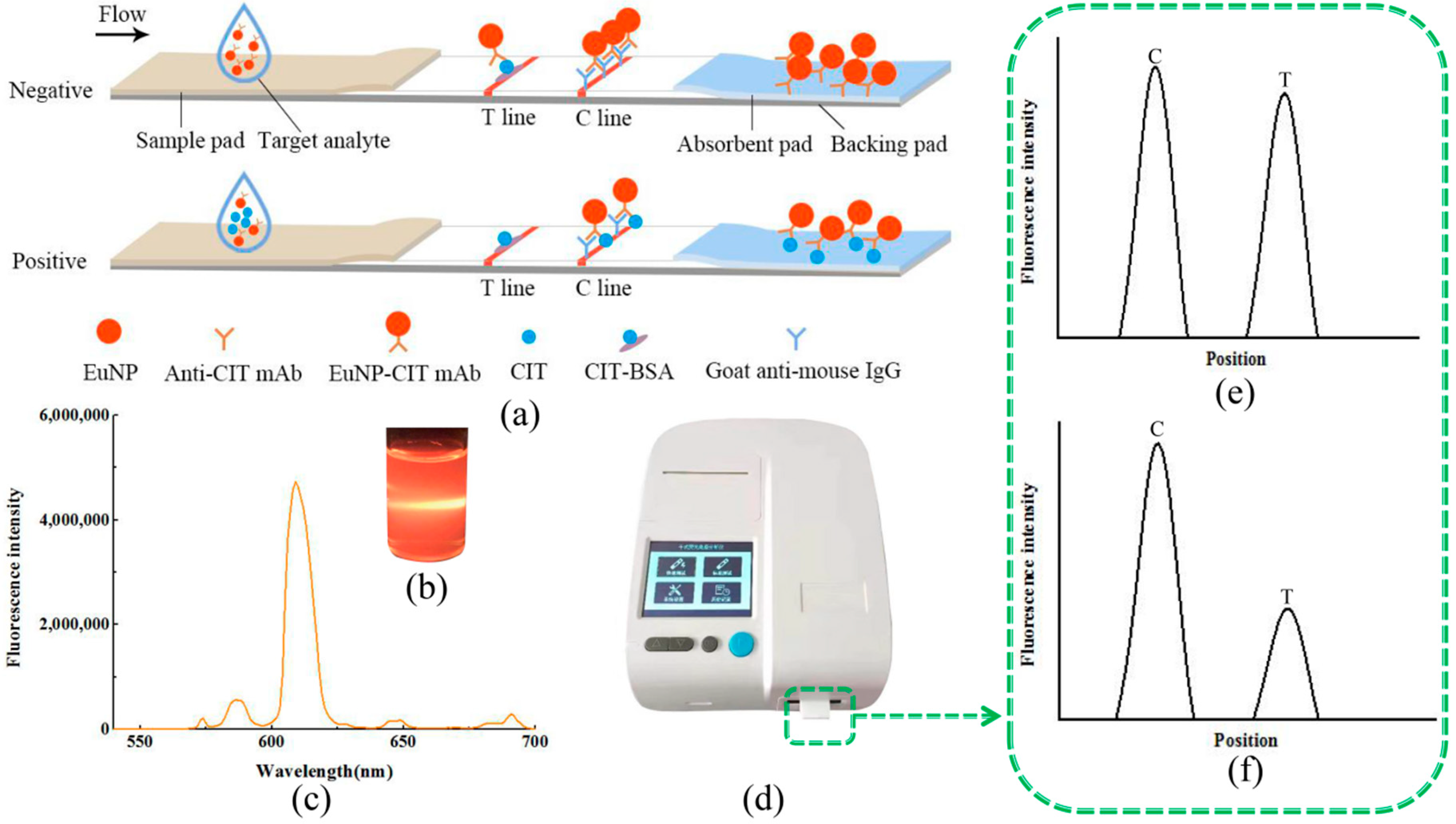
2.1. Detection Principle
2.2. Identification of CIT Artificial Antigen and Monoclonal Antibody
2.3. Optimization of FICTS Test Strip Parameters
2.4. Comparison of FICTS and CG-ICTS
2.5. Specific Analysis of FICTS
2.6. Matrix Interference in Real Samples
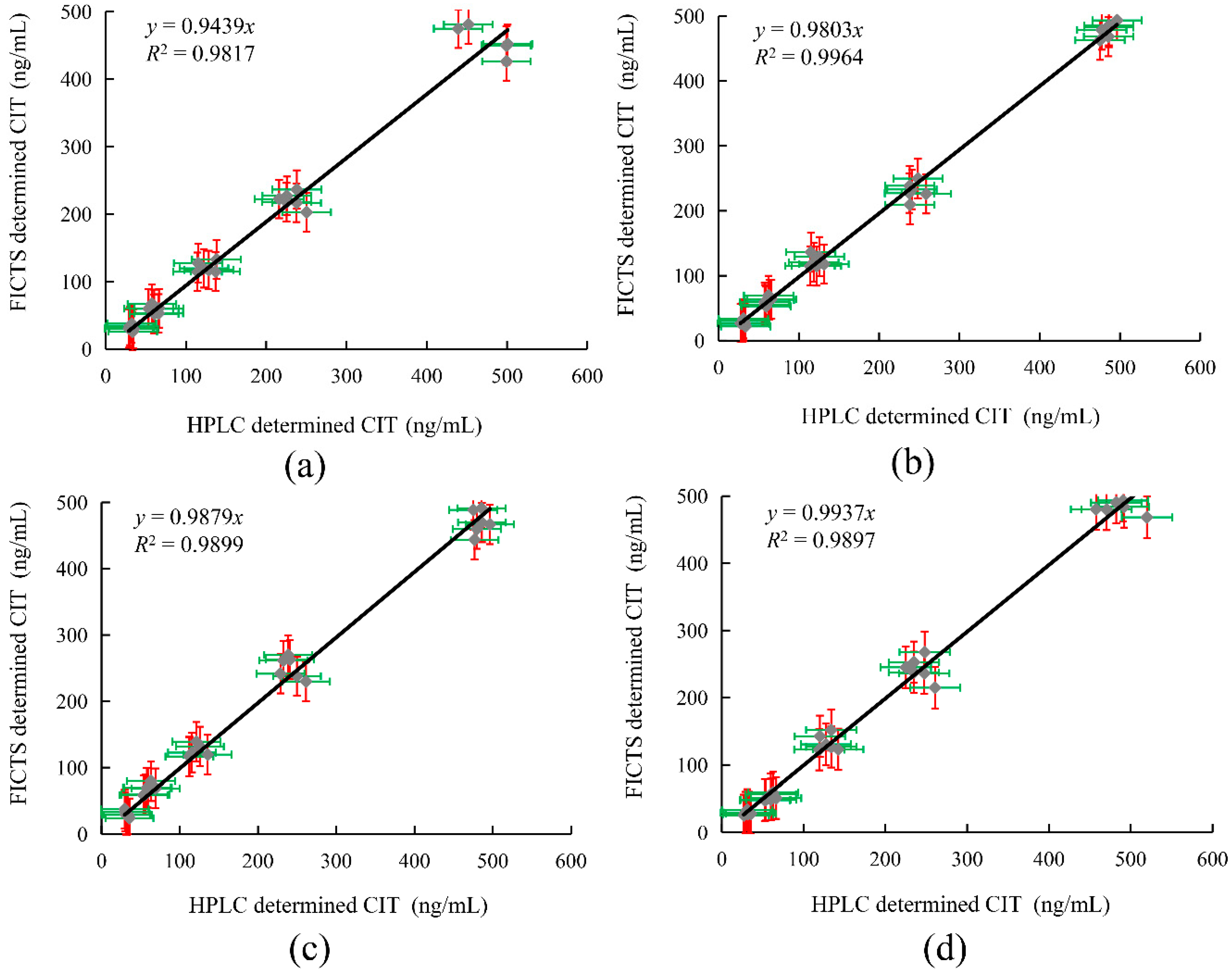
2.7. Detection of Spiked Samples by FICTS and HPLC
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Chemicals
5.2. Apparatus
5.3. Preparation of CIT–BSA and CIT–HSA
5.4. Production of Monoclonal Antibody
5.5. Preparation of Colloidal Gold-mAb
5.6. Preparation of EuNP mAb Probes
5.7. Preparation of the Test Strip
5.8. FICTS Detection Procedure
5.9. Standard Curves and Specificity
5.10. Sample Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2013, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.S. Mycotoxins: Food contamination, mechanism, carcinogenic potential and preventive measures. Mutat. Res. 1991, 259, 291–306. [Google Scholar] [CrossRef]
- Hetherington, A.C.; Raistrick, H. Studies in the biochemistry of micro-organisms. XIV. On the production and chemical constitution of a new yellow colouring mater, citrinin, produced from glucose by Penicillium citrinum, Thom. Philos. Trans. R. Soc. Ser. B 1931, 220, 269–295. [Google Scholar] [CrossRef]
- Pleadin, J.; Zadravec, M.; Lešić, T.; Vahčić, N.; Frece, J.; Mitak, M.; Markov, K. Co-occurrence of ochratoxin a and citrinin in unprocessed cereals established during a three-year investigation period. Food Addit. Contam. B. 2018, 11, 20–25. [Google Scholar] [CrossRef]
- Cheng, H.W.; Yang, Y.; Chen, Y.F.; Chen, X.Q.; Cai, Z.Z.; Du, A.F. Novel monoclonal antibody-based immunochromatographic strip for detecting citrinin in fruit from zhejiang province, china. PLoS ONE 2018, 13, e0197179. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Cumova, M.; Kyrova, V.; Toman, J.; Grosse, Y.; Pospichalova, M.; Ruprich, J. Investigation of patulin and citrinin in grape must and wine from grapes naturally contaminated by strains of, Penicillium expansum. Food Chem. Toxicol. 2018, 118, 805–811. [Google Scholar] [CrossRef]
- Visconti, A.; Bottalico, A. High levels of ochratoxins a and b in moldy bread responsible for mycotoxicosis in farm animals. J. Agric. Food Chem. 1983, 31, 1122–1123. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Rodríguez-Estévez, V.; Arenas-Fernández, P.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of Mycotoxins in Swine Feeding from Spain. Toxins 2019, 11, e342. [Google Scholar] [CrossRef]
- Wang, T.H.; Lin, T.F. Monascus rice products. In Advances in Food and Nutrition Research; University of Nebraska, Academic Press: Lincoln, NE, USA, 2007; Volume 53, pp. 123–159. [Google Scholar]
- Li, Y.; Zhou, Y.-C.; Yang, M.-H.; Ou-Yang, Z. Natural occurrence of citrinin in widely consumed traditional chinese food red yeast rice, medicinal plants and their related products. Food Chem. 2012, 132, 1040–1045. [Google Scholar] [CrossRef]
- Li, F.; Xu, G.; Li, Y.; Chen, Y.; Ji, R. Natural occurrence of citrinin in Monascus products. Wei Sheng Yan Jiu (J. Hyg. Res.) 2005, 34, 451–454. [Google Scholar]
- Liu, B.H.; Wu, T.S.; Su, M.C.; Chung, C.P.; Yu, F.Y. Evaluation of citrinin occurrence and cytotoxicity in Monascus fermentation products. J. Agric. Food Chem. 2005, 53, 170–175. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; Santos, J.V.O.; de Alencar, M.V.O.B.; Júnior, A.L.G.; Paz, M.F.C.J.; de Brito, M.D.R.M.; E Sousa, J.M.C.; et al. A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Naturally Occurring and Synthetic Food Components, Furocoumarins and Ultraviolet Radiation. In Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1986; pp. 83–98. [Google Scholar]
- Liao, C.D.; Chen, Y.C.; Lin, H.Y.; Chiueh, L.C.; Shih, D.Y.C. Incidence of citrinin in red yeast rice and various commercial Monascus products in Taiwan from 2009 to 2012. Food Control. 2014, 38, 178–183. [Google Scholar] [CrossRef]
- GB.5009.222-2016. National Food Safety Standards for Determination of Citrinin in Monascus Products; Standards Press of China: Beijing, China, 2017. [Google Scholar]
- Marley, E.; Brown, P.; Leeman, D.; Donnelly, C. Analysis of citrinin in cereals, red yeast rice dietary supplement, and animal feed by immunoaffinity column cleanup and lc with fluorescence detection. J. AOAC Int. 2016, 99, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Vrabcheva, T.; Usleber, E.; Dietrich, R.; Märtlbauer, E. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J. Agric. Food Chem. 2000, 48, 2483–2488. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, H.W.; Yao, S.; Zhu, P.J. Detection of trace amounts of citrinin in dried orange peel by using an optimized extraction method coupled with ultra-performance liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2018, 32, e4237. [Google Scholar] [CrossRef]
- Wei, F.; Liu, X.; Liao, X.; Shi, L.; Zhang, S.; Lu, J.; Zhou, L.; Kong, W. Simultaneous determination of 19 mycotoxins in lotus seed using a multimycotoxin UFLC-MS/MS method. J. Pharm. Pharmacol. 2019, 71, 1172–1183. [Google Scholar] [CrossRef]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. High-throughput determination of citrinin in rice by ultra-high-performance liquid chromatography and fluorescence detection (UHPLC-FL). Food Addit. Contam. Part A 2015, 32, 1352–1357. [Google Scholar] [CrossRef]
- Shu, P.-Y.; Lin, C.-H. Simple and sensitive determination of citrinin in Monascus by GC-selected ion monitoring mass spectrometry. Anal. Sci. 2002, 18, 283–287. [Google Scholar] [CrossRef]
- Yirga, S.K.; Ling, S.M.; Yang, Y.L.; Yuan, J.; Wang, S.H. The preparation and identification of a monoclonal antibody against citrinin and the development of detection via indirect competitive elisa. Toxins 2017, 56, 488–495. [Google Scholar] [CrossRef]
- Zhang, C.X.; Zhang, Q.; Tang, X.Q.; Zhang, W.; Li, P.W. Development of an anti-idiotypic VHH antibody and toxin-free enzyme immunoassay for ochratoxin A in cereals. Toxins 2019, 11, e280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.H.; Fang, Y.; Sun, R.J.; Cao, T.; Paudyal, N.; Fang, W.H.; Song, H.H. Antibody microarray immunoassay for simultaneous quantification of multiple mycotoxins in corn samples. Toxins 2018, 10, e415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, H.B. Chemiluminescence immunoassay using magnetic nanoparticles with targeted inhibition for the determination of ochratoxin A. Talanta 2015, 140, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Vanrell, L.; Gonzalez-Techera, A.; Hammock, B.D.; Gonzalez-Sapienza, G. Nanopeptamers for the development of small-analyte lateral flow tests with a positive readout. Anal. Chem. 2013, 85, 1177–1182. [Google Scholar] [CrossRef]
- Wang, Z.; Zhi, D.; Zhao, Y.; Zhang, H.; Wang, X.; Ru, Y.; Li, H. Lateral flow test strip based on colloidal selenium immunoassay for rapid detection of melamine in milk, milk powder, and animal feed. Int. J. Nanomed. 2014, 9, 1699–1707. [Google Scholar] [CrossRef]
- Liu, B.H.; Tsao, Z.J.; Wang, J.J.; Yu, F.Y. Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Anal. Chem. 2008, 80, 7029–7035. [Google Scholar] [CrossRef]
- Wang, Y.K.; Shi, Y.B.; Zou, Q.; Sun, J.H.; Chen, Z.F.; Wang, H.A.; Li, S.Q.; Yan, Y.X. Development of a rapid and simultaneous immunochromatographic assay for the determination of zearalenone and fumonisin B1 in corn, wheat and feedstuff samples. Food Control. 2013, 31, 180–188. [Google Scholar] [CrossRef]
- Wang, S.; Quan, Y.; Lee, N.A.; Kennedy, I.R. Rapid determination of fumonisin B 1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. J. Agric. Food Chem. 2006, 54, 2491–2495. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.B.; He, Q.H.; Deng, S.Z.; Li, L.S.; Li, Y.P. Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chemistry. 2010, 119, 834–839. [Google Scholar] [CrossRef]
- Huang, Z.B.; Xu, Y.; Li, L.S.; Li, Y.P.; Zhang, H.; He, Q.H. Development of an immunochromatographic strip test for the rapid simultaneous detection of deoxynivalenol and zearalenone in wheat and maize. Food Control. 2012, 28, 7–12. [Google Scholar] [CrossRef]
- Majdinasab, M.; Zareian, M.; Zhang, Q.; Li, P. Development of a new format of competitive immunochromatographic assay using secondary antibody–EuNPsropium nanoparticle conjugates for ultrasensitive and quantitative determination of ochratoxin A. Food Chem. 2019, 275, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Nankoberanyi, S.; Mbogo, G.W.; LeClair, N.P.; Conrad, M.D.; Tumwebaze, P.; Tukwasibwe, S.; Kamya, M.R.; Tappero, J.; Nsobya, S.L.; Rosenthal, P.J. Validation of the ligase detection reaction fluorescent microsphere assay for the detection of Plasmodium falciparum resistance mediating polymorphisms in Uganda. Malar. J. 2014, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.J.; Bao, D.T.; Seo, G.E.; Bui, C.T.; Kim, H.; Anh, N.T.V.; Trinh, T.; Nguyen, L.; Sohn, H.J.; Sohn, C.K.; et al. Improvement of a rapid diagnostic application of monoclonal antibodies against avian influenza h7 subtype virus using EuNPsropium nanoparticles. Sci. Rep. 2017, 7, 7933. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Yu, Y.A.; Liu, B.H.; Yu, F.Y. Development of a sensitive enzyme-linked immunosorbent assay and rapid gold nanoparticle immunochromatographic strip for detecting citrinin in monascus fermented food. Toxins 2018, 10, e354. [Google Scholar] [CrossRef]
- Gong, Y.F.; Zhang, M.Z.; Wang, M.Z.; Chen, Z.L.; Xi, X. Development of immuno-based methods for detection of melamine. Arab. J. Sci. Eng. 2014, 39, 5315–5324. [Google Scholar] [CrossRef]
- Kong, D.; Xie, Z.; Liu, L.; Song, S.; Kuang, H. Development of ic-elisa and lateral-flow immunochromatographic assay strip for the detection of citrinin in cereals. Food Agric. Immunol. 2017, 28, 1–13. [Google Scholar] [CrossRef]



| Sample | Dilution | Standard Curve | R2 | IC50 (ng/mL) | Matrix Effect |
|---|---|---|---|---|---|
| Red yeast powder | 50 | y = 39.57x + 62.13 | 0.97 | 0.49 | 121.12% |
| 100 | y = 38.63x + 69.93 | 0.98 | 0.31 | 74.70% | |
| 200 | y = 37.70x + 67.49 | 0.99 | 0.34 | 84.20% | |
| 400 | y = 40.68x + 66.03 | 0.98 | 0.40 | 98.95% | |
| Red rice wine | 50 | y = 34.20x + 68.65 | 0.99 | 0.29 | 67.84% |
| 100 | y = 42.77x + 70.21 | 0.99 | 0.35 | 77.90% | |
| 200 | y = 41.14x + 62.97 | 0.97 | 0.48 | 111.75% | |
| 400 | y = 39.89x + 63.52 | 0.96 | 0.46 | 105.81% | |
| Red rice vinegar | 50 | y = 30.31x + 60.38 | 0.96 | 0.46 | 77.57% |
| 100 | y = 40.23x + 62.69 | 0.98 | 0.48 | 82.54% | |
| 200 | y = 18.16x + 54.74 | 0.99 | 0.52 | 88.35% | |
| 400 | y = 34.05x + 57.32 | 0.99 | 0.61 | 103.96% | |
| Rose fermented bean curd | 50 | y = 22.67x + 60.32 | 0.99 | 0.35 | 71.82% |
| 100 | y = 17.81x + 57.51 | 0.95 | 0.38 | 77.57% | |
| 200 | y = 18.16x + 54.74 | 0.99 | 0.55 | 112.36% | |
| 400 | y = 24.68x + 58.61 | 0.99 | 0.45 | 91.81% |
| Sample | Spiked Level (ng/mL) | FICTS | HPLC | ||||
|---|---|---|---|---|---|---|---|
| Detected Amount (ng/mL) | Recovery Rate | RSD (n = 6) | Detected Amount (ng/mL) | Recovery Rate | RSD (n = 6) | ||
| Red yeast powder | 30 | 31.8 | 106.0% | 12.7% | 31.6 | 105.3% | 7.5% |
| 60 | 58.9 | 98.2% | 9.4% | 60.7 | 101.1% | 8.2% | |
| 120 | 121.2 | 101.0% | 6.1% | 125.9 | 104.9% | 8.2% | |
| 240 | 220.6 | 91.9% | 5.1% | 232.2 | 96.8% | 5.2% | |
| 480 | 455.6 | 94.9% | 4.3% | 481.6 | 100.3% | 5.9% | |
| Red rice wine | 30 | 27.5 | 91.8% | 14.6% | 30.8 | 102.5% | 7.7% |
| 60 | 60.8 | 101.3% | 9.9% | 61.0 | 101.7% | 5.5% | |
| 120 | 122.4 | 102.0% | 7.1% | 121.1 | 100.9% | 5.5% | |
| 240 | 230.6 | 96.1% | 5.9% | 243.7 | 101.5% | 3.4% | |
| 480 | 478.3 | 99.7% | 2.4% | 484.4 | 100.9% | 1.6% | |
| Red rice vinegar | 30 | 30.8 | 102.7% | 15.3% | 32.0 | 106.7% | 7.0% |
| 60 | 67.8 | 113.0% | 11.3% | 59.9 | 99.7% | 9.3% | |
| 120 | 124.4 | 103.7% | 7.4% | 120.5 | 100.4% | 7.6% | |
| 240 | 250.6 | 104.4% | 6.5% | 241.9 | 100.8% | 5.0% | |
| 480 | 469.9 | 97.9% | 3.8% | 483.2 | 100.7% | 1.6% | |
| Rose fermented bean curd | 30 | 28.9 | 96.4% | 9.7% | 31.3 | 104.3% | 9.7% |
| 60 | 52.1 | 86.8% | 9.2% | 59.3 | 98.8% | 9.0% | |
| 120 | 133.0 | 110.8% | 8.9% | 129.4 | 107.9% | 7.0% | |
| 240 | 242.4 | 101.0% | 7.3% | 241.9 | 100.8% | 5.2% | |
| 480 | 482.7 | 100. 6% | 1.8% | 485.4 | 101.1% | 4.4% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, E.; Xu, Y.; Ma, B.; Cui, H.; Sun, C.; Zhang, M. Carboxyl-Functionalized, Europium Nanoparticle-Based Fluorescent Immunochromatographic Assay for Sensitive Detection of Citrinin in Monascus Fermented Food. Toxins 2019, 11, 605. https://doi.org/10.3390/toxins11100605
Chen E, Xu Y, Ma B, Cui H, Sun C, Zhang M. Carboxyl-Functionalized, Europium Nanoparticle-Based Fluorescent Immunochromatographic Assay for Sensitive Detection of Citrinin in Monascus Fermented Food. Toxins. 2019; 11(10):605. https://doi.org/10.3390/toxins11100605
Chicago/Turabian StyleChen, Erjing, Ying Xu, Biao Ma, Haifeng Cui, Chuanxin Sun, and Mingzhou Zhang. 2019. "Carboxyl-Functionalized, Europium Nanoparticle-Based Fluorescent Immunochromatographic Assay for Sensitive Detection of Citrinin in Monascus Fermented Food" Toxins 11, no. 10: 605. https://doi.org/10.3390/toxins11100605





