Prey and Venom Efficacy of Male and Female Wandering Spider, Phoneutria boliviensis (Araneae: Ctenidae)
Abstract
:1. Introduction
2. Results
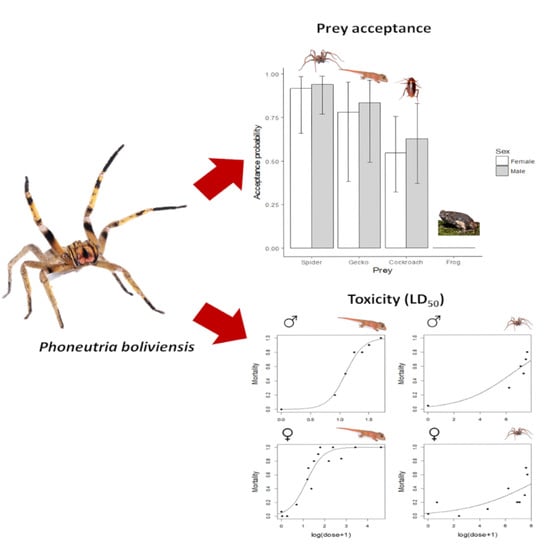
2.1. Prey Acceptance and Immobilization Time
2.2. Venom Volume
2.3. Toxicity
3. Discussion
4. Materials and Methods
4.1. Specimen Collection and Housing
4.2. Prey Acceptance and Immobilization
4.3. Venom Extraction and Volume
4.4. Toxicity Bioassays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuhn-Nentwig, L.; Stöcklin, R.; Nentwig, W. Venom composition and strategies in spiders: Is everything possible? In Spider Physiology and Behaviour; Elsevier: London, UK, 2011; Volume 1, pp. 2–86. [Google Scholar]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kardong, K.V. Snake toxins and venoms: An evolutionary perspective. Herpetologica 1996, 52, 36–46. [Google Scholar]
- Walker, A.A.; Madio, B.; Jin, J.; Undheim, E.A.B.; Fry, B.G.; King, G.F. Melt with this kiss: Paralyzing and liquefying venom of the assassin bug Pristhesancus plagipennis (Hemiptera: Reduviidae). Mol. Cell. Proteom. 2017, 16, 552–566. [Google Scholar] [CrossRef]
- Schulz, J.R.; Norton, A.G.; Gilly, W.F. The projectile tooth of a fish-hunting cone snail: Conus catus injects venom into fish prey using a high-speed ballistic mechanism. Biol. Bull. 2004, 207, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P.; Sixberry, N.M.; Heyborne, W.H.; Fritts, T. Venom of the brown treesnake, Boiga irregularis: Ontogenetic shifts and taxa-specific toxicity. Toxicon 2006, 47, 537–548. [Google Scholar] [CrossRef]
- Pawlak, J.; Mackessy, S.P.; Fry, B.G.; Bhatia, M.; Mourier, G.; Fruchart-Gaillard, C.; Servent, D.; Ménez, R.; Stura, E.; Ménez, A.; et al. Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J. Biol. Chem. 2006, 281, 29030–29041. [Google Scholar] [CrossRef]
- Starkov, V.G.; Osipov, A.V.; Utkin, Y.N. Toxicity of venoms from vipers of Pelias group to crickets Gryllus assimilis and its relation to snake entomophagy. Toxicon 2007, 49, 995–1001. [Google Scholar] [CrossRef]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 2443–2449. [Google Scholar] [CrossRef] [Green Version]
- Healy, K.; Carbone, C.; Jackson, A.L. Snake venom potency and yield are associated with prey-evolution, predator metabolism and habitat structure. Ecol. Lett. 2019, 22, 527–537. [Google Scholar] [CrossRef]
- Pekár, S.; Coddington, J.A.; Blackledge, T.A. Evolution of stenophagy in spiders (Araneae): Evidence based on the comparative analysis of spider diets. Evolution 2012, 66, 776–806. [Google Scholar] [CrossRef]
- Foelix, R.F. Biology of Spiders, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2011. [Google Scholar]
- King, G.F.; Hardy, M.C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Pekár, S.; Líznarová, E.; Bočánek, O.; Zdráhal, Z. Venom of prey-specialized spiders is more toxic to their preferred prey: A result of prey-specific toxins. J. Anim. Ecol. 2018, 87, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.; Polis, G.A. Arthropods that prey on vertebrates. Biol. Rev. 1982, 57, 29–58. [Google Scholar] [CrossRef]
- Bücherl, W.; Buckley, E.E. (Eds.) Venomous Animals and Their Venoms; Academic Press, Inc.: New York, NY, USA, 1971; Volume 3, pp. 197–277. [Google Scholar]
- Nyffeler, M.; Knörnschild, M. Bat predation by spiders. PLoS ONE 2013, 8, e58120. [Google Scholar] [CrossRef]
- Von May, R.; Biggi, E.; Cárdenas, H.; Diaz, M.I.; Alarcón, C.; Herrera, V.; Santa-Cruz, R.; Tomasinelli, F.; Westeen, E.P.; Sánchez-Paredes, C.M.; et al. Ecological interactions between arthropods and small vertebrates in a lowland Amazon rainforest. Amph. Reptil. Conserv. 2019, 13, 65–77. [Google Scholar]
- Malta-Borges, L.; Mario-da-Rosa, C.; Dri, G.F.; Bertani, R. Predation of the snake Erythrolamprus almadensis (Wagler, 1824) by the tarantula Grammostola quirogai Montes De Oca, D’Elía & Pérez-Miles, 2016. J. Herpetol. 2016, 9, 321–322. [Google Scholar]
- Menin, M.; Rodrigues, D.D.J.; Azevedo, C.S. De Predation on amphibians by spiders (Arachnida, Araneae) in the Neotropical region. Phyllomedusa J. Herpetol. 2005, 4, 39. [Google Scholar] [CrossRef]
- Nyffeler, M.; Pusey, B.J. Fish predation by semi-aquatic spiders: A global pattern. PLoS ONE 2014, 9, e99459. [Google Scholar] [CrossRef]
- Garb, J.E.; Hayashi, C.Y. Molecular evolution of α-latrotoxin, the exceptionally potent vertebrate neurotoxin in black widow spider venom. Mol. Biol. Evol. 2013, 30, 999–1014. [Google Scholar] [CrossRef]
- Dunbar, J.P.; Ennis, E.; Gandola, R.; Dugon, M.M. Biting off more than one can chew: First record of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) feeding on the native viviparous lizard Zootoca vivipara (Lichtenstein, 1823) in Ireland. Biol. Environ. 2018, 118, 45–48. [Google Scholar]
- Bucaretchi, F.; Bertani, R.; De Capitani, E.M.; Hyslop, S. Envenomation by Wandering Spiders (Genus Phoneutria). Clin. Tox. 2016, 63, 1–49. [Google Scholar]
- Sheumack, D.D.; Baldo, B.A.; Carroll, P.R.; Hampson, F.; Howden, M.E.H.; Skorulis, A. A comparative study of properties and toxic constituents of funnel web spider (A T&4X) venoms. Sci. Rep. 2018, 8, 1–7. [Google Scholar]
- Binford, G.J. An analysis of geographic and intersexual chemical variation in venoms of the spider Tegenaria agrestis (Agelenidae). Toxicon 2001, 39, 955–968. [Google Scholar] [CrossRef]
- Herzig, V.; John Ward, R.; Ferreira dos Santos, W. Intersexual variations in the venom of the Brazilian ‘armed’ spider Phoneutria nigriventer (Keyserling, 1891). Toxicon 2002, 40, 1399–1406. [Google Scholar] [CrossRef]
- De Lima, M.E.; Figueiredo, S.G.; Matavel, A.; Nunes, K.P.; da Silva, C.N.; de Marco Almeida, F.; Diniz, M.R.V.; do Cordeiro, M.N.; Stankiewicz, M.; Beirão, P.S.L. Phoneutria nigriventer venom and toxins: A review. In Spider Venoms; Gopalakrishnakone, P., Corzo, G.A., Diego-Garcia, E., de Lima, M.E., Eds.; Springer: Dordrecht, The Netherland, 2015; pp. 1–24. [Google Scholar]
- Diniz, M.R.V.; Paiva, A.L.B.; Guerra-Duarte, C.; Nishiyama, M.Y., Jr.; Mudadu, M.A.; de Oliveira, U.; Borges, M.H.; Yates, J.R.; Junqueira-de-Azevedo, I.D.L. An overview of Phoneutria nigriventer spider venom using combined transcriptomic and proteomic approaches. PLoS ONE 2018, 13, e0200628. [Google Scholar] [CrossRef]
- Valenzuela-Rojas, J.C.; González-Gómez, J.C.; Guevara, G.; Franco, L.M.; Reinoso-Flórez, G.; García, L.F. Notes on the feeding habits of the “Wandering spiders” Phoneutria boliviensis (Arachnida: Ctenidae). J. Arachnol. accepted.
- Walker, S.E.; Rypstra, A.L. Sexual dimorphism in trophic morphology and feeding behavior of wolf spiders (Araneae: Lycosidae) as a result of differences in reproductive roles. Can. J. Zool. 2002, 80, 679–688. [Google Scholar] [CrossRef]
- Pekár, S.; Toft, S. Trophic specialisation in a predatory group: The case of prey-specialised spiders (Araneae). Biol. Rev. 2015, 90, 744–761. [Google Scholar] [CrossRef]
- Melo-Sampaio, P.R.; Maciel, J.M.L.; Oliveira, C.M.B.; Moura, R.S.; Silva, L.C.B.; Silva, T.R.B. Scinax ruber (Red Snouted Treefrog). Herpetol. Rev. 2012, 43, 636–637. [Google Scholar]
- Delfino, G.; Giachi, F.; Malentacchi, C.; Nosi, D. Ultrastructural evidence of serous gland polymorphism in the skin of the Tungara Frog Engystomops pustulosus (Anura Leptodactylidae). Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2015, 298, 1659–1667. [Google Scholar] [CrossRef]
- Taylor, P.W.; Soley, F.G. Ploys and counterploys of assassin bugs and their dangerous spider prey. Behaviour 2013, 150, 397–425. [Google Scholar] [CrossRef]
- Wigger, E.; Kuhn-Nentwig, L.; Nentwig, W. The venom optimisation hypothesis: A spider injects large venom quantities only into difficult prey types. Toxicon 2002, 40, 749–752. [Google Scholar] [CrossRef]
- García, L.F.; Franco, V.; Robledo-Ospina, L.E.; Viera, C.; Lacava, M.; Willemart, R.H. The predation strategy of the recluse spider Loxosceles rufipes (Lucas, 1834) against four prey species. J. Insect Behav. 2016, 29, 515–526. [Google Scholar] [CrossRef]
- García, L.F.; Viera, C.; Pekár, S. Comparison of the capture efficiency, prey processing, and nutrient extraction in a generalist and a specialist spider predator. Sci. Nat. 2018, 105, 30. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, M.C.; Sibly, R.M. Optimal sting use in the feeding behavior of the scorpion Hadrurus spadix. J. Arachnol. 2010, 38, 123–125. [Google Scholar] [CrossRef]
- Dugon, M.M.; Wallace, A. Prey orientation and the role of venom availability in the predatory behaviour of the centipede Scolopendra subspinipes mutilans (Arthropoda: Chilopoda). J. Insect Physiol. 2012, 58, 874–880. [Google Scholar] [CrossRef]
- Herzig, V.; Ward, R.J.; dos Santos, W.F. Ontogenetic changes in Phoneutria nigriventer (Araneae, Ctenidae) spider venom. Toxicon 2004, 44, 635–640. [Google Scholar] [CrossRef]
- Herzig, V.; Hodgson, W.C. Intersexual variations in the pharmacological properties of Coremiocnemis tropix (Araneae, Theraphosidae) spider venom. Toxicon 2009, 53, 196–205. [Google Scholar] [CrossRef]
- De Oliveira, K.C.; Gonçalves de Andrade, R.M.; Giusti, A.L.; da Silva, W.D.; Tambourgi, D.V. Sex-linked variation of Loxosceles intermedia spider venoms. Toxicon 1999, 37, 217–221. [Google Scholar] [CrossRef]
- Estrada-Gomez, S.; Muñoz, L.; Lanchero, P.; Latorre, C. Partial characterization of venom from the colombian spider Phoneutria boliviensis (Aranae:Ctenidae). Toxins 2015, 7, 2872–2887. [Google Scholar] [CrossRef]
- Santana, R.; Perez, D.; Dobson, J.; Panagides, N.; Raven, R.; Nouwens, A.; Jones, A.; King, G.; Fry, B. Venom profiling of a population of the theraphosid spider Phlogius crassipes reveals continuous ontogenetic changes from juveniles through adulthood. Toxins 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Weirauch, C.; Fry, B.; King, G. Venoms of heteropteran insects: A treasure trove of diverse pharmacological toolkits. Toxins 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Fortes-Dias, C.L.; Schaffert, P.P.; Carvalho Botelho, A.C.; Nacif-Pimenta, R.; Estevão-Costa, M.I.; Cordeiro, M.D.N.; Paolucci Pimenta, P.F. Developmental biology of the Brazilian ‘Armed’ spider Phoneutria nigriventer (Keyserling, 1891): Microanatomical and molecular analysis of the embryonic stages. Toxicon 2011, 57, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Pimenta, A.M.C.; Bemquerer, M.P.; Santoro, M.M.; Beirao, P.S.L.; Lima, M.E.; Figueiredo, S.G.; Bloch, C.; Vasconcelos, E.A.R.; Campos, F.A.P.; et al. Comparison of the partial proteomes of the venoms of Brazilian spiders of the genus Phoneutria. Toxicol. Pharmacol. 2006, 142, 173–187. [Google Scholar] [CrossRef]
- McCitorre, J.D. Comparative lethality of several Latrodectus venoms. Toxicon 1964, 2, 201–203. [Google Scholar]
- Nyffeler, M.; Vetter, R.S. Black widow spiders, Latrodectus spp. (Araneae: Theridiidae), and other spiders feeding on mammals. J. Arachnol. 2018, 46, 541–548. [Google Scholar] [CrossRef]
- Schenberg, S.; Lima, F.A. Pharmacology of the polypeptides from the venom of the spider Phoneutria fera. Mem. Inst. Butatan 1966, 33, 627–638. [Google Scholar]
- Gallego-Carmona, C.A.; Forero-Rodríguez, J.S.; Castro-Arango, J.A.; Castellanos-Vargas, C. Engystomops pustulosus (Túngara Frog). Predation. Herpetol. Rev. 2017, 48, 408. [Google Scholar]
- Lucas, S. Spiders in Brazil. Toxicon 1988, 26, 759–772. [Google Scholar] [CrossRef]
- Hazzi, N.A. Natural history of Phoneutria boliviensis (Araneae: Ctenidae): Habitats, reproductive behavior, postembryonic development and prey-wrapping. J. Arachnol. 2014, 42, 303–310. [Google Scholar] [CrossRef]
- Beaupre, S.J.; Jacobson, J.R.; Lillywhite, H.B.; Zamudio, K. Guidelines for Use of Live Amphibians and Reptiles in Field and Laboratory Research, 2nd ed.; Allen Media Press: South Hadley, MA, USA, 2004. [Google Scholar]
- Simone, Y.; Garcia, L.F.; Lacava, M.; van der Meijden, A.; Viera, C. Predatory versatility in females of the scorpion Bothriurus bonariensis (Scorpiones: Bothriuridae): Overcoming prey with different defensive mechanisms. J. Insect Behav. 2018, 31, 402–415. [Google Scholar] [CrossRef]
- Pekár, S.; García, L.F.; Viera, C. Trophic niches and trophic adaptations of prey-specialized spiders from the neotropics: A guide. In Behaviour and Ecology of Spiders; Viera, C., Gonzaga, M.O., Eds.; Springer: Cham, Switzerland, 2017; pp. 247–274. [Google Scholar]
- Yamashita, S. Photoreceptor cells in the spider eye: Spectral sensitivity and efferent control. In Neurobiology of Arachnids; Barth, F.G., Ed.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 103–117. [Google Scholar]
- Yan, J.; Fine, J. Estimating equations for association structures. Stat. Med. 2004, 23, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Pekár, S.; Brabec, M. Generalized estimating equations: A pragmatic and flexible approach to the marginal GLM modelling of correlated data in the behavioural sciences. Ethology 2018, 124, 86–93. [Google Scholar] [CrossRef]
- Valenzuela-Rojas, J.C. Comportamiento Depredador y Aspectos Toxinológicos del Veneno de la “Araña Bananera” Phoneutria boliviensis F.O. Pickard-Cambridge, 1897. Master’s Thesis, Universida del Tolima, Ibagué, Colombia, 2019. [Google Scholar]
- Garcia, L.F.; Pedrosa, L.H.A.; Rosada, D.R.B. An easy method for handling the genus Phoneutria (Araneae, Ctenidae) for venom extraction. J. Arachnol. 2008, 36, 604–605. [Google Scholar] [CrossRef]
- Hayes, W.K.; Fox, G.A.; Nelsen, D.R. Venom collection from spiders and snakes: Voluntary and involuntary extractions (“milking”) and venom gland extractions. In Snake and Spider Toxins. Methods and Protocols; Priel, A., Ed.; Humana Press: Hatfield, UK, 2019; pp. 53–71. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Pekár, S.; Brabec, M. Modern Analysis of Biological Data: Generalized Linear Models in R; Masaryk University Press: Brno, Czech Republic, 2016; ISBN 978-80-210-8019-5. [Google Scholar]
- Kerr, D.R.; Meador, J.P. Modeling dose response using generalized linear models. Environ. Toxicol. Chem. 1996, 15, 395–401. [Google Scholar] [CrossRef]
- R Development Core Team. R: Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2012. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer: Berlín, Germany, 2013. [Google Scholar]




| PREY | SEX | |
|---|---|---|
| Female | Male | |
| Gecko | 2.19 (1.57, 2.96) | 2.03 (1.92, 2.16) |
| Spider | 4229 (460, 38,745) | 639 (248, 1636) |
| Species | Prey | |||||
|---|---|---|---|---|---|---|
| Sex | Mouse | Dog | Spider | Gecko | Fly | |
| P. nigriventer [27] | F | 0.63 | - | - | - | - |
| P. nigriventer [27] | M | 1.57 | - | - | - | - |
| P. nigriventer [48] | M/F | 0.6 | - | - | - | 22.40 |
| P. keyserlingi [48] | M/F | 0.9 | - | - | - | |
| P. reidyi [48] | M/F | 0.11 | - | - | - | 0.85 |
| P. fera [51] | M/F | 0.76 | 0.20 | - | - | - |
| P. boliviensis * | M | - | - | 639 | 2.03 | - |
| P. boliviensis * | F | - | - | 4229 | 2.20 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Rojas, J.C.; González-Gómez, J.C.; van der Meijden, A.; Cortés, J.N.; Guevara, G.; Franco, L.M.; Pekár, S.; García, L.F. Prey and Venom Efficacy of Male and Female Wandering Spider, Phoneutria boliviensis (Araneae: Ctenidae). Toxins 2019, 11, 622. https://doi.org/10.3390/toxins11110622
Valenzuela-Rojas JC, González-Gómez JC, van der Meijden A, Cortés JN, Guevara G, Franco LM, Pekár S, García LF. Prey and Venom Efficacy of Male and Female Wandering Spider, Phoneutria boliviensis (Araneae: Ctenidae). Toxins. 2019; 11(11):622. https://doi.org/10.3390/toxins11110622
Chicago/Turabian StyleValenzuela-Rojas, Juan Carlos, Julio César González-Gómez, Arie van der Meijden, Juan Nicolás Cortés, Giovany Guevara, Lida Marcela Franco, Stano Pekár, and Luis Fernando García. 2019. "Prey and Venom Efficacy of Male and Female Wandering Spider, Phoneutria boliviensis (Araneae: Ctenidae)" Toxins 11, no. 11: 622. https://doi.org/10.3390/toxins11110622