Vipera berus berus Venom from Russia: Venomics, Bioactivities and Preclinical Assessment of Microgen Antivenom
Abstract
:1. Introduction
2. Results and Discussion
2.1. Toxic and Enzymatic Activities of V. b. berus Venom and their Neutralization by Microgen Antivenom
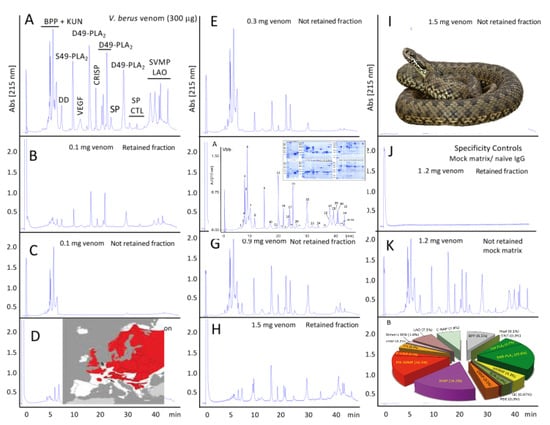
2.2. Quantification of Venom-Specific Antivenom Antibodies using Third-Generation Antivenomics
2.2.1. Characterization of the V. b. berus Venom Proteome
2.2.2. Third-Generation Antivenomics
3. Conclusions
4. Materials and Methods
4.1. Venom and Antivenom
4.2. Animals
4.3. Toxic and Enzymatic Venom Activities
4.3.1. Lethality
4.3.2. Hemorrhagic Activity
4.3.3. Phospholipase Activity
4.4. Fractionation and Proteomics Characterization of V. b. berus (Russia) Venom
4.5. Third-Generation Antivenomics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lenk, P.; Kalyabina, S.; Wink, M.; Joger, U. Evolutionary relationships among the true vipers (Reptilia: Viperidae) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2001, 19, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, T.; Dauga, C.; Ferquel, E.; Choumet, V.; Failloux, A.-B. Molecular phylogeny of Vipera Laurenti, 1768 and the related genera Macrovipera (Reuss, 1927) and Daboia (Gray, 1842), with comments about neurotoxic Vipera aspis aspis populations. Mol. Phylogenet. Evol. 2005, 35, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Szyndlar, Z.; Rage, J.C. Oldest fossil vipers (Serpentes: Viperidae) from the old world. In: Phylogeny and Systematics of the Viperidae. Kaupia 1999, 8, 9–20. [Google Scholar]
- Mallow, D.; Ludwig, D.; Nilson, G. True Vipers: Natural History and Toxinology of Old World Vipers; Krieger Publishing Company: Malabar, FL, USA, 2003; ISBN 0-89464-877-2. [Google Scholar]
- De Smedt, J. The Vipers of Europe; JDS Verlag: Halblech, Germany, 2006; ISBN 3-00-008196-8. [Google Scholar]
- Phelps, T. Old World Vipers. A Natural History of the Azemiopinae and Viperinae; Chimaira: Frankfurt am Main, Germany, 2010; ISBN 978-89973-470-6. [Google Scholar]
- Tuniyev, B.S.; Ostrovskikh, S. Two new species of vipers of “kaznakovi” complex (Ophidia, Viperinae) from the Western Caucasus. Russ. J. Herpetol. 2001, 8, 117–126. [Google Scholar]
- Tuniyev, S.B.; Orlov, N.L.; Tuniyev, B.S.; Kidov, F.F. On the taxonomical status of steppe viper from foothills of the south macroslope of the East Caucasus. Russ. J. Herpetol. 2013, 20, 129–146. [Google Scholar]
- Uetz, P.; Freed, P.; Hoek, J. The Reptile Database. 2016. Available online: http://www.reptile-database.org (accessed on 4 December 2018).
- Kovalchuk, S.I.; Ziganshin, R.H.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Quantitative Proteomic Analysis of Venoms from Russian Vipers of Pelias Group: Phospholipases A2 are the Main Venom Components. Toxins 2016, 8, 105. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae Per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentis, Synomymis; Locis, Tomus I, Editio Decima, Reformata; Impensis Direct, Laurentii Salvii: Holmiae, Sweden, 1758. [Google Scholar]
- Cui, S.; Luo, X.; Chen, D.; Sun, J.; Chu, H.; Li, C.; Jiang, Z. The adder (Vipera berus) in Southern Altay Mountains: Population characteristics, distribution, morphology and phylogenetic position. Peer J. 2016, 4, e2342. [Google Scholar] [CrossRef]
- Zinenko, O.I. Habitats of Vipera berus nikolskii in Ukraine. In Proceedings of the 13th Congress of the Societas Europaea Herpetologica, Bonn, Germany, 27 September–2 October 2005; pp. 205–209. [Google Scholar]
- Malina, T.; Krecsák, L.; Westerström, A.; Szemán-Nagy, G.; Gyémánt, G.; M-Hamvas, M.; Rowan, E.G.; Harvey, A.L.; Warrell, D.A.; Pál, B.; et al. Individual variability of venom from the European adder (Vipera berus berus) from one locality in Eastern Hungary. Toxicon 2017, 135, 59–70. [Google Scholar] [CrossRef]
- Nilson, G.; Andrén, C.; Szynlar, Z. The systematic position of the Common Adder, Vipera berus, in North Korea and adjacent regions. Bonner Zoologische Beiträge 1994, 45, 49–56. [Google Scholar]
- Ursenbacher, S.; Carlsson, M.; Helfer, V.; Tegelström, H.; Fumagalli, L. Phylogeography and Pleistocene refugia of the adder (Vipera berus) as inferred from mitochondrial DNA sequence data. Mol. Ecol. 2006, 15, 3425–3437. [Google Scholar] [CrossRef]
- Kalyabina-Hauf, S.; Schweiger, S.; Joger, U.; Mayer, W.; Wink, M. Phylogeny and systematics of adders (Vipera berus complex). Mertensiella 2004, 15, 7–16. [Google Scholar]
- Ulrich, J.; Fritz, U.; Guicking, D.; Kalyabina-Hauf, S.; Nagy, Z.T.; Wink, T. Phylogeography of western Palaearctic reptiles—Spatial and temporal speciation patterns. Zool. Anz.-A J. Comp. Zool. 2007, 246, 293–313. [Google Scholar]
- Chippaux, J.-P. Epidemiology of snakebites in Europe: A systematic review of the literature. Toxicon 2012, 59, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.J. Incidence, pathology, and treatment of adder (Vipera berus L.) bites in man. J. Accid. Emerg. Med. 1996, 13, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Minton, S.A., Jr. Venom Diseases; CC Thomas Publ.: Springfield, IL, USA, 1974; ISBN 978-0-398-03051-3. [Google Scholar]
- Warrell, D.A. Treatment of bites by adders and exotic venomous snakes. Br. Med. J. 2005, 331, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Karlson-Stiber, C.; Salmonson, H.; Persson, H. A Nationwide Study of Vipera berus bites during one year—Epidemiology and morbidity of 231 cases. Clin. Toxicol. 2006, 44, 25–30. [Google Scholar] [CrossRef]
- Magdalan, J.; Trocha, M.; Merwid-Lad, A.; Sozański, T.; Zawadzki, M. Vipera berus bites in the Region of Southwest Poland—A clinical analysis of 26 cases. Wilderness Environ. Med. 2010, 21, 114–119. [Google Scholar] [CrossRef]
- Malina, T.; Krecsak, L.; Warrell, D.A. Neurotoxicity and hypertension following European adder (Vipera berus berus) bites in Hungary: Case report and review. QJM 2008, 101, 801–806. [Google Scholar] [CrossRef]
- Weinelt, W.; Sattler, R.W.; Mebs, D. Persistent paresis of the facialis muscle after European adder (Vipera berus) bite on the forehead. Toxicon 2002, 40, 1627–1629. [Google Scholar] [CrossRef]
- Malina, T.; Babocsay, G.; Krecsák, L.; Erdész, C. Further clinical evidence for the existence of neurotoxicity in a population of the European adder (Vipera berus berus) in eastern Hungary: Second authenticated case. Wilderness Environ. Med. 2013, 24, 378–383. [Google Scholar] [CrossRef]
- Westerström, A.; Petrov, B.; Tzankov, N. Envenoming following bites by the Balkan adder Vipera berus bosniensis-first documented case series from Bulgaria. Toxicon 2010, 56, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Marquart, H. Recherches statistiques sur les accidents par morsures de serpents au Danemark et en Suède de 1900 à 1947. Presse Méd. 1951, 59, 1110–1111. [Google Scholar] [PubMed]
- Persson, H.; Irestedt, B. A study of 136 cases of adder bite treated in Swedish hospitals during one year. Acta Med. Scand. 1981, 210, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Boels, D.; Hamel, J.F.; Bretaudeau Deguigne, M.; Harry, P. European viper envenomings: Assessment of Viperfav™ and other symptomatic treatments. Clin. Toxicol. 2012, 50, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Reddi, K.R.; Laing, G.; Theakston, R.G.; Landon, J. An affinity purified ovine antivenom for the treatment of Vipera berus envenoming. Toxicon 1992, 30, 865–871. [Google Scholar] [CrossRef]
- Casewell, N.R.; Al-Abdulla, I.; Smith, D.; Coxon, R.; Landon, J. Immunological cross-reactivity and neutralisation of European viper venoms with the monospecific Vipera berus antivenom ViperaTAb. Toxins 2014, 6, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.; de Haro, L.; Lonati, D.; Brvar, M.; Eddleston, M. Antivenom for European Vipera species envenoming. Clin. Toxicol. 2017, 55, 557–568. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, Á.; Vargas, M.; Villalta, M.; Sánchez, A.; Sanz, L.; Lomonte, B.; León, G.; et al. Preclinical evaluation of the efficacy of antivenoms for snakebite envenoming: State-of-the-art and challenges ahead. Toxins 2017, 9, 163. [Google Scholar] [CrossRef]
- Pla, D.; Rodríguez, Y.; Calvete, J.J. Third generation antivenomics: Pushing the limits of the in vitro preclinical assessment of antivenoms. Toxins 2017, 9, 158. [Google Scholar] [CrossRef]
- Calvete, J.J.; Rodríguez, Y.; Quesada-Bernat, S.; Pla, D. Toxin-resolved antivenomics-guided assessment of the immunorecognition landscape of antivenoms. Toxicon 2018, 148, 107–122. [Google Scholar] [CrossRef]
- Calderón, L.; Lomonte, B.; Gutiérrez, J.M.; Tarkowski, A.; Hanson, L.A. Biological and biochemical activities of Vipera berus (European viper) venom. Toxicon 1993, 31, 743–753. [Google Scholar] [CrossRef]
- Araujo, H.P.; Bourguignon, S.C.; Boller, M.A.; Dias, A.A.; Lucas, E.P.; Santos, I.C.; Delgado, I.F. Potency evaluation of antivenoms in Brazil: The national control laboratory experience between 2000 and 2006. Toxicon 2008, 51, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Next-generation snake venomics: Protein-locus resolution through venom proteome decomplexation. Expert Rev. Proteomics 2014, 11, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, S.; Sanz, L.; Calvete, J.J.; Pla, D. Constructing comprehensive venom proteome reference maps for integrative venomics. Expert Rev. Proteomics 2015, 12, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Bocian, A.; Urbanik, M.; Hus, K.; Łyskowski, A.; Petrilla, V.; Andrejčáková, Z.; Petrillová, M.; Legath, J. Proteome and Peptidome of Vipera berus berus Venom. Molecules 2016, 21, 1398. [Google Scholar] [CrossRef] [PubMed]
- Latinović, Z.; Leonardi, A.; Šribar, J.; Sajevic, T.; Žužek, M.C.; Frangež, R.; Halassy, B.; Trampuš-Bakija, A.; Pungerčar, J.; Križaj, I. Venomics of Vipera berus berus to explain differences in pathology elicited by Vipera ammodytes ammodytes envenomation: Therapeutic implications. J. Proteomics 2016, 146, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Calvete, J.J. Strategies in ‘snake venomics’ aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J. Venom Anim. Toxins Incl. Trop. Dis. 2017, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Quesada-Bernat, S.; Chen, P.Y.; Lee, C.D.; Chiang, J.R.; Calvete, J.J. Translational Venomics: Third-Generation Antivenomics of Anti-Siamese Russell’s Viper, Daboia siamensis, Antivenom Manufactured in Taiwan CDC’s Vaccine Center. Trop. Med. Infect. Dis. 2018, 3, 66. [Google Scholar] [CrossRef]
- Whiteley, G.; Casewell, N.R.; Pla, D.; Quesada-Bernat, S.; Logan, R.A.E.; Bolton, F.M.S.; Wagstaff, S.C.; Gutiérrez, J.M.; Calvete, J.J.; Harrison, R.A. Defining the pathogenic threat of envenoming by South African shield-nosed and coral snakes (genus Aspidelaps), and revealing the likely efficacy of available antivenom. J. Proteomics 2018. [Google Scholar] [CrossRef]
- Pla, D.; Sanz, L.; Molina-Sánchez, P.; Zorita, V.; Madrigal, M.; Flores-Díaz, M.; Alape-Girón, A.; Núñez, V.; Andrés, V.; Gutiérrez, J.M.; et al. Snake venomics of Lachesis muta rhombeata and genus-wide antivenomics assessment of the paraspecific immunoreactivity of two antivenoms evidence the high compositional and immunological conservation across Lachesis. J. Proteomics 2013, 89, 112–123. [Google Scholar] [CrossRef]
- Segura, A.; Herrera, M.; Villalta, M.; Vargas, M.; Gutiérrez, J.M.; León, G. Assessment of snake antivenom purity by comparing physicochemical and immunochemical methods. Biologicals 2013, 41, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Burgasov, P.N. Handbook on Vaccination and Seroprophylaxis; Medicine: Moscow, Russia, 1978; p. 439. (In Russian) [Google Scholar]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17079. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis; A Statistical Treatment of the Sigmoid Response Curve; Macmillan: Oxford, UK, 1947; ISBN 978-0521135900. [Google Scholar]
- Segura, Á.; Castillo, M.C.; Núñez, V.; Yarlequé, A.; Gonçalves, L.R.C.; Villalta, M.; Bonilla, C.; Herrera, M.; Vargas, M.; Fernández, M.; Yano, M.Y.; et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon 2010, 56, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Gené, J.A.; Rojas, G.; Cerdas, L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 1985, 23, 887–893. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B.; Chaves, F.; Moreno, E.; Cerdas, L. Pharmacological activities of a toxic phospholipase A2 isolated from the venom of the snake Bothrops asper. Comp. Biochem. Physiol. C 1986, 84, 159–164. [Google Scholar] [CrossRef]
- Howard, G.C.; Kaser, M.R. Making and Using Antibodies: A Practical Handbook, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; ISBN 9781439869086. [Google Scholar]
- Morais, V.; Ifran, S.; Berasain, P.; Massaldi, H. Antivenoms: Potency or median effective dose, which to use? J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 191–193. [Google Scholar] [CrossRef]
- WHO. Progress in the Characterization of Venoms and Standardization of Antivenoms; WHO Offset Publication: Geneva, Switzerland, 1981; Available online: http://apps.who.int/iris/handle/10665/37282 (accessed on 30 December 2018).


| Toxic Activity | Activity | Neutralization by Antivenom |
|---|---|---|
| Lethality (LD50, i.p.) | 19.8 (10.7–30.3) a µg per mouse | ED50: 3.1 (1.5–4.7) a mgV/mL AV |
| Hemorrhagic (MHD) | 2.00 ± 0.79 µg per mouse | 8.0 ± 0.1 mgV/mL AV |
| PLA2 Activity | 48 ± 20 µeq/mg/min | 1.70 ± 0.06 mgV/mL AV |
| Vipera berus berus (Russia) Total Venom Proteins (µg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RP-HPLC Fraction | 100 | 300 | 600 | 900 | 1200 | 1500 | Toxins in RP-HPLC fraction | ||
| 1, 2, 5 | µg TOTAL | 6.68 | 20.04 | 40.08 | 60.12 | 80.16 | 100.2 | BPP + KUN | |
| µg RET | 0.21 | 0.64 | 1.10 | 0.84 | 0.80 | 0.64 | |||
| 3, 4, 6, 7 | µg TOTAL | 18.97 | 56.91 | 113.82 | 170.73 | 227.64 | 288.55 | KUN [P00991, P00992] | |
| µg RET | 3.05 | 7.08 | 11.93 | 17.13 | 12.30 | 11.57 | |||
| 8 | µg TOTAL | 2.79 | 8.38 | 16.75 | 25.13 | 33.50 | 41.88 | Dimeric Disintegrin [P0C6A6] | |
| µg RET | 2.54 | 7.29 | 10.13 | 10.41 | 10.42 | 8.46 | |||
| 9 | µg TOTAL | 6.60 | 19.80 | 39.60 | 59.40 | 79.20 | 99.00 | S49-PLA2 [CAE47248] | |
| µg RET | 6.58 | 14.96 | 19.56 | 19.86 | 18.09 | 16.89 | |||
| 10 | µg TOTAL | 3.02 | 9.06 | 18.12 | 27.18 | 36.24 | 45.30 | VEGF [P83942] | |
| µg RET | 3.02 | 7.25 | 9.44 | 9.87 | 11.06 | 9.88 | |||
| 11–14 | µg TOTAL | 15.94 | 31.88 | 63.76 | 95.64 | 127.52 | 159.40 | D49-PLA2 [P31854] | |
| µg RET | 15.94 | 33.84 | 44.21 | 46.32 | 49.33 | 38.33 | |||
| 16 | µg TOTAL | 5.43 | 16.29 | 32.58 | 48.87 | 65.16 | 81.45 | CRISP [ B7FDI1] | |
| µg RET | 5.42 | 15.37 | 15.78 | 15.92 | 15.12 | 14.85 | |||
| 17–19 | µg TOTAL | 8.00 | 24.01 | 48.01 | 72.02 | 96.02 | 120.03 | D49-PLA2 [AAN59990] | |
| µg RET | 7.98 | 11.65 | 15.09 | 12.01 | 11.01 | 10.75 | |||
| 20, 21 | µg TOTAL | 1.65 | 4.96 | 9.92 | 14.89 | 19.85 | 24.81 | SVSP [E5AJX2] + D49-PLA2 | |
| µg RET | 1.65 | 4.62 | 5.65 | 6.22 | 6.04 | 5.79 | |||
| 22 | µg TOTAL | 9.95 | 29.84 | 59.68 | 89.51 | 119.35 | 149.19 | ||
| µg RET | 9.39 | 24.27 | 28.24 | 26.77 | 27.02 | 24.09 | |||
| 23 | µg TOTAL | 0.83 | 2.49 | 4.99 | 7.48 | 9.97 | 12.47 | PIII-SVMP | |
| µg RET | 0.83 | 2.24 | 2.95 | 3.18 | 2.90 | 2.74 | |||
| 24 | µg TOTAL | 0.98 | 2.94 | 5.88 | 8.82 | 11.76 | 14.70 | SVSP + CTL | |
| µg RET | 0.87 | 1.63 | 5.56 | 8.31 | 7.55 | 7.68 | |||
| 25–28 | µg TOTAL | 12.92 | 38.76 | 77.52 | 116.28 | 155.04 | 193.8 | LAO + PIII-SVMP + CTL | |
| µg RET | 12.17 | 36.96 | 64.92 | 71.65 | 82.54 | 78.98 | |||
| 29–34 | µg TOTAL | 4.01 | 12.03 | 24.06 | 36.09 | 49.02 | 61.05 | LAO + PIII-SVMP + SVSP | |
| µg RET | 3.78 | 12.00 | 22.21 | 34.78 | 45.51 | 49.34 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shekhadat, R.I.; Lopushanskaya, K.S.; Segura, Á.; Gutiérrez, J.M.; Calvete, J.J.; Pla, D. Vipera berus berus Venom from Russia: Venomics, Bioactivities and Preclinical Assessment of Microgen Antivenom. Toxins 2019, 11, 90. https://doi.org/10.3390/toxins11020090
Al-Shekhadat RI, Lopushanskaya KS, Segura Á, Gutiérrez JM, Calvete JJ, Pla D. Vipera berus berus Venom from Russia: Venomics, Bioactivities and Preclinical Assessment of Microgen Antivenom. Toxins. 2019; 11(2):90. https://doi.org/10.3390/toxins11020090
Chicago/Turabian StyleAl-Shekhadat, Ruslan I., Ksenia S. Lopushanskaya, Álvaro Segura, José María Gutiérrez, Juan J. Calvete, and Davinia Pla. 2019. "Vipera berus berus Venom from Russia: Venomics, Bioactivities and Preclinical Assessment of Microgen Antivenom" Toxins 11, no. 2: 90. https://doi.org/10.3390/toxins11020090