The Pressure of Fusarium Disease and Its Relation with Mycotoxins in The Wheat Grain and Malt
Abstract
:1. Introduction
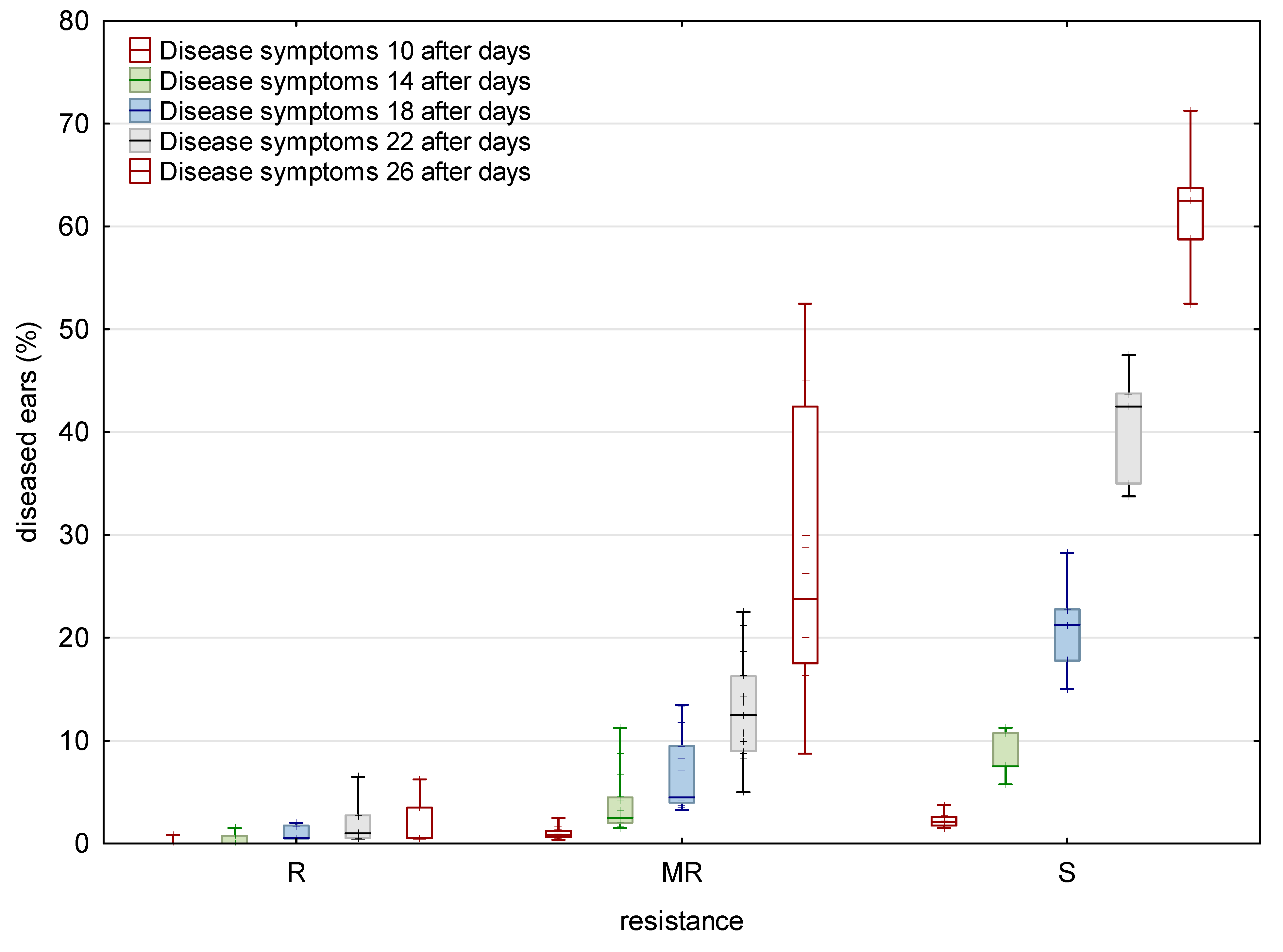
2. Results
3. Discussion
3.1. Relationship of Toxin Measurement to Resistance
3.2. Development of Mycotoxins in the Wheat Grain and Malt
4. Conclusions
5. Materials and Methods
5.1. Inoculum Production
5.2. Field Trials
5.3. Malting
5.4. Mycotoxin Analyses
5.5. Method Validation
5.6. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Ameye, M.; De Saeger, S.; Audenaert, K.; Haesaert, G. Biological control of mycotoxigenic fungi and their toxins: An update for the pre-harvest approach. In Mycotoxins—Socio-Economic and Health Impact as well as Pre- and Postharvest Management Strategies; Berka, N.P., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ohmichi, K.; Sakamoto, S.; Sago, Y.; Kushiro, M.; Nagashima, H.; Yoshida, M.; Nakajima, T. Detection of a new Fusarium masked mycotoxin in wheat grain by high-resolution LC-OrbitrapTMMS. Food Addit. Contam. 2011, 28, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). FAO Food and Nutrition Papers 81: Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO: Rome, Italy; Available online: http://www.fao.org/docrep/007/y5499e/y5499e00.HTM (accessed on 10 July 2018).
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food, Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 38, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Bondy, G.S. Immunotoxic effects of mycotoxins. In Mycotoxins in Grain-Compounds other than Aflatoxin; Miller, J.D., Trenholm, H.L., Eds.; Eagan Press: St. Paul, MN, USA, 1994; pp. 339–358. [Google Scholar]
- Desjardins, A.E. Trichotechenes. In Fusarium Mycotoxins-Chemistry, Genetics and Biology; Desjardins, A.E., Ed.; American Phytopathological Society Press: St. Paul, MN, USA, 2007; pp. 13–64. [Google Scholar]
- Steiner, B.; Lemmens, M.; Griesser, M.; Scholz, U.; Schondelmaier, J.; Buerstmayr, H. Molecular mapping of resistance to Fusarium head blight in the spring wheat cultivar Frontana. Theor. Appl. Genet. 2004, 109, 215–224. [Google Scholar] [CrossRef]
- Mergoum, M.; Frohberg, R.C.; Stack, R.W. Breeding Hard Red Spring Wheat for Fusarium Head Blight Resistance. Wheat Prod. Stressed Environ. 2007, 12, 161–167. [Google Scholar] [CrossRef]
- Spanic, V.; Marcek, T.; Abicic, I.; Sarkanj, B. Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum. Toxins 2018, 10, 17. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Liu, S.; Hall, M.D.; Griffey, C.A.; McKendry, A.L. Meta-analysis of QTL associated with Fusarium head blight resistance in wheat. Crop Sci. 2009, 49, 1955–1968. [Google Scholar] [CrossRef]
- Miller, J.D.; Arnison, P.G. Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Spanic, V.; Lemmens, M.; Drezner, G. Variability in Components of Fusarium Head Blight Resistance among Wheat Genotypes. Cereal Res. Commun. 2013, 41, 420–430. [Google Scholar] [CrossRef]
- Xu, X. Effects of Environmental Conditions on the Development of Fusarium Ear Blight. Eur. J. Plant Pathol. 2003, 109, 683–689. [Google Scholar] [CrossRef]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium head blight incidence and mycotoxin accumulation in three durum wheat cultivars in relation to sowing date and density. Naturwissenschaften 2018, 105, 2. [Google Scholar] [CrossRef]
- Mesterhazy, A. Breeding for resistance to Fusarium head blight of wheat. In Fusarium Head Blight: Global Status and Future Prospects; Dubin, H.J., Gilchrist, L., Reeves, J., McNab, A., Eds.; CIMMYT: El Batan, Mexico, 1997; pp. 79–85. [Google Scholar]
- Snijders, C.H.A.; Perkowski, J. Effects of Head Blight Caused by Fusarium culmorum on toxin content and weight of wheat kernels. Phytopathology 1990, 80, 566–570. [Google Scholar] [CrossRef]
- Mesterházy, Á. Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. Eur. J. Plant Pathol. 2002, 108, 675–684. [Google Scholar] [CrossRef]
- Berger, G.; Green, A.; Khatibi, P.; Brooks, W.; Rosso, L.; Liu, S.; Chao, S.; Griffey, C.; Schmale, D. Characterization of Fusarium head blight resistance and deoxynivalenol accumulation in hulled and hulless winter barley. Plant Dis. 2014, 98, 599–606. [Google Scholar] [CrossRef]
- He, X.; Osman, M.; Helm, J.; Capettini, F.; Singh, P.K. Evaluation of Canadian barley breeding lines for Fusarium head blight resistance. Can. J. Plant Sci. 2015, 95, 923–925. [Google Scholar] [CrossRef]
- Cwalina-Ambroziak, B.; Kurowski, T.P.; Waśkiewicz, A.; Goliński, P.; Stępień, A.; Głosek-Sobieraj, M.; Perczak, A. The effect of fertiliser treatments on the severity of Fusarium head blight and mycotoxin biosynthesis in winter rye. Arch. Hig. Rada Toksikol. 2017, 68, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers, D.J.; Fedak, G.; Savard, M. Molecular mapping of novel genes controlling Fusarium head blight resistance and deoxynivalenol accumulation in spring wheat. Genome 2003, 46, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Quarta, A.; Mita, G.; Haidukowski, M.; Santino, A.; Mulè, G.; Visconti, A. Assessment of trichothecene chemotypes of Fusarium culmorum occurring in Europe. Food Addit. Contam. 2005, 22, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Nakajima, T. Deoxynivalenol and nivalenol accumulation in wheat infected with Fusarium graminearum during grain development. Phytopathology 2010, 100, 763–773. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-mycotoxins fusalproliferin, beauvericin, enniatins and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Mastanjević, K.; Lukinac, J.; Jukić, M.; Šarkanj, B.; Krstanović, V.; Mastanjević, K. Multi-(myco)toxins in Malting and Brewing By-Products. Toxins 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Junge, N.; Schumacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 246–267. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef]
- Palacios, S.A.; Ramirez, M.L.; Zalazar, M.C.; Farnochi, M.C.; Zappacosta, D.; Chiacchiera, S.M.; Reynoso, M.M.; Chulze, S.N.; Torres, A.M. Occurrence of Fusarium spp. and Fumonisin in Durum Wheat Grains. Agric. Food Chem. 2011, 59, 12264–12269. [Google Scholar] [CrossRef]
- Chrpová, J.; Šíp, V.; Matějová, E.; Sýkorová, S. Resistance of Winter Wheat Varieties Registered in the Czech Republic to Mycotoxin Accumulation in Grain Following Inoculation with Fusarium culmorum. Czech J. Genet. Plant Breed. 2007, 43, 44–52. [Google Scholar] [CrossRef]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Horsley, R.D.; Steffenson, B.J.; Salas, B.; Barr, J.M. Quality Risks Associated with the Utilization of Fusarium Head Blight Infected Malting Barley. J. Am. Soc. Brew. Chem. 2006, 64, 1–7. [Google Scholar] [CrossRef]
- Váňová, M.; Hajšlová, J.; Havlová, P.; Matušinsky, P.; Lancová, K.; Spitzerová, D. Effect of spring barley protection on the production of Fusarium spp. mycotoxins in grain and malt using fungicides in field trials. Plant Soil Environ. 2004, 50, 447–455. [Google Scholar] [CrossRef]
- Duffeck, M.R.; Tibola, C.S.; Guarienti, E.M.; Del Ponte, E.M. Survey of mycotoxins in Southern Brazilian wheat and evaluation of immunoassay methods. Sci. Agric. 2017, 74, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Bryla, M.; Ksieniewicz-Wozniak, E.; Waskiewicz, A.; Szymczyk, K.; Jedrzejczak, R. Natural Occurrence of Nivalenol, Deoxynivalenol, and Deoxynivalenol-3-Glucoside in Polish Winter Wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Vrabcheva, T.M.; Geßler, R.; Usleber, E.; Märtlbauer, E.P. First survey on the natural occurrence of Fusarium mycotoxins in Bulgarian wheat. Mycopathologia 1996, 136, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Curtui, V.; Usleber, E.; Dietrich, R.B.; Lepschy, J.; Märtlbauer, E. A survey on the occurrence of mycotoxins in wheat and maize from western Romania. Mycopathologia 1998, 143, 97–103. [Google Scholar] [CrossRef]
- Brera, C.; Bertazzoni, V.; Debegnach, F.; Gregori, E.; Pranter, E.; De Santis, B. Expossure Assessment for Italian Population Groups to Deoxynivalenol Deriving from Pasta Consumption. Toxins 2013, 5, 2293–2309. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Hall, C.E.; Schwarz, P.B. Mycotoxins and fermentation—Beer production. Adv. Exp. Med. Biol. 2002, 504, 217–226. [Google Scholar] [CrossRef]
- Stanciu, O.; Banc, R.; Cozma, A.; Filip, L.; Miere, D.; Mañes, J.; Loghin, F. Occurence of Fusarium Mycotoxins in Wheat from Europe—A Review. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 35–60. [Google Scholar] [CrossRef]
- Lorenz, N.; Dänicke, S.; Edler, L.; Gottschalk, C.; Lassek, E.; Marko, D.; Rychlik, M.; Mally, A. A critical evaluation of health risk assessment of modified mycotoxins with a special focus on zearalenone. Mycotoxin Res. 2019, 35, 27–46. [Google Scholar] [CrossRef]
- Abbas, H.K.; Yoshizawa, T.; Shier, W.T. Cytotoxicity and phytotoxicity of trichothecene mycotoxins produced by Fusarium spp. Toxicon 2013, 74, 68–75. [Google Scholar] [CrossRef]
- Habler, K.; Hofer, K.; Geißinger, C.; Schüler, J.; Hückelhoven, R.; Hess, M.; Gastl, M.; Rychlik, M. Fate of Fusarium Toxins during the Malting Process. J. Agric. Food Chem. 2018, 64, 1377–1384. [Google Scholar] [CrossRef]
- Galaverna, G.; Dall’asta, C.; Mangia, M.A.; Dossena, A.; Marchelli, R. Masked Mycotoxins: An Emerging Issue for Food Safety. Czech J. Food Sci. 2009, 27, S89–S92. [Google Scholar] [CrossRef]
- Edwards, S. Zearalenone risk in European wheat. World Mycotoxin J. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.O.; Savi, G.D.; Carnielli-Queiroz, L.; Almeida, F.G.; Minella, E.; Correa, B. Occurrence of deoxynivalenol and zearalenone in brewing barley grains from Brazil. Mycotoxin Res. 2018, 34, 173–178. [Google Scholar] [CrossRef]
- Gratz, S.W. Do Plant-Bound Masked Mycotoxins Contribute to Toxicity? Toxins 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef]
- De Boevre, M.; Landschoot, S.; Audenaert, K.; Maene, P.; Di Mavungu, J.D.; Eeckhout, M.; Haesaert, G.; De Saeger, S. Occurrence and within field variability of Fusarium mycotoxins and their masked forms in maize crops in Belgium. World Mycotoxin J. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Maul, R.; Müller, C.; Rieß, S.; Koch, M.; Methner, F.J.; Nehls, I. Germination induces the glucosylation of the Fusarium mycotoxin deoxynivalenol in various grains. Food Chem. 2012, 131, 274–279. [Google Scholar] [CrossRef]
- Malachová, A.; Cerkal, R.; Ehrenbergerova, J.; Hajslova, J. The fate of mycotoxins during malting and brewing. In Proceedings of the International Ph.D. Students Conference on MendelNet 2010, Brno, Czech Republic, 24 November 2010; Skarpa, P., Ed.; Mendel University in Brno: Brno, Chezch Republic, 2010; pp. 708–715. [Google Scholar]
- Trombete, F.; Barros, A.; Vieira, M.; Saldanha, T.; Venâncio, A.; Fraga, M. Simultaneous Determination of Deoxynivalenol, Deoxynivalenol-3-Glucoside and Nivalenol in Wheat Grains by HPLC-PDA with Immunoaffinity Column Cleanup. Food Anal. Methods 2016, 9, 2579–2586. [Google Scholar] [CrossRef] [Green Version]
- Malachová, A.; Dzuman, Z.; Veprikova, Z.; Vaclavikova, M.; Zachariasova, M.; Hajslova, J. Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: The major mycotoxins found in cereal-based products on the Czech market. J. Agric. Food Chem. 2011, 59, 12990–12997. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; van Dam, R.; van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Haim, K.; Lew, H.; Ruckenbauer, P. The effect of nitrogen fertilization on Fusarium head blight development and deoxynivalenol contamination in wheat. Phytopathology 2004, 152, 1–8. [Google Scholar] [CrossRef]
- Snijders, C.H.A.; Van Eeuwijk, F.A. Genotype X strain interactions for resistance to Fusarium head blight caused by Fusarium culmorum in winter wheat. Theor. Appl. Genet. 1991, 81, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzac, F.C.A. Decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Shao, S.; Cai, Z.; Feng, L.; Pan, H.; Wang, Z. Simultaneous determination of multi-component mycotoxin contaminants in foods and feeds by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. 2007, 1143, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Suman, M.; Bergamini, E.; Catellani, D.; Manzitti, A. Development and validation of a liquid chromatography/linear ion trap mass spectrometry method for the quantitative determination of deoxynivalenol-3-glucoside in processed cereal derived products. Food Chem. 2013, 136, 1568–1576. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990; p. 532. [Google Scholar]






| DON-grain | 3-AcDON-grain | NIV-grain | DON-malt | 3-AcDON-malt | ZEN-malt | NIV-malt | AUDPC-GR | AUDPC-IN | |
|---|---|---|---|---|---|---|---|---|---|
| DON-grain | 1 | ||||||||
| 3-AcDON-grain | 0.8239 ** | 1 | |||||||
| NIV-grain | 0.4879 * | 0.5570 ** | 1 | ||||||
| DON-malt | 0.8462 ** | 0.8597 ** | 0.6138 ** | 1 | |||||
| 3-AcDON-malt | 0.7981 ** | 0.7883 ** | 0.5153 ** | 0.8451 ** | 1 | ||||
| ZEN-malt | 0.5513 ** | 0.5054 ** | 0.6491 ** | 0.6682 ** | 0.6337 ** | 1 | |||
| NIV-malt | 0.4992 ** | 0.6007 ** | 0.5214 ** | 0.6661 ** | 0.4709 * | 0.5743 ** | 1 | ||
| AUDPC-GR | 0.9085 ** | 0.7786 ** | 0.4561 * | 0.8339 ** | 0.7942 ** | 0.6328 ** | 0.4745 * | 1 | |
| AUDPC-IN | 0.8185 ** | 0.7073 ** | 0.4289 * | 0.7662 ** | 0.7087 ** | 0.6309 ** | 0.3962 * | 0.9585 ** | 1 |
| Varieties | Origin | Year of Release |
|---|---|---|
| U1 | HR, AIO | 1936 |
| APACHE | FRA | 1998 |
| SIRBAN PROLIFIC | HU | 1905 |
| RENAN | FRA | 1991 |
| DIVANA | HR, JS | 1995 |
| GRAINDOR | FRA | 2006 |
| OS OLIMPIJA | HR, AIO | 2009 |
| BEZOSTAYA-1 | Former USSR | 1955 |
| FLAMURA 85 | ROM | 1989 |
| KRALJICA | HR, AIO | 2010 |
| VULKAN | HR, AIO | 2009 |
| DROPIA | ROM | 2006 |
| ZITARKA | HR, AIO | 1985 |
| ANTONIJA | HR, AIO | 2011 |
| SANA | HR, BC | 1983 |
| LUCIJA | HR, AIO | 2001 |
| OS ALKA | HR, AIO | 2003 |
| KATARINA | HR, AIO | 2006 |
| SRPANJKA | HR, AIO | 1989 |
| BC ANICA | HR, BC | 2010 |
| GOLUBICA | HR, AIO | 1997 |
| RENATA | HR, AIO | 2006 |
| BASTIDE | FRA | 2003 |
| FELIX | HR, AIO | 2007 |
| SUPER ZITARKA | HR, AIO | 1997 |
| Analyte | Polarity | Retention Time (min) | RE (%) | RA (%) | SSE (%) | RSD Interday | RSD Intraday | LOD Matrix (ng g−1) | LOQ Matrix (ng g−1) |
|---|---|---|---|---|---|---|---|---|---|
| NIV | − | 2.4 | 81 | 70 | 70 | 5.3% | 9.6% | 6.1 | 21.0 |
| DON | − | 2.5 | 102 | 101 | 99 | 6.0% | 10.4% | 4.8 | 15.1 |
| D3G | − | 2.4 | 88 | 68 | 78 | 8.2% | 13.5% | 5.2 | 15.2 |
| 3-AcDON | + | 3.1 | 92 | 88 | 96 | 5.1% | 7.1% | 5.15 | 16.2 |
| ZEN | − | 19.8 | 73 | 82 | 109 | 11.2% | 13.6% | 1.2 | 3.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanic, V.; Zdunic, Z.; Drezner, G.; Sarkanj, B. The Pressure of Fusarium Disease and Its Relation with Mycotoxins in The Wheat Grain and Malt. Toxins 2019, 11, 198. https://doi.org/10.3390/toxins11040198
Spanic V, Zdunic Z, Drezner G, Sarkanj B. The Pressure of Fusarium Disease and Its Relation with Mycotoxins in The Wheat Grain and Malt. Toxins. 2019; 11(4):198. https://doi.org/10.3390/toxins11040198
Chicago/Turabian StyleSpanic, Valentina, Zvonimir Zdunic, Georg Drezner, and Bojan Sarkanj. 2019. "The Pressure of Fusarium Disease and Its Relation with Mycotoxins in The Wheat Grain and Malt" Toxins 11, no. 4: 198. https://doi.org/10.3390/toxins11040198





