Conversion of Deoxynivalenol-3-Glucoside to Deoxynivalenol during Chinese Steamed Bread Processing
Abstract
:1. Introduction
2. Results
2.1. Conversion of D3G during CSB Processing
2.2. Role of Enzymes in the Conversion of D3G during CSB Processing
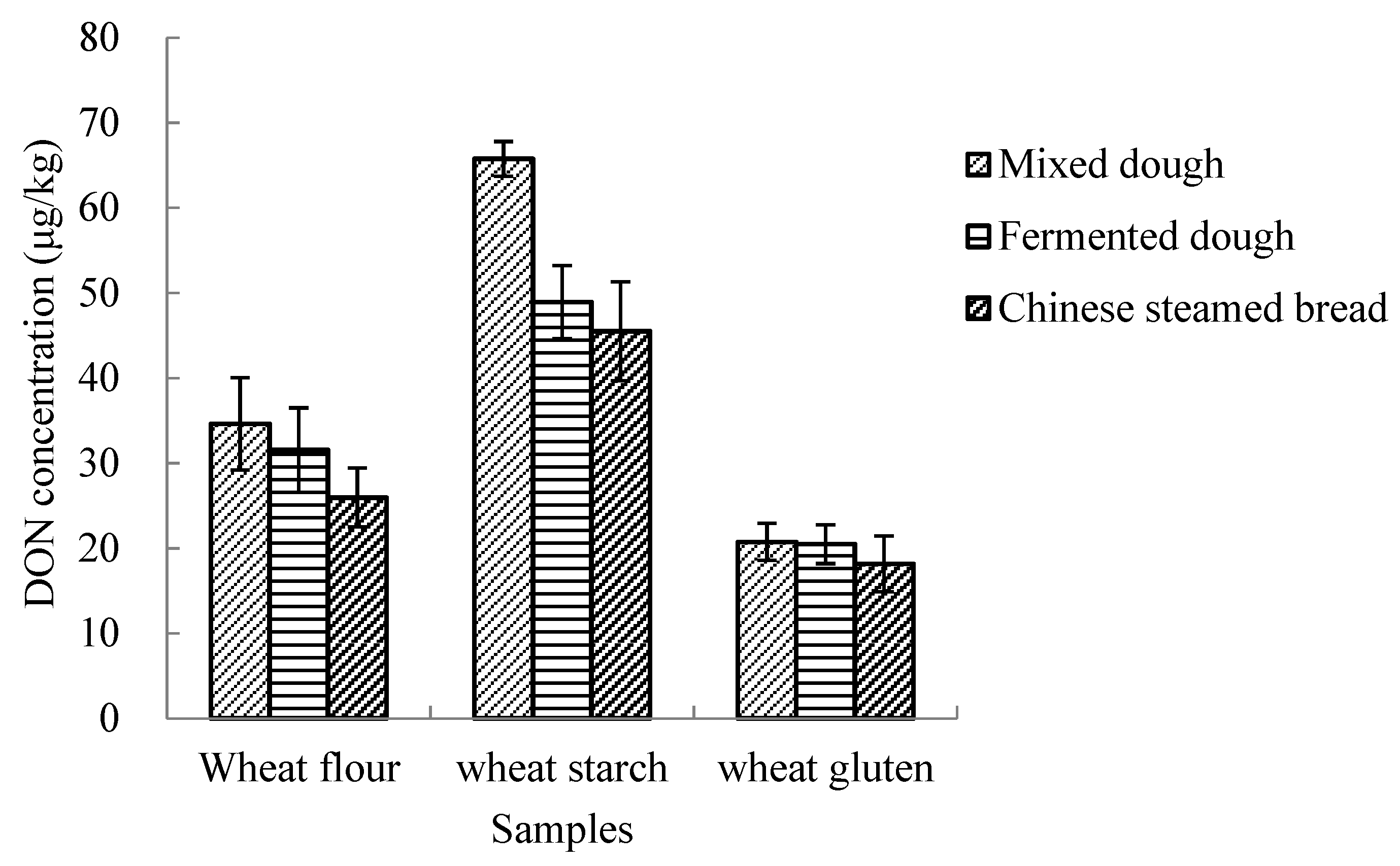
2.3. Conversion of D3G in Different Wheat Compositions during CSB Processing
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of D3G-Contaminated Wheat Flour/Enzyme-Deactivated Wheat Flour/Wheat Starch/Wheat Gluten
4.3. Preparation of Chinese Steamed Bread
4.4. Sample Treatment and UPLC-MS/MS Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 137, 283–298. [Google Scholar] [CrossRef]
- Engelhardt, G.; Ruhland, M.; Wallnöfer, P.R. Metabolism of mycotoxins in plants. Adv. Food Sci. 1999, 21, 71–78. [Google Scholar]
- Karlovsky, P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Berthiller, F.; Werner, U.; Adam, G.; Krska, R.; Lemmens, M.; Sulyok, M.; Hauser, M.T.; Schuhmacher, R. Bildung von maskierten Fusarium mykotoxinen in Pflanzen. Ernaehrung 2006, 30, 477–481. [Google Scholar]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, A.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweiger, W.; Boddu, J.; Shin, S.; Poppenberger, B.; Berthiller, F.; Lemmens, M.; Muehlbauer, G.J.; Adam, G. Validation of a candidate deoxynivalenol-inactivating UDP-glucosyltransferase from barley by heterologous expression in yeast. Mol. Plant Microbe Interact. 2010, 23, 977–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirlini, M.; Dall’Asta, C.; Galaverna, G. Hyphenated chromatographic techniques for structural characterization and determination of masked mycotoxins. J. Chromatogr. A. 2012, 1255, 145–152. [Google Scholar] [CrossRef]
- Kostelanska, M.; Hajslova, J.; Zachariasova, M.; Malachova, A.; Kalachova, K.; Poustka, J.; Fiala, J.; Scott, P.M.; Berthiller, F.; Krska, R. Occurrence of deoxynivalenol and its major conjugate, deoxynivalenol-3-glucoside, in beer and some brewing intermediates. J. Agr. Food Chem. 2009, 57, 3187–3194. [Google Scholar] [CrossRef]
- Malachova, A.; Dzuman, Z.; Veprikova, Z.; Vaclavikova, M.; Zachariasova, M.; Hajslova, J. Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: The major mycotoxins found in cereal-based products on the Czech market. J. Agr. Food Chem. 2011, 59, 12990–12997. [Google Scholar] [CrossRef]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal based food by LCMS/MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Sanchis, V.; Ramos, A.J. Mycotoxin bioaccessibility/absorption using in vitro digestion model: A review. World Mycotoxin J. 2013, 6, 167–184. [Google Scholar] [CrossRef] [Green Version]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, 959. 2011. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_959_eng.pdf (accessed on 25 January 2014).
- Bretz, M.; Beyer, M.; Cramer, B.; Humpf, H.U. Synthesis of stable isotope labeled 3-acetyldeoxynivalenol. Mol. Nutr. Food Res. 2005, 49, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Dall’asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-beta-D-glucoside in wheat and maize. Food Addit. Contam. 2009, 26, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef]
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostalek, P.; Sachambula, L. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. 2008, 25, 732–744. [Google Scholar] [CrossRef] [Green Version]
- Ksieniewicz-Woźniak, E.; Bryła, M.; Waśkiewicz, A.; Yoshinari, T.; Szymczyk, K. Selected Trichothecenes in barley malt and beer from Poland and an assessment of dietary risks associated with their consumption. Toxins 2019, 11, 715. [Google Scholar]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agr. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef]
- Sasanya, J.J.; Hall, C.; Wolf-Hall, C. Analysis of deoxynivalenol, masked deoxynivalenol, and Fusarium graminearum pigment in wheat samples, using liquid chromatography–UV–mass spectrometry. J. Food Protect. 2008, 71, 1205–1213. [Google Scholar] [CrossRef]
- Li, F.Q.; Yu, C.C.; Shao, B.; Wang, W.; Yu, H.X. Natural occurrence of masked deoxynivalenol and multi-mycotoxins in cereals from China harvested in 2007 and 2008. Chin. J. Prev. Med. 2011, 45, 57–63. [Google Scholar]
- Berthiller, F.; Schuhmacher, R.; Adam, G.; Krska, R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009, 395, 1243–1252. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Snook, M.E. Stability of the mycotoxin deoxynivalenol (DON) during the production of flour-based foods and wheat flake cereal. Food Addit. Contam. 2010, 27, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Burgess, K.; Whitney, K.L.; Gu, Y.; Qian, S.Y. Analysis of deoxynalenol and deoxynivalenol-3-glucoside in wheat. Food Control 2012, 26, 287–292. [Google Scholar] [CrossRef]
- Young, J.; Fulcher, R.; Hayhoe, J.; Scott, P.M.; Dexter, J. Effect of milling and baking on deoxynivalenol (vomitoxin) content of eastern Canadian wheats. J. Agr. Food Chem. 1984, 32, 659–664. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Zhang, H.J.; Wang, B.J. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during wheat milling and Chinese steamed bread processing. Food Control 2014, 44, 86–91. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, B.J. Fates of deoxynivalenol and deoxynivalenol-3-glucoside during bread and noodle processing. Food Control 2015, 50, 754–757. [Google Scholar] [CrossRef]
- Wu, L.; Wang, B.J. Evaluation on levels and conversion profiles of spiked DON, 3-ADON and 15-ADON during bread making process. Food Chem. 2015, 185, 509–516. [Google Scholar] [CrossRef]
- Rani, K.U.; Prasada Rao, U.J.S.; Leelavathi, K.; Haridas Rao, P. Distribution of enzymes in wheat flour mill streams. J. Cereal Sci. 2001, 34, 233–242. [Google Scholar] [CrossRef]
- Samar, M.M.; Neira, M.S.; Resnik, S.L.; Pacin, A. Effect of fermentation on naturally occurring deoxynivalenol (DON) in Argentinean bread processing technology. Food Addit. Contam. 2001, 18, 1004–1010. [Google Scholar] [CrossRef]
- Carlier, L.; Baron, M.; Chamayou, A.; Couarraze, G. Greener pharmacy using solvent-free synthesis: Investigation of the mechanism in the case of dibenzophenazine. Powder Technol. 2013, 240, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Carlier, L.; Baron, M.; Chamayou, A.; Couarraze, G. Use of co-grinding as a solvent-free solid state method to synthesize dibenzophenazines. Tetrahedron Lett. 2011, 52, 4686–4689. [Google Scholar] [CrossRef] [Green Version]

| Samples | Spiking Levels (μg/kg) | ||
|---|---|---|---|
| 300 | 500 | 800 | |
| Mixed dough | 26.02 ± 2.07a | 34.64 ± 5.42a | 61.75 ± 4.88a |
| Fermented dough | 26.37 ± 2.11a | 31.55 ± 4.96a | 54.42 ± 5.16a |
| Chinese steamed bread | 21.33 ± 3.37a | 25.96 ± 3.46a | 52.16 ± 5.69a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wu, L.; Li, W.; Zhang, Y.; Li, J.; Hu, X.; Sun, L.; Du, W.; Wang, B. Conversion of Deoxynivalenol-3-Glucoside to Deoxynivalenol during Chinese Steamed Bread Processing. Toxins 2020, 12, 225. https://doi.org/10.3390/toxins12040225
Zhang H, Wu L, Li W, Zhang Y, Li J, Hu X, Sun L, Du W, Wang B. Conversion of Deoxynivalenol-3-Glucoside to Deoxynivalenol during Chinese Steamed Bread Processing. Toxins. 2020; 12(4):225. https://doi.org/10.3390/toxins12040225
Chicago/Turabian StyleZhang, Huijie, Li Wu, Weixi Li, Yan Zhang, Jingmei Li, Xuexu Hu, Lijuan Sun, Wenming Du, and Bujun Wang. 2020. "Conversion of Deoxynivalenol-3-Glucoside to Deoxynivalenol during Chinese Steamed Bread Processing" Toxins 12, no. 4: 225. https://doi.org/10.3390/toxins12040225




