Cyclophilin D Promotes Acute, but Not Chronic, Kidney Injury in a Mouse Model of Aristolochic Acid Toxicity
Abstract
:1. Introduction
2. Results
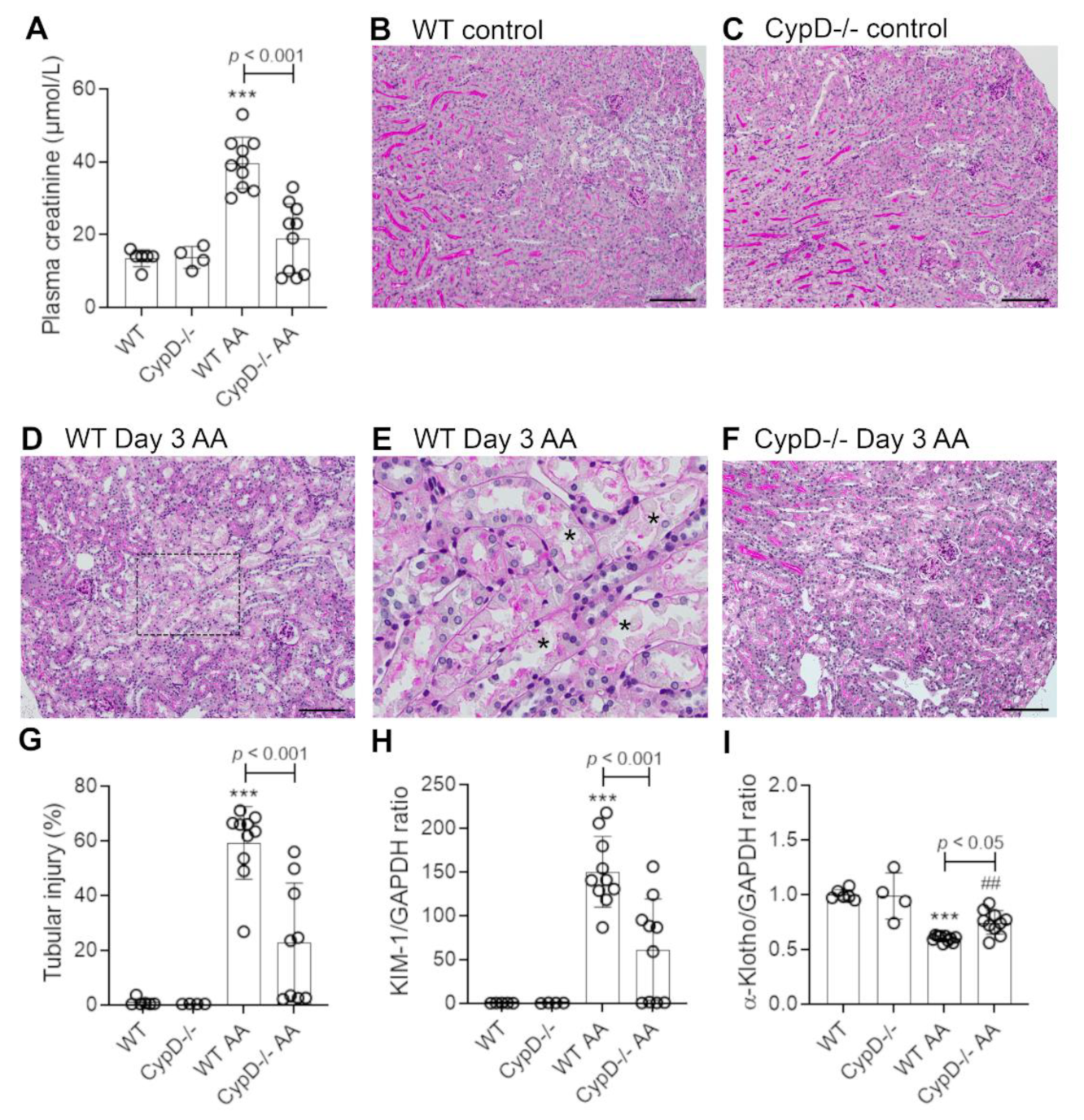
2.1. Cyclophilin D Deletion Protects against Aristolochic Acid-Induced Acute Kidney Injury
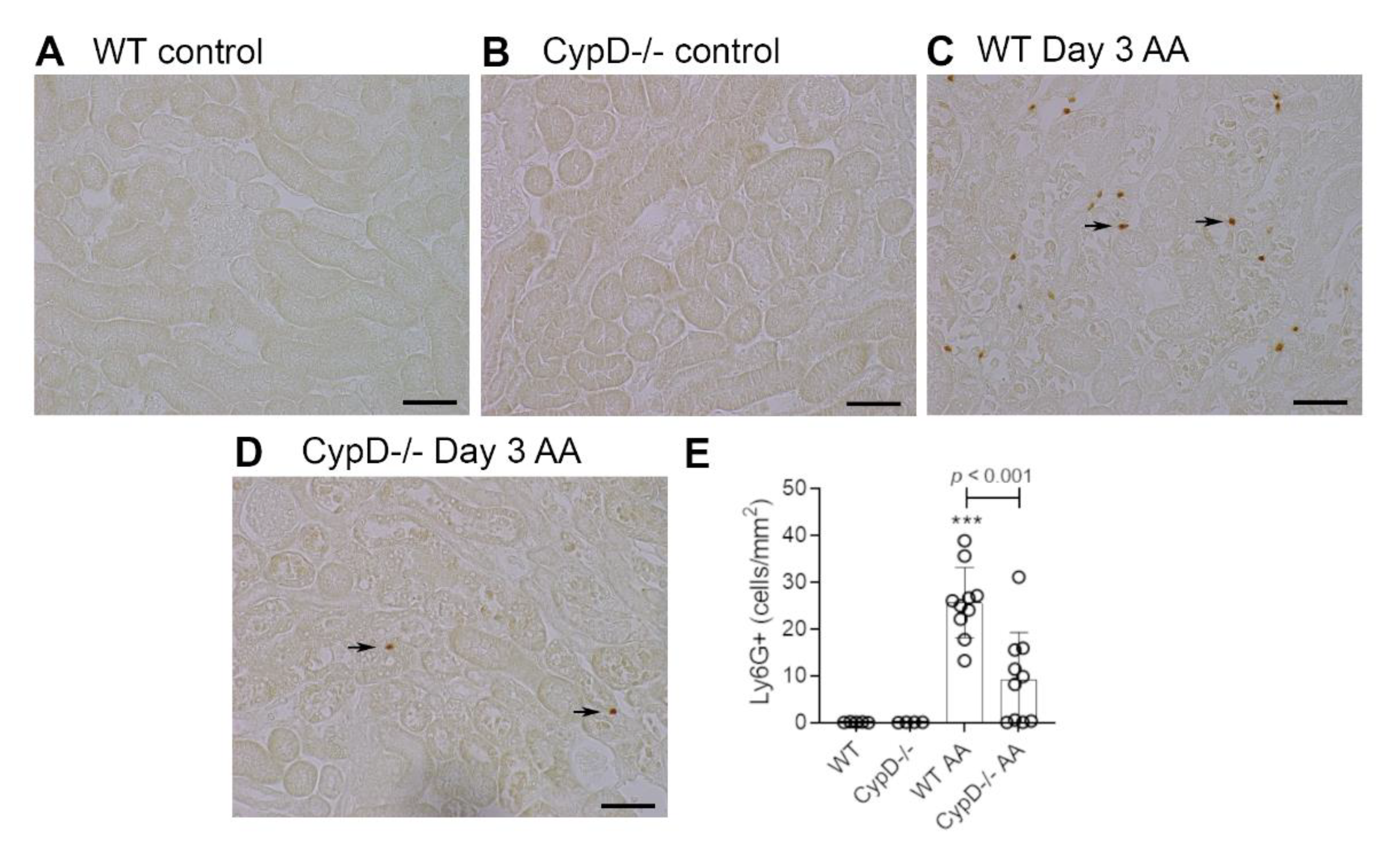
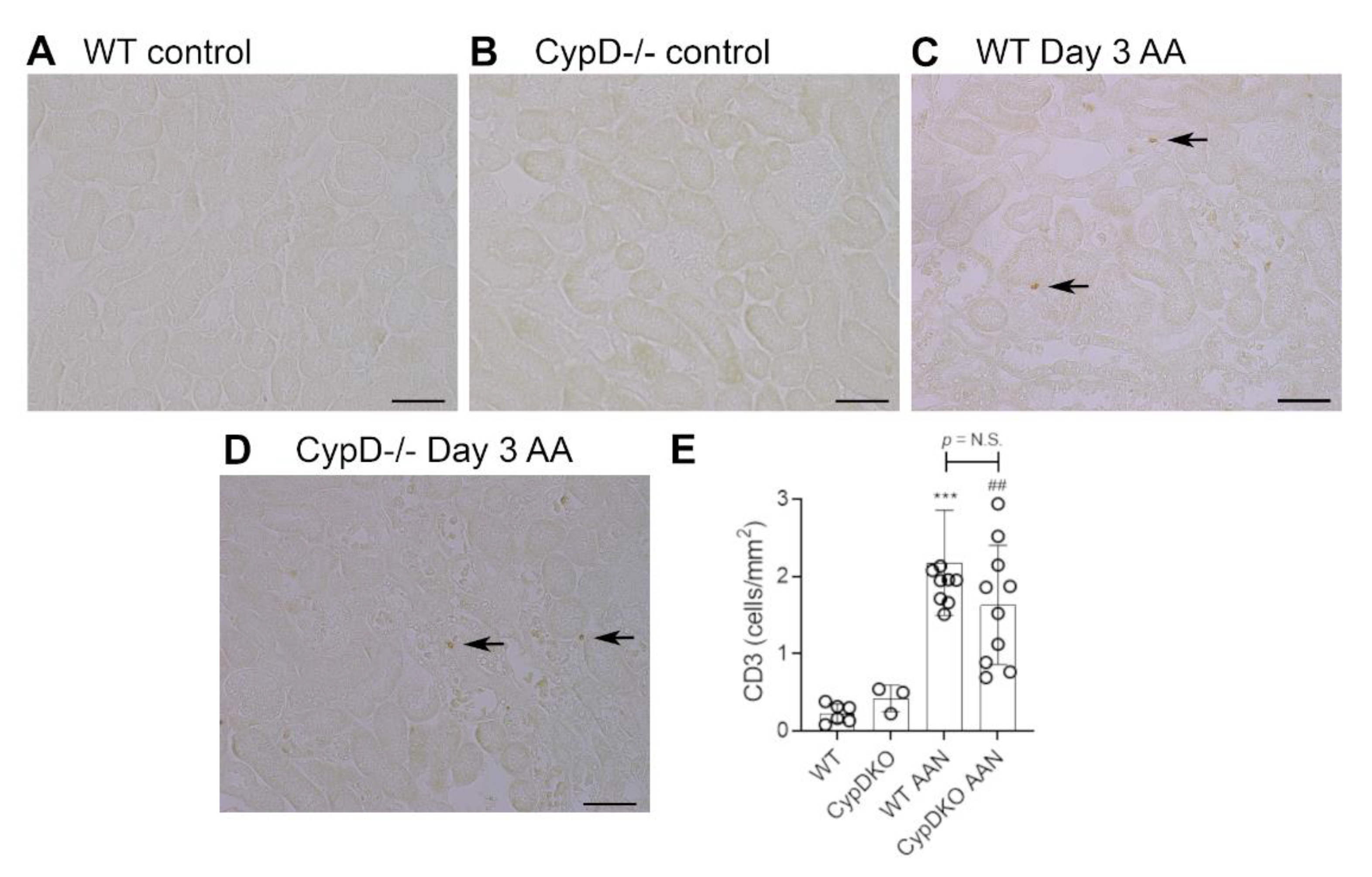
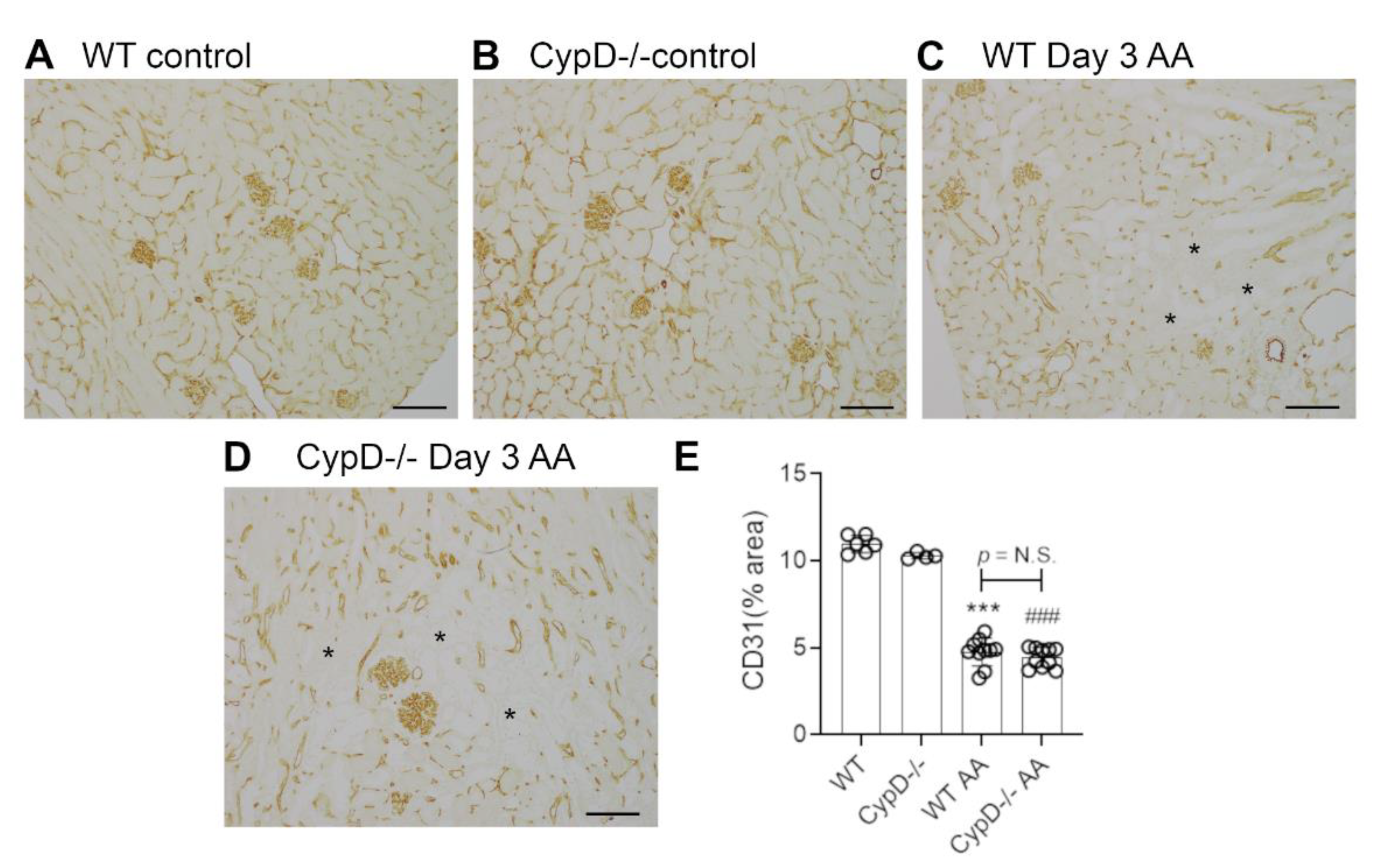
2.2. Cyclophilin D Deletion Protects against Acute Aristolochic Acid-Induced Leukocyte Infiltration
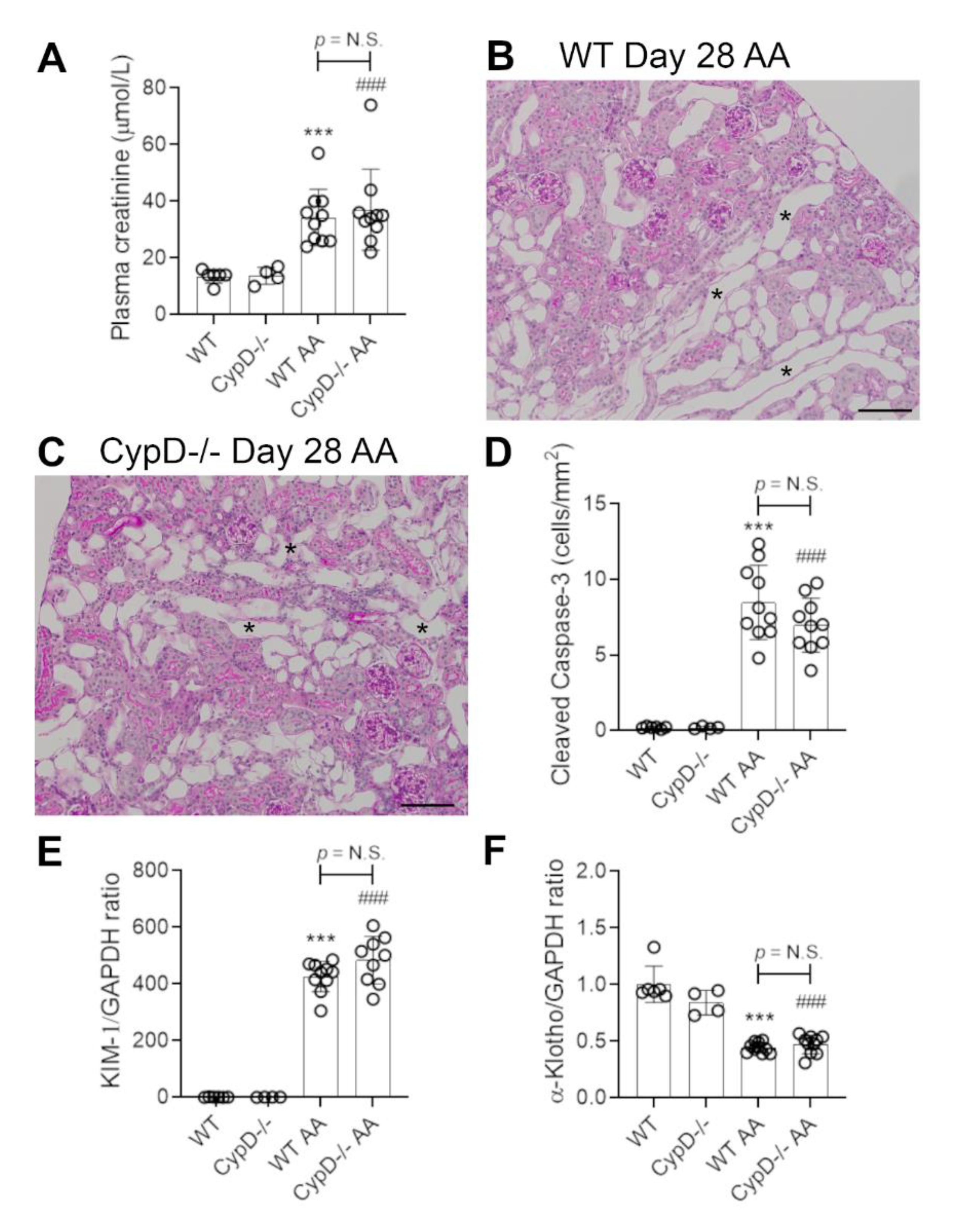
2.3. Cyclophilin D Deletion Does Not Protect against Chronic Aristolochic Acid-Induced Kidney Disease
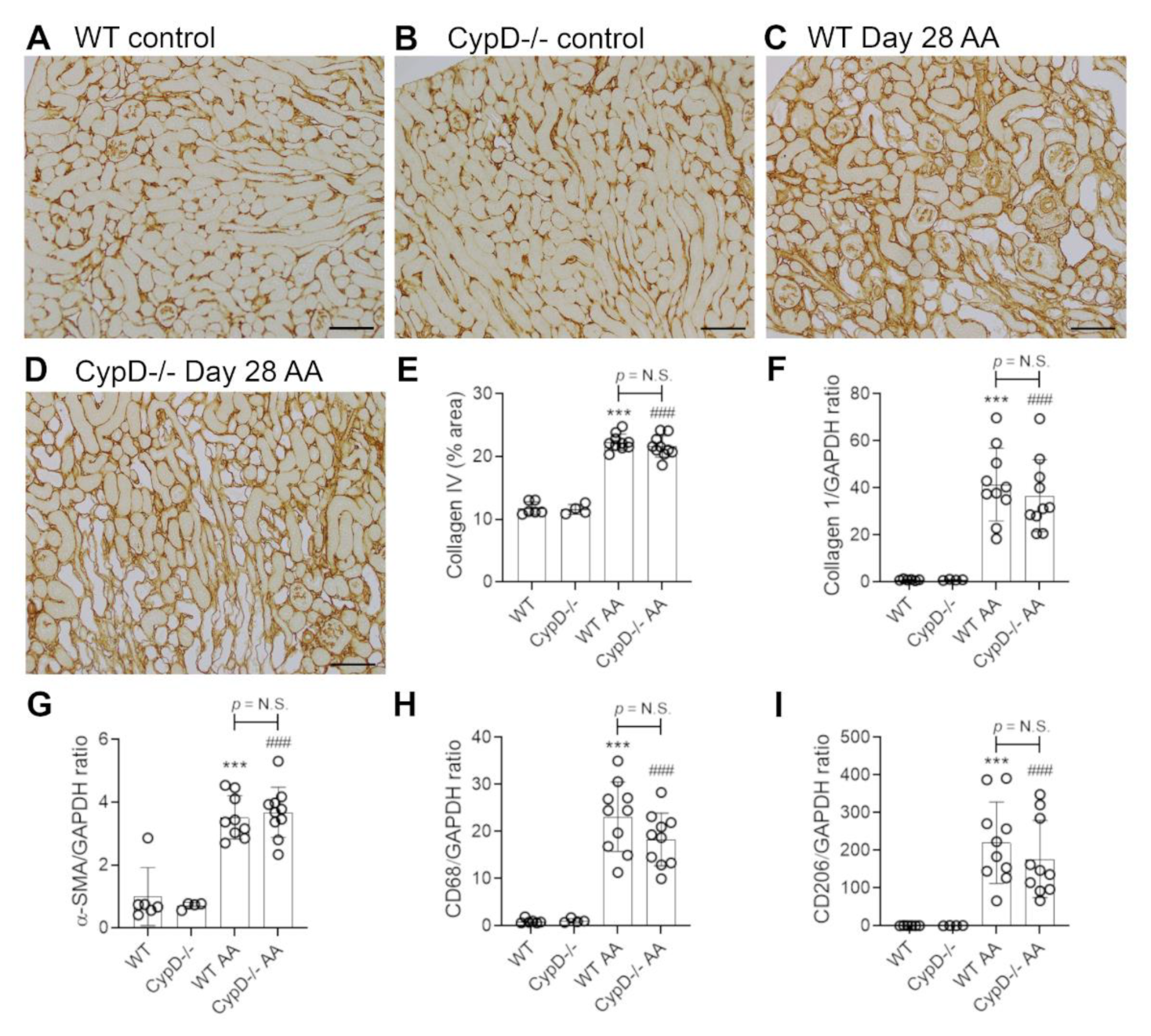
2.4. Cyclophilin D Deletion Does Not Protect against Chronic Aristolochic Acid-Induced Renal Fibrosis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Mice
5.3. Aristolochic Acid-Induced Acute and Chronic Kidney Injury
5.4. Histology
5.5. Immunostaining
5.6. Real Time Polymerase Chain Reaction
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jadot, I.I.; Declèves, A.-E.; Nortier, J.; Caron, N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debelle, F.D.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Xie, Y.; Guo, M.; Rosner, M.H.; Yang, H.; Ronco, C. Nephrotoxicity and Chinese Herbal Medicine. Clin. J. Am. Soc. Nephrol. 2018, 13, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Su, T.; Li, X.-M.; Wang, X.; Cai, S.-Q.; Meng, L.-Q.; Zou, W.-Z.; Wang, H.-Y. Aristolochic acid nephropathy: Variation in presentation and prognosis. Nephrol. Dial. Transplant. 2011, 27, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhiya, N.; Arlt, V.M.; Bahn, A.; Burckhardt, G.; Phillips, D.; Glatt, H. Molecular evidence for an involvement of organic anion transporters (OATs) in aristolochic acid nephropathy. Toxicology 2009, 264, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Y.; Wu, T.S.; Chen, T.W.; Liu, B.H. Aristolochic acid I induced oxidative DNA damage associated with glutathione depletion and ERK1/2 activation in human cells. Toxicol. In Vitro 2011, 25, 810–816. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Chung, A.C.K.; Lai, K.N.; Lan, H.Y. Mechanism of chronic aristolochic acid nephropathy: Role of Smad3. Am. J. Physiol. Physiol. 2010, 298, F1006–F1017. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Lai, K.N.; Lan, H.Y. Activation of p53 Promotes Renal Injury in Acute Aristolochic Acid Nephropathy. J. Am. Soc. Nephrol. 2009, 21, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Elrod, J.W.; Molkentin, J.D. Physiologic Functions of Cyclophilin D and the Mitochondrial Permeability Transition Pore. Circ. J. 2013, 77, 1111–1122. [Google Scholar] [CrossRef] [Green Version]
- Devalaraja-Narashimha, K.; Diener, A.M.; Padanilam, B.J. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am. J. Physiol. Physiol. 2009, 297, F749–F759. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.-S.; Noh, M.; Jung, E.-M.; Kim, W.-Y.; Southekal, S.; Guda, C.; Foster, K.W.; Oupicky, D.; Ferrer, F.A.; Padanilam, B.J. Proximal tubule cyclophilin D regulates fatty acid oxidation in cisplatin-induced acute kidney injury. Kidney Int. 2020, 97, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Bräsen, J.H.; Darding, M.; Jin, M.K.; Sanz, A.B.; Heller, J.-O.; De Zen, F.; Weinlich, R.; Ortiz, A.; Walczak, H.; et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2013, 110, 12024–12029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Pasupulati, R.; Feldkamp, T.; Roeser, N.F.; Weinberg, J.M. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am. J. Physiol. Physiol. 2011, 301, F134–F150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, W.; Leong, K.G.; Ozols, E.; Tesch, G.H.; Nikolic-Paterson, D.J.; Ma, F.Y. Cyclophilin D promotes tubular cell damage and the development of interstitial fibrosis in the obstructed kidney. Clin. Exp. Pharmacol. Physiol. 2017, 45, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.K.; Nikolic-Paterson, D.J.; Lan, H.-Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martínek, A.; Zadrazil, J.; Teplan, V. Acute toxic kidney injury. Ren. Fail. 2019, 41, 576–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Bao, Q.; Sun, L.; Huang, X.; Wang, T.; Zhang, S.; Li, H.; Zhang, L. Possible role of mtDNA depletion and respiratory chain defects in aristolochic acid I-induced acute nephrotoxicity. Toxicol. Appl. Pharmacol. 2013, 266, 198–203. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Liu, F.; Dong, Z. Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch. Toxicol. 2014, 88, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wu, J.; Liu, X.; Wu, H.; Fan, J.; Yang, X. The protective role of Nrf2 against aristolochic acid-induced renal tubular epithelial cell injury. Toxicol. Mech. Methods 2020, 30, 580–589. [Google Scholar] [CrossRef]
- Klausner, J.M.; Paterson, I.S.; Goldman, G.; Kobzik, L.; Rodzen, C.; Lawrence, R.; Valeri, C.R.; Shepro, D.; Hechtman, H.B. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am. J. Physiol. Physiol. 1989, 256, F794–F802. [Google Scholar] [CrossRef]
- Ryan, J.; Kanellis, J.; Blease, K.; Ma, F.Y.; Nikolic-Paterson, D.J. Spleen Tyrosine Kinase Signaling Promotes Myeloid Cell Recruitment and Kidney Damage after Renal Ischemia/Reperfusion Injury. Am. J. Pathol. 2016, 186, 2032–2042. [Google Scholar] [CrossRef] [Green Version]
- Singbartl, K.; Ley, K. Protection from ischemia-reperfusion induced severe acute renal failure by blocking E-selectin. Crit. Care Med. 2000, 28, 2507–2514. [Google Scholar] [CrossRef]
- Leong, K.G.; Ozols, E.; Kanellis, J.; Badal, S.S.; Liles, J.T.; Nikolic-Paterson, D.J.; Ma, F.Y. Cyclophilin Inhibition Protects Against Experimental Acute Kidney Injury and Renal Interstitial Fibrosis. Int. J. Mol. Sci. 2020, 22, 271. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ozols, E.; Ma, F.Y.; Leong, K.G.; Tesch, G.H.; Jiang, X.; Nikolic-Paterson, D.J. c-Jun Amino Terminal Kinase Signaling Promotes Aristolochic Acid-Induced Acute Kidney Injury. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, R.S.; Higgins, G.C.; Nguyen, T.-V.; Arnstein, M.; Henstridge, D.C.; Granata, C.; Snelson, M.; Thallas-Bonke, V.; Cooper, M.E.; Forbes, J.M.; et al. Delineating a role for the mitochondrial permeability transition pore in diabetic kidney disease by targeting cyclophilin D. Clin. Sci. 2020, 134, 239–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, K.G.; Ozols, E.; Kanellis, J.; Ma, F.Y.; Nikolic-Paterson, D.J. Cyclophilin D Promotes Acute, but Not Chronic, Kidney Injury in a Mouse Model of Aristolochic Acid Toxicity. Toxins 2021, 13, 700. https://doi.org/10.3390/toxins13100700
Leong KG, Ozols E, Kanellis J, Ma FY, Nikolic-Paterson DJ. Cyclophilin D Promotes Acute, but Not Chronic, Kidney Injury in a Mouse Model of Aristolochic Acid Toxicity. Toxins. 2021; 13(10):700. https://doi.org/10.3390/toxins13100700
Chicago/Turabian StyleLeong, Khai Gene, Elyce Ozols, John Kanellis, Frank Y. Ma, and David J. Nikolic-Paterson. 2021. "Cyclophilin D Promotes Acute, but Not Chronic, Kidney Injury in a Mouse Model of Aristolochic Acid Toxicity" Toxins 13, no. 10: 700. https://doi.org/10.3390/toxins13100700




