Estrogen Receptor β Participates in Alternariol-Induced Oxidative Stress in Normal Prostate Epithelial Cells
Abstract
:1. Introduction
2. Results
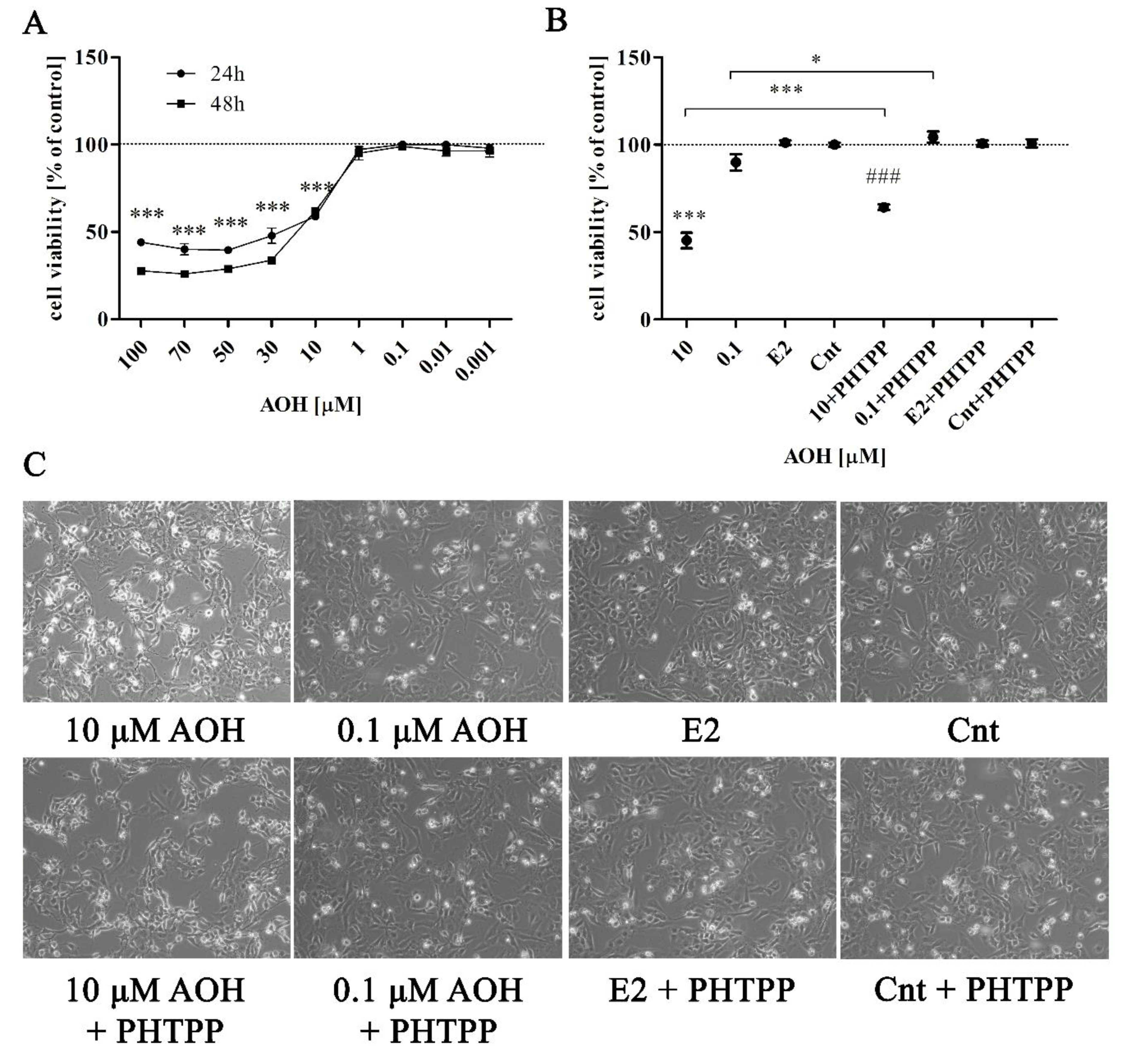
2.1. AOH Significantly Affects Viability of Prostate Epithelial Cells
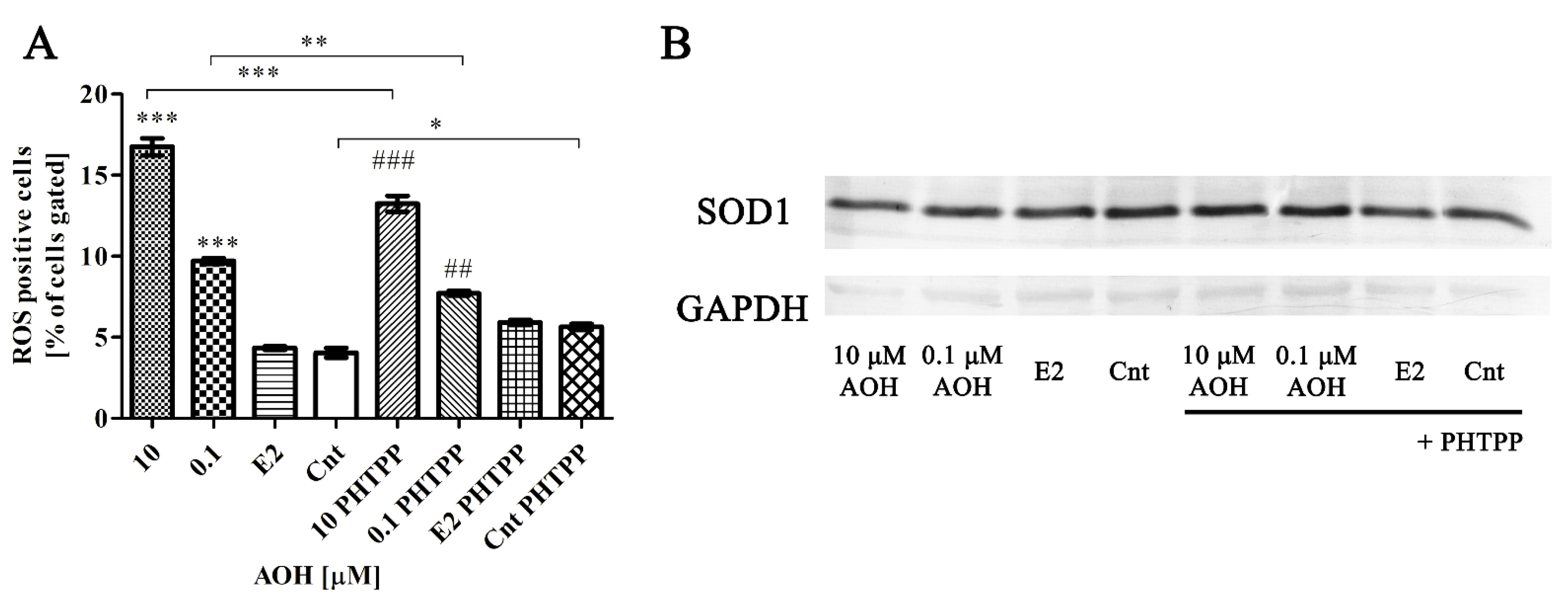
2.2. AOH Induces Oxidative Stress in PNT1A Cells
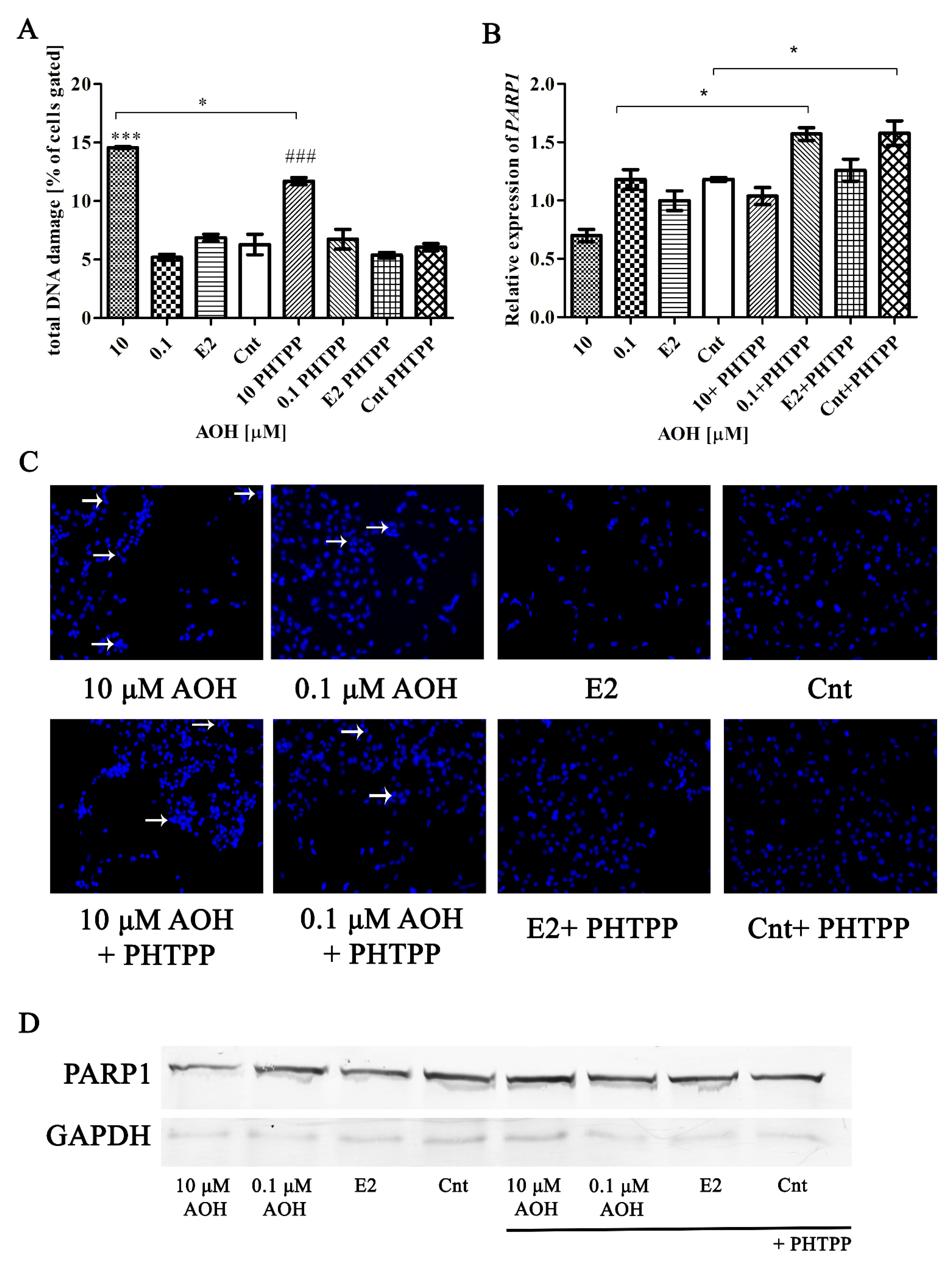
2.3. AOH Induces DNA Damage in PNT1A Cells
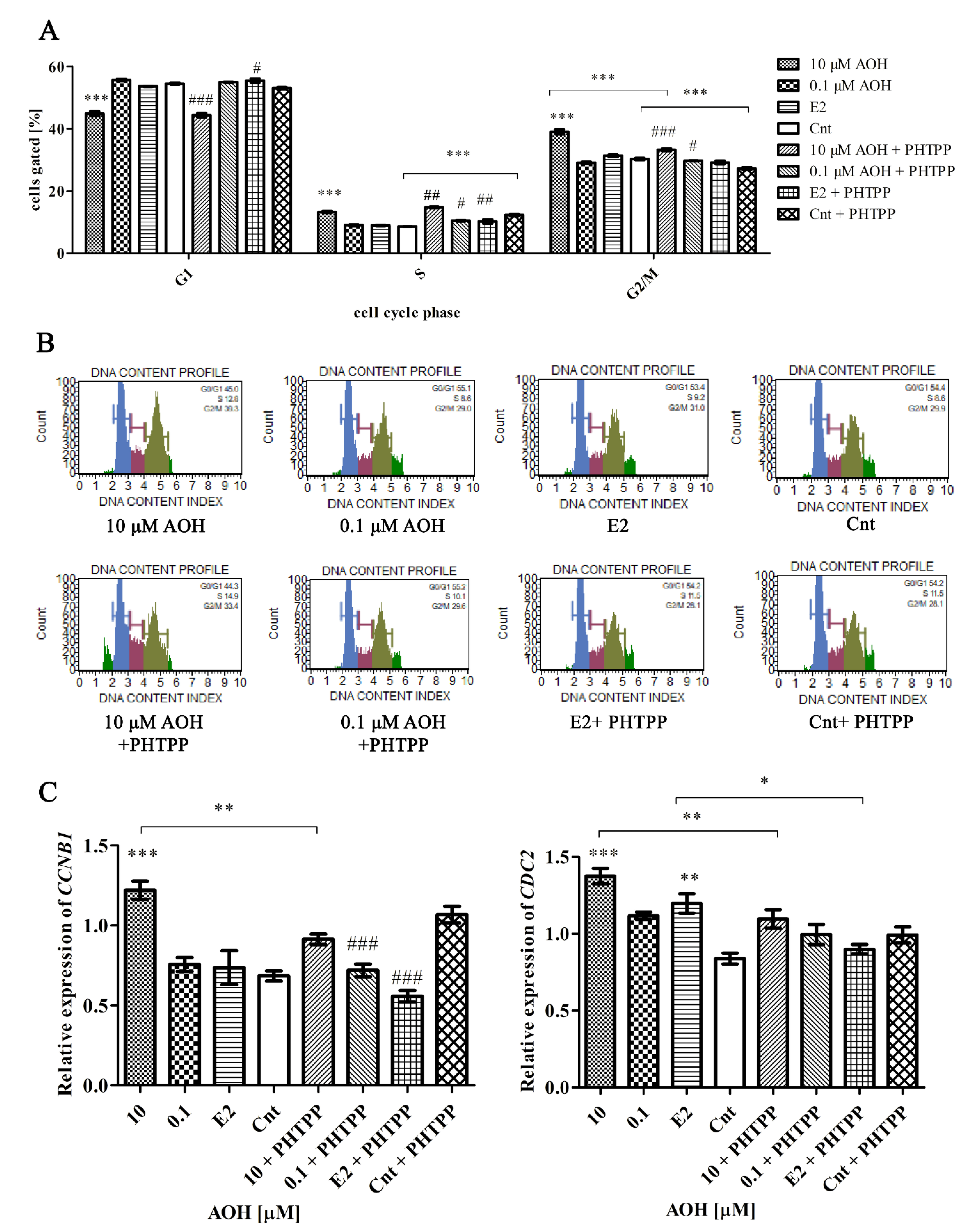
2.4. AOH Induces Cell Cycle Arrest in G2/M Cell Cycle Phase
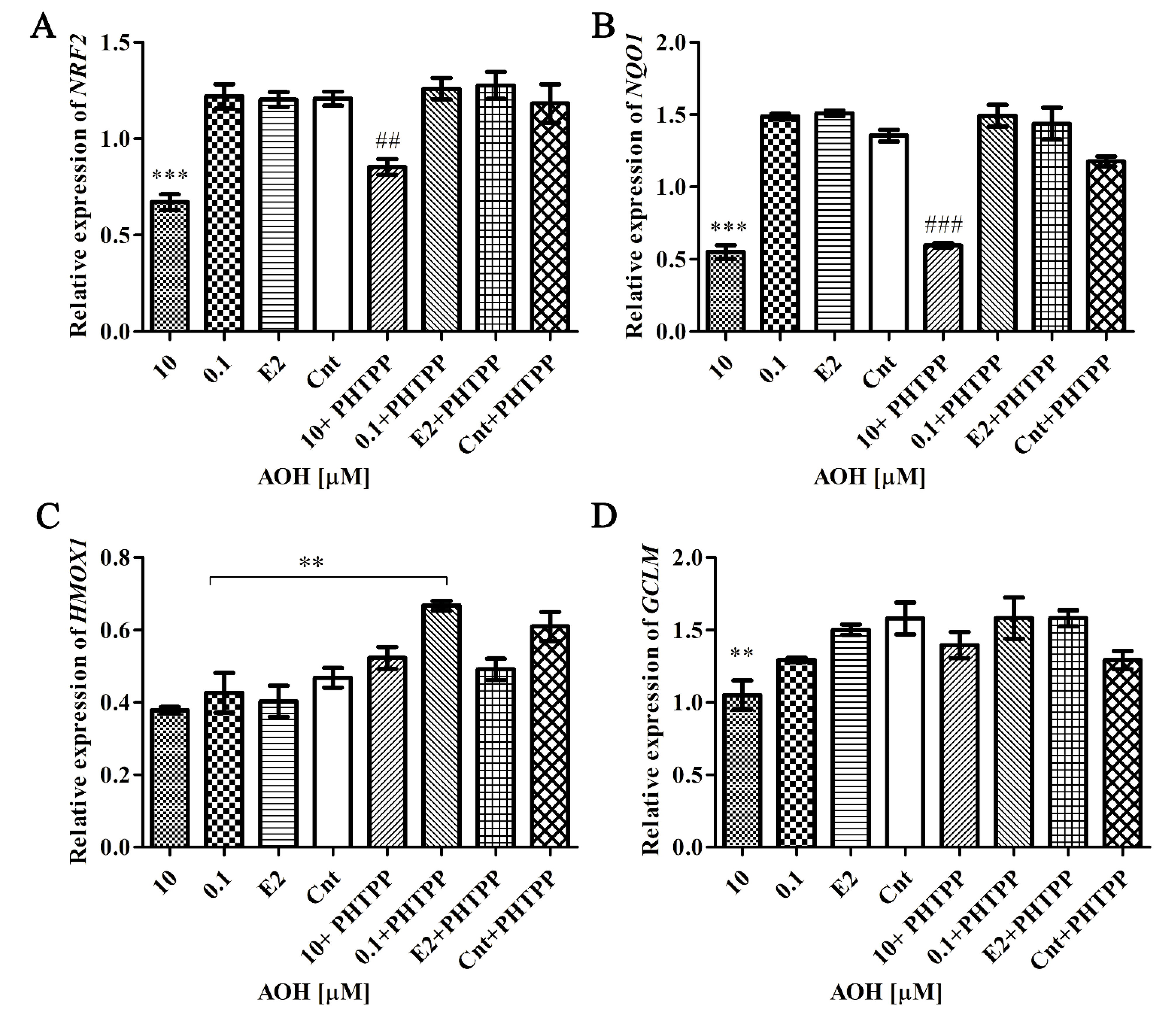
2.5. AOH Modulates Nrf2 Signaling Pathway
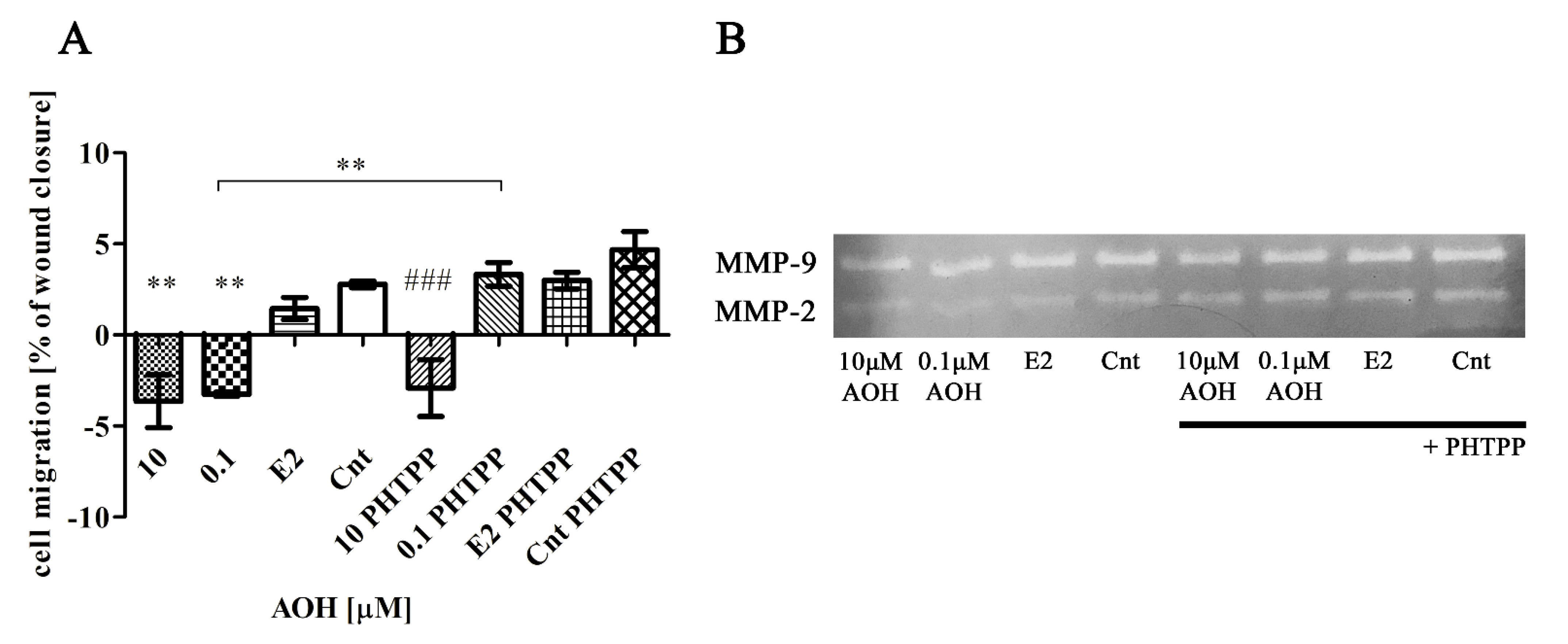
2.6. AOH Modulates Motility of Prostate Epithelial Cells
2.7. AOH Affects Inflammatory Response in PNT1A Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture
5.2. Reagents & Treatments
5.3. Cell Viability and Morphology
5.4. Oxidative Stress, DNA Damage and Cell Cycle Distribution
5.5. Enzyme-Linked Immunosorbent Assay (ELISA)
5.6. Scratch Assay
5.7. Gelatin Zymography
5.8. Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
5.9. Western Blot
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aichinger, G.; Favero, G.; Del Warth, B.; Marko, D. Alternaria toxins—Still emerging? Compr. Rev. Food Sci. Food Saf. 2021, 20, 4390–4406. [Google Scholar] [CrossRef]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol acts as a topoisomerase poison, preferentially affecting the IIα isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef]
- Puntscher, H.; Aichinger, G.; Grabher, S.; Attakpah, E.; Krüger, F.; Tillmann, K.; Motschnig, T.; Hohenbichler, J.; Braun, D.; Plasenzotti, R.; et al. Bioavailability, metabolism, and excretion of a complex Alternaria culture extract versus altertoxin II: A comparative study in rats. Arch. Toxicol. 2019, 93, 3153–3167. [Google Scholar] [CrossRef] [Green Version]
- Solhaug, A.; Vines, L.L.; Ivanova, L.; Spilsberg, B.; Holme, J.A.; Pestkab, J.; Collins, A.; Eriksen, G.S. Mechanisms involved in alternariol-induced cell cycle arrest. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 2012, 738–739, 1–11. [Google Scholar] [CrossRef]
- Solhaug, A.; Wisbech, C.; Christoffersen, T.E.; Hult, L.O.; Lea, T.; Eriksen, G.S.; Holme, J.A. The mycotoxin alternariol induces DNA damage and modify macrophage phenotype and inflammatory responses. Toxicol. Lett. 2015, 239, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, S.; Lawrence, C.B. The Alternaria alternata mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation. Int. J. Mol. Sci. 2017, 18, 1577. [Google Scholar] [CrossRef] [Green Version]
- Stypuła-Trębas, S.; Minta, M.; Radko, L.; Jedziniak, P.; Posyniak, A. Nonsteroidal mycotoxin alternariol is a full androgen agonist in the yeast reporter androgen bioassay. Environ. Toxicol. Pharmacol. 2017, 55, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Wagner, J.; Metzler, M. Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem. Toxicol. 2006, 44, 398–408. [Google Scholar] [CrossRef]
- Aichinger, G.; Krüger, F.; Puntscher, H.; Preindl, K.; Warth, B.; Marko, D. Naturally occurring mixtures of Alternaria toxins: Anti-estrogenic and genotoxic effects in vitro. Arch. Toxicol. 2019, 93, 3021–3031. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2011, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Udensi, U.K.; Tchounwou, P.B. Oxidative stress in prostate hyperplasia and carcinogenesis. J. Exp. Clin. Cancer Res. 2016, 35, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Kusnadi, E.P.; Furic, L.; Selth, L.A. Regulation of mRNA Translation by Hormone Receptors in Breast and Prostate Cancer. Cancers 2021, 13, 3254. [Google Scholar] [CrossRef]
- Božović, A.; Mandušić, V.; Todorović, L.; Krajnović, M. Estrogen Receptor Beta: The Promising Biomarker and Potential Target in Metastases. Int. J. Mol. Sci. 2021, 22, 1656. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, G.; Dellafiora, L.; Pantazi, F.; Del Favero, G.; Galaverna, G.; Dall’Asta, C.; Marko, D. Alternaria toxins as casein kinase 2 inhibitors and possible consequences for estrogenicity: A hybrid in silico/in vitro study. Arch. Toxicol. 2020, 94, 2225–2237. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Domińska, K.; Sakowicz, A.; Piastowska-Ciesielska, A.W. Estrogen receptor β plays a protective role in zearalenone-induced oxidative stress in normal prostate epithelial cells. Ecotoxicol. Environ. Saf. 2019, 172, 504–513. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Urbanek, K.A.; Piastowska-Ciesielska, A.W. ERβ and NFκB—modulators of zearalenone-induced oxidative stress in human prostate cancer cells. Toxins 2020, 12, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollarova, J.; Cenk, E.; Schmutz, C.; Marko, D. The mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation in THP-1 derived macrophages targeting the NF-κB signalling pathway. Arch. Toxicol. 2018, 92, 3347–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, 4654. [Google Scholar]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef]
- Ricke, W.A.; McPherson, S.J.; Bianco, J.J.; Cunha, G.R.; Wang, Y.; Risbridger, G.P. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008, 22, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Christoforou, P.; Christopoulos, P.F.; Koutsilieris, M. The role of estrogen receptor β in prostate cancer. Mol. Med. 2014, 20, 427–434. [Google Scholar] [CrossRef]
- Miró, A.M.; Sastre-Serra, J.; Pons, D.G.; Valle, A.; Roca, P.; Oliver, J. 17β-Estradiol regulates oxidative stress in prostate cancer cell lines according to ERalpha/ERbeta ratio. J. Steroid Biochem. Mol. Biol. 2011, 123, 133–139. [Google Scholar] [CrossRef]
- Aichinger, G.; Beisl, J.; Marko, D. Genistein and delphinidin antagonize the genotoxic effects of the mycotoxin alternariol in human colon carcinoma cells. Mol. Nutr. Food Res. 2017, 61, 1600462. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.J. Oxidative DNA damage and disturbance of antioxidant capacity by alternariol in Caco-2 cells. Toxicol. Lett. 2015, 235, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, C.; Fehr, M.; Schwarz, C.; Baechler, S.; Domnanich, K.; Böttler, U.; Pahlke, G.; Marko, D. Modulation of the cellular redox status by the Alternaria toxins alternariol and alternariol monomethyl ether. Toxicol. Lett. 2013, 216, 23–30. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Kozieł, M.J.; Urbanek, K.A.; Domińska, K.; Piastowska-Ciesielska, A.W. Mycotoxin alternariol (AOH) affects viability and motility of mammary breast epithelial cells. Int. J. Mol. Sci. 2021, 22, 696. [Google Scholar] [CrossRef]
- Chotiner, J.Y.; Wolgemuth, D.J.; Wang, P.J. Functions of cyclins and CDKs in mammalian gametogenesis. Biol. Reprod. 2019, 101, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Mu, P.; Deng, Y. Aflatoxin b1 induces neurotoxicity through reactive oxygen species generation, dna damage, apoptosis, and s-phase cell cycle arrest. Int. J. Mol. Sci. 2020, 21, 6517. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.; Li, J.; Samanta, S.; Mercurio, A.M. ERB2 regulation of NF-kB activation in prostate cancer is mediated by HIF-1. Oncotarget 2015, 6, 40247–40254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, H.; Schnherr, E. Proteoglycans of the extracellular matrix and growth control. J. Cell. Physiol. 2001, 189, 266–274. [Google Scholar] [CrossRef]
- Cancemi, P.; Aiello, A.; Accardi, G.; Caldarella, R.; Candore, G.; Caruso, C.; Ciaccio, M.; Cristaldi, L.; Di Gaudio, F.; Siino, V.; et al. The Role of Matrix Metalloproteinases (MMP-2 and MMP-9) in Ageing and Longevity: Focus on Sicilian Long-Living Individuals (LLIs). Mediat. Inflamm. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Leung, Y.-K.; Lam, H.-M.; Wu, S.; Song, D.; Levin, L.; Cheng, L.; Wu, C.-L.; Ho, S.-M. Estrogen receptor β2 and β5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr. Relat. Cancer 2010, 17, 675. [Google Scholar] [CrossRef]
- Powell, I.J.; Chinni, S.R.; Reddy, S.S.; Zaslavsky, A.; Gavande, N. Pro-inflammatory cytokines and chemokines initiate multiple prostate cancer biologic pathways of cellular proliferation, heterogeneity and metastasis in a racially diverse population and underlie the genetic/biologic mechanism of racial disparity: Update. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 34–40. [Google Scholar]
- Schmutz, C.; Cenk, E.; Marko, D. The Alternaria Mycotoxin Alternariol Triggers the Immune Response of IL-1β-stimulated, Differentiated Caco-2 Cells. Mol. Nutr. Food Res. 2019, 63, e1900341. [Google Scholar] [CrossRef] [Green Version]
- Erb, H.H.H.; Langlechner, R.V.; Moser, P.L.; Handle, F.; Casneuf, T.; Verstraeten, K.; Schlick, B.; Schäfer, G.; Hall, B.; Sasser, K.; et al. IL6 sensitizes prostate cancer to the antiproliferative effect of IFNα2 through IRF9. Endocr. Relat. Cancer 2013, 20, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Handle, F.; Puhr, M.; Schaefer, G.; Lorito, N.; Hoefer, J.; Gruber, M.; Guggenberger, F.; Santer, F.; Marques, R.B.; van Weerden, W.M.; et al. The STAT3 inhibitor galiellalactone reduces IL6-mediated AR activity in benign and malignant prostate models. Mol. Cancer Ther. 2018, 17, 2722–2731. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Luo, Y.; Tai, R.; Zhang, N. Estrogen receptor β suppresses inflammation and the progression of prostate cancer. Mol. Med. Rep. 2019, 49, 3555–3563. [Google Scholar] [CrossRef]
- Bansal, M.; Singh, N.; Alam, S.; Pal, S.; Satyanarayana, G.N.V.; Singh, D.; Ansari, K.M. Alternariol induced proliferation in primary mouse keratinocytes and inflammation in mouse skin is regulated via PGE2/EP2/cAMP/p-CREB signaling pathway. Toxicology 2019, 412, 79–88. [Google Scholar] [CrossRef]
- Del Favero, G.; Mayer, R.M.; Dellafiora, L.; Janker, L.; Niederstaetter, L.; Dall’Asta, C.; Gerner, C.; Marko, D. Structural Similarity with Cholesterol Reveals Crucial Insights into Mechanisms Sustaining the Immunomodulatory Activity of the Mycotoxin Alternariol. Cells 2020, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Dellafiora, L.; Warth, B.; Schmidt, V.; Del Favero, G.; Mikula, H.; Fröhlich, J.; Marko, D. An integrated in silico/in vitro approach to assess the xenoestrogenic potential of Alternaria mycotoxins and metabolites. Food Chem. 2018, 248, 253–261. [Google Scholar] [CrossRef]

| 10 µM AOH | 0.1 µM AOH | E2 | Cnt | 10 µM AOH + PHTPP | 0.1 µM AOH + PHTPP | E2 + PHTPP | Cnt + PHTPP | |
|---|---|---|---|---|---|---|---|---|
| SOD1 | 1.652 | 1.288 | 1.029 | 1.000 | 0.870 | 0.935 | 1.159 | 1.224 |
| Gene | 10 µM AOH | 0.1 µM AOH | E2 | Cnt | 10 µM AOH + PHTPP | 0.1 µM AOH + PHTPP | E2 + PHTPP | Cnt + PHTPP |
|---|---|---|---|---|---|---|---|---|
| SOD1 | 1.97 | 2.45 | 2.25 | 2.37 | 2.04 | 2.98 | 2.51 | 2.58 |
| HIF-1α | 0.74 | 0.94 | 0.80 | 0.86 | 1.07 ** | 0.99 | 0.84 | 0.83 |
| RelA | 13.91 | 21.08 | 16.61 | 18.08 | 27.61 *** | 29.09 | 22.83 | 32.63 |
| 10 µM AOH | 0.1 µM AOH | E2 | Cnt | 10 µM AOH + PHTPP | 0.1 µM AOH + PHTPP | E2 + PHTPP | Cnt + PHTPP | |
|---|---|---|---|---|---|---|---|---|
| Cleaved-PARP1 | 0.616 | 0.732 | 0.625 | 1.000 | 0.767 | 0.868 | 0.847 | 0.846 |
| Gene | 10 µM AOH | 0.1 µM AOH | E2 | Cnt | 10 µM AOH + PHTPP | 0.1 µM AOH + PHTPP | E2 + PHTPP | Cnt + PHTPP |
|---|---|---|---|---|---|---|---|---|
| MMP-2 | 81.86 | 69.76 | 86.22 | 100.0 | 79.47 | 84.82 | 93.27 | 91.63 |
| MMP-9 | 91.16 | 94.22 | 100.9 | 100.0 | 98.74 | 110.6 | 98.44 | 96.52 |
| Gene | Sequence (5′-3′) | Product Size [bp] |
|---|---|---|
| CDC2 | For TTTTCAGAGCTTTGGGCACT Rev AGGCTTCCTGGTTTCCATTT | 100 |
| CCNB1 | For ACCTATGCTGGTGCCAGTG Rev GGCTTGGAGAGCAGTA | 128 |
| GCLM | For TGTGCAACTCCAAGGACTGA Rev ACAGCGAGGAGCTTCATGAT | 247 |
| HIF-1α | For TTACTCATCCATGTGACCATGA Rev AGTTCTTCCTCGGCTAGTTAG | 140 |
| HMOX1 | For CAGCTCCTGCAACTCCTCAAA Rev TTCTTCACCTTCCCCAACATTG | 165 |
| H3F3A | For AGGACTTTAAAAGATCTGCGCTTCCAGAG Rev ACCAGATAGGCCTCACTTGCCTCCTGC | 74 |
| IL-1β | For GGCAATGAGGATGACTTGTT Rev TGCTGTAGTGGTGGTCGGA | 127 |
| IL6 | For GGATGCTTCCAATCTGGATTCA Rev TCTGGCTTGTTCCTCACTACT | 126 |
| NQO1 | For CCAGGATTTGAATTCGGGCG Rev AGGACCCTTCCGGAGTAAGA | 212 |
| NRF2 | For GTCACATCGAGAGCCCAGTC Rev ACCATGGTAGTCTCAACCAGC | 193 |
| PARP1 | For TCTTCAAGAGCGATGCCTATT Rev TGAGGTAAGAGATTTCTCGGAA | 129 |
| RelA | For GCACAGATACCACCAAGACC Rev TCAGCCTCATAGAAGCCATC | 157 |
| RPLP0 | For ACGGATTACACCTTCCCACTTGCTAAAAGGTC Rev AGCCACAAAGGCAGATGGATCAGCCAAG | 69 |
| RPS17 | For AAGCGCGTGTGCGAGGAGATCG Rev TCGCTTCATCAGATGCGTGACATAACCTG | 87 |
| SOD1 | For GCGTGGCCTAGCGAGTTAT Rev. ACACCTTCACTGGTCCATTACT | 114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, K.; Kozieł, M.J.; Urbanek, K.A.; Habrowska-Górczyńska, D.E.; Domińska, K.; Piastowska-Ciesielska, A.W. Estrogen Receptor β Participates in Alternariol-Induced Oxidative Stress in Normal Prostate Epithelial Cells. Toxins 2021, 13, 766. https://doi.org/10.3390/toxins13110766
Kowalska K, Kozieł MJ, Urbanek KA, Habrowska-Górczyńska DE, Domińska K, Piastowska-Ciesielska AW. Estrogen Receptor β Participates in Alternariol-Induced Oxidative Stress in Normal Prostate Epithelial Cells. Toxins. 2021; 13(11):766. https://doi.org/10.3390/toxins13110766
Chicago/Turabian StyleKowalska, Karolina, Marta Justyna Kozieł, Kinga Anna Urbanek, Dominika Ewa Habrowska-Górczyńska, Kamila Domińska, and Agnieszka Wanda Piastowska-Ciesielska. 2021. "Estrogen Receptor β Participates in Alternariol-Induced Oxidative Stress in Normal Prostate Epithelial Cells" Toxins 13, no. 11: 766. https://doi.org/10.3390/toxins13110766






