First Report of Domoic Acid Production from Pseudo-nitzschia multistriata in Paracas Bay (Peru)
Abstract
:1. Introduction
2. Results
2.1. Morphological Analysis
2.2. Molecular Analysis
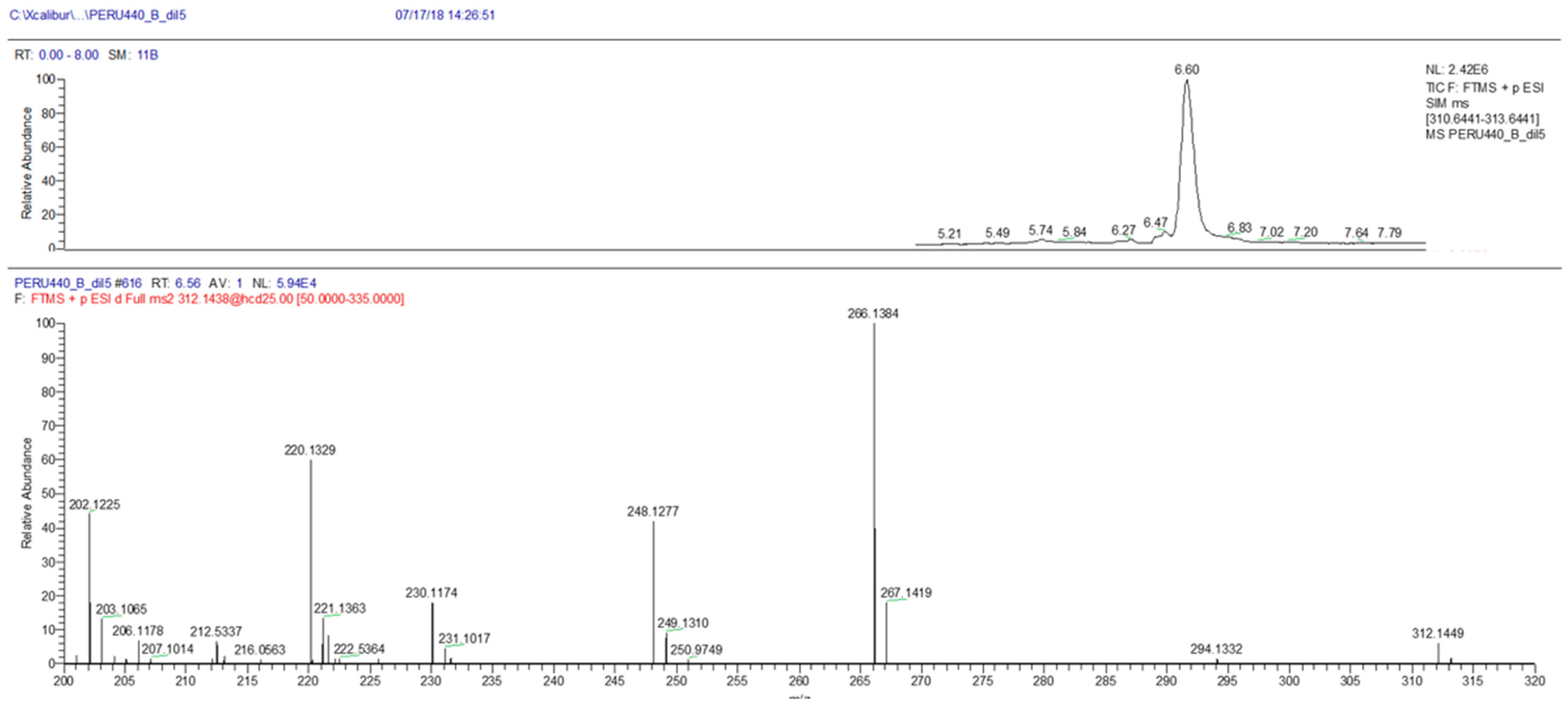
2.3. Toxin Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Biological Samples and Establishment of Cultures
5.2. Morphological Analysis
5.3. Molecular and Phylogenetic Analysis
5.4. Sample Preparation and Toxin Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasle, G.R. Are most of the domoic acid-producing species of the diatom genus Pseudo-nitzschia cosmopolites? Harmful Algae 2002, 1, 137–146. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Chen, X.M.; Pang, J.X.; Huang, C.X.; Lundholm, N.; Teng, S.T.; Li, A.; Li, Y. Two New and Nontoxigenic Pseudo-nitzschia species (Bacillariophyceae) from Chinese Southeast Coastal Waters. J. Phycol. 2021, 57, 335–344. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Available online: http://www.algaebase.org (accessed on 1 March 2021).
- Lundholm, N. Bacillariophyceae, in IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Available online: http://www.marinespecies.org/hab (accessed on 25 February 2021).
- Coleman, A.W. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet. 2003, 19, 370–375. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.I.; Garcés, E.; Lundholm, N.; Moestrup, Ø.; Andree, K.; Camp, J. Morphology, physiology, molecular phylogeny and sexual compatibility of the cryptic Pseudo-nitzschia delicatissima complex (Bacillariophyta), including the description of P. arenysensis sp. nov. Phycologia 2009, 48, 492–509. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.I.; Olivos-Ortiz, A.; Garcia-Mendoza, E.; Sánchez-Bravo, Y.; Sosa-Avalos, R.; Salas Marias, N.; Lim, H.C. Phylogenetic relationships of Pseudo-nitzschia subpacifica (Bacillariophyceae) from the Mexican Pacific, and its production of domoic acid in culture. PLoS ONE 2020, 15, e0231902. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, N.; Moestrup, Ø.; Hasle, G.R.; Hoef-Emden, K. A study of the Pseudonitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): What is P. pseudodelicatissima? J. Phycol. 2003, 39, 797–813. [Google Scholar] [CrossRef]
- Lundholm, N.; Bates, S.S.; Baugh, K.A.; Bill, B.D.; Connell, L.B.; Léger, C.; Trainer, V.L. Cryptic and pseudo-cryptic diversity in diatoms—with descriptions of pseudo-nitzschia hasleana sp. nov. and p. fryxelliana sp. nov. 1. J. Phycol. 2012, 48, 436–454. [Google Scholar] [CrossRef]
- Amato, A.; Kooistra, W.H.; Ghiron, J.H.L.; Mann, D.G.; Pröschold, T.; Montresor, M. Reproductive isolation among sympatric cryptic species in marine diatoms. Protist 2007, 158, 193–207. [Google Scholar] [CrossRef]
- Bates, S.; Bird, C.J.; Freitas, A.d.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; Mc Culloch, A.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Perl, T.M.; Bédard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl. J. Med. 1990, 322, 1775–1780. [Google Scholar] [CrossRef]
- Wright, J.; Boyd, R.; Freitas, A.d.; Falk, M.; Foxall, R.; Jamieson, W.; Laycock, M.; McCulloch, A.; McInnes, A.; Odense, P. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- Bates, S. Toxic phytoplankton on the Canadian east coast: Implications for aquaculture. Bull. Aquacult. Assoc. Can. 1997, 97, 9–18. [Google Scholar]
- La Barre, S.; Bates, S.S.; Quilliam, M.A. Domoic acid. In Outstanding Marine Molecules: Chemistry, Biology, Analysis; La Barre, S., Kornprobst, J.M., Eds.; Wiley-VCH Verlag GmbH & KgaA: Weinheim, Germany, 2014; pp. 189–216. [Google Scholar]
- Goldberg, J.D. Domoic Acid in the Benthic Food Web of Monterey Bay, California. Master’s Thesis, University Monterey Bay, Seaside, CA, USA, 2003. [Google Scholar]
- Zabaglo, K.; Chrapusta, E.; Bober, B.; Kaminski, A.; Adamski, M.; Bialczyk, J. Environmental roles and biological activity of domoic acid: A review. Algal Res. 2016, 13, 94–101. [Google Scholar] [CrossRef]
- Bates, S.S.; Garrison, D.L.; Horner, R.A. Bloom dynamics and physiology of domoic-acid-producing Pseudo-nitzschia species. Nato Asi Ser. G Ecol. Sci. 1998, 41, 267–292. [Google Scholar]
- Lefebvre, K.A.; Frame, E.R.; Kendrick, P.S. Domoic acid and fish behavior: A review. Harmful Algae 2012, 13, 126–130. [Google Scholar] [CrossRef]
- D’Agostino, V.C.; Degrati, M.; Sastre, V.; Santinelli, N.; Krock, B.; Krohn, T.; Dans, S.L.; Hoffmeyer, M.S. Domoic acid in a marine pelagic food web: Exposure of southern right whales Eubalaena australis to domoic acid on the Peninsula Valdes calving ground, Argentina. Harmful Algae 2017, 68, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Di Liberto, T. This summer’s West Coast algal bloom was unusual. What would Usual Look Like? 30 September 2015. [Google Scholar]
- McCabe, R.M.; Hickey, B.M.; Kudela, R.M.; Lefebvre, K.A.; Adams, N.G.; Bill, B.D.; Gulland, F.M.; Thomson, R.E.; Cochlan, W.P.; Trainer, V.L. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10366–310376. [Google Scholar] [CrossRef]
- Ritzman, J.; Brodbeck, A.; Brostrom, S.; McGrew, S.; Dreyer, S.; Klinger, T.; Moore, S.K. Economic and sociocultural impacts of fisheries closures in two fishing-dependent communities following the massive 2015 US West Coast harmful algal bloom. Harmful Algae 2018, 80, 35–45. [Google Scholar] [CrossRef]
- Du, X.; Peterson, W.; Fisher, J.; Hunter, M.; Peterson, J. Initiation and development of a toxic and persistent Pseudo-nitzschia bloom off the Oregon coast in spring/summer 2015. PLoS ONE 2016, 11, e0163977. [Google Scholar] [CrossRef]
- Arévalo, F.; Bermúdez de la Puente, M.; Salgado, C. Seguimiento de biotoxinas marinas en las Rías Gallegas: Control y evolución durante los años 1995–1996. V Reunión Ibérica de Fitoplancton Tóxico y Biotoxinas. ANFACO-CECOPESCA, Vigo 1997, 90–101. [Google Scholar]
- Blanco, J.; Acosta, C.; De La Puente, M.B.; Salgado, C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop Pecten maximus. Aquat. Toxicol. 2002, 60, 111–121. [Google Scholar] [CrossRef]
- Mauriz, A.; Blanco, J. Distribution and linkage of domoic acid (amnesic shellfish poisoning toxins) in subcellular fractions of the digestive gland of the scallop Pecten maximus. Toxicon 2010, 55, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Mauríz, A.; Álvarez, G. Distribution of Domoic Acid in the Digestive Gland of the King Scallop Pecten maximus. Toxins 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Calienes, R.; Guillén, O.; Lostaunau, N. Variabilidad espacio-temporal de clorofila, producción primaria y nutrientes frente a la costa peruana. Boletin Instituto del Mar del Peru 1985, 10, 1–44. [Google Scholar]
- Graco, M.; Ledesma, J.; Flores, G.; Giron, M. Nutrients, oxygen and biogeochemical processes in the Humboldt upwelling current system off Peru. Rev. Peru. Biol 2007, 14, 117–128. [Google Scholar]
- Echevin, V.; Gévaudan, M.; Espinoza-Morriberón, D.; Tam, J.; Aumont, O.; Gutierrez, D.; Colas, F. Physical and biogeochemical impacts of RCP8. 5 scenario in the Peru upwelling system. Biogeosciences 2020, 17, 3317–3341. [Google Scholar] [CrossRef]
- Bakun, A.; Weeks, S.J. The marine ecosystem off Peru: What are the secrets of its fishery productivity and what might its future hold? Prog. Oceanogr. 2008, 79, 290–299. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Jiménez, A.B.; Kudela, R.M.; Reguera, B. Harmful algal blooms in eastern boundary upwelling systems. Oceanography 2010, 30, 22. [Google Scholar] [CrossRef]
- Trainer, V.L.; Pitcher, G.C.; Reguera, B.; Smayda, T.J. The distribution and impacts of harmful algal bloom species in eastern boundary upwelling systems. Prog. Oceanogr. 2010, 85, 33–52. [Google Scholar] [CrossRef]
- Hasle, G.R. Nitzschia and Fragilariopsis species studied in the light and electron microscopes. II. The group Pseudonitzschia. Skr. Nor. Vidensk-Akad. I. Mat.-Nat. Kl. Ny Ser. 1965, 18, 1–45. [Google Scholar]
- Tenorio, C.; Uribe, E.; Gil-Kodaka, P.; Blanco, J.; Álvarez, G. Morphological and toxicological studies ofPseudo-nitzschiaspecies from the central coast of Peru. Diatom Res. 2016, 31, 331–338. [Google Scholar] [CrossRef]
- Álvarez, G.; Uribe, E.; Quijano-Scheggia, S.; López-Rivera, A.; Mariño, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, A.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol 2019. [Google Scholar] [CrossRef]
- Fire, S.E.; Adkesson, M.J.; Wang, Z.; Jankowski, G.; Cárdenas-Alayza, S.; Broadwater, M. Peruvian fur seals (Arctocephalus australis ssp.) and South American sea lions (Otaria byronia) in Peru are exposed to the harmful algal toxins domoic acid and okadaic acid. Mar. Mammal Sci. 2017, 33, 630–644. [Google Scholar] [CrossRef]
- Kluger, L.C.; Taylor, M.H.; Wolff, M.; Stotz, W.; Mendo, J. From an open-access fishery to a regulated aquaculture business: The case of the most important Latin American bay scallop (Argopecten purpuratus). Rev. Aquac. 2019, 11, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Lü, S.; Li, Y.; Lundholm, N.; Ma, Y.; Ho, K. Diversity, taxonomy and biogeographical distribution of the genus Pseudo-nitzschia (Bacillariophyceae) in Guangdong coastal waters, South China Sea. Nova Hedwig. 2012, 95, 123–152. [Google Scholar] [CrossRef]
- Sahraoui, I.; Grami, B.; Bates, S.S.; Bouchouicha, D.; Chikhaoui, M.A.; Mabrouk, H.H.; Hlaili, A.S. Response of potentially toxic Pseudo-nitzschia (Bacillariophyceae) populations and domoic acid to environmental conditions in a eutrophied, SW Mediterranean coastal lagoon (Tunisia). Estuar. Coast. Shelf Sci. 2012, 102, 95–104. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.; Garcés, E.; Andree, K.B.; De la Iglesia, P.; Diogène, J.; Fortuño, J.M.; Camp, J. Pseudo-nitzschia species on the Catalan coast: Characterization and contribution to the current knowledge of the distribution of this genus in the Mediterranean Sea. Sci. Mar. 2010, 74, 395–410. [Google Scholar] [CrossRef]
- Quijano-Sheggia, S.; Garcés, E.; Sampedro, N.; Van Lenning, K.; Flo Arcas, E.; Andree, K.; Fortuño Alós, J.M.; Camp, J. Identification and characterisation of the dominant Pseudo-nitzschia species (Bacillariophyceae) along the NE Spanish coast (Catalonia, NW Mediterranean). Sci. Mar. 2008, 72, 343–359. [Google Scholar]
- Orsini, L.; Sarno, D.; Procaccini, G.; Poletti, R.; Dahlmann, J.; Montresor, M. Toxic Pseudo-nitzschia multistriata (Bacillariophyceae) from the Gulf of Naples: Morphology, toxin analysis and phylogenetic relationships with other Pseudo-nitzschia species. Eur. J. Phycol. 2002, 37, 247–257. [Google Scholar] [CrossRef]
- Sarno, D. Production of domoic acid in another species of Pseudo-nitzschia: P. multistriata in the Gulf of Naples (Mediterranean Sea). Harmful Algal News 2000, 21, 5. [Google Scholar]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L. Toxin levels and profiles in microalgae from the North-Western Adriatic Sea—15 years of studies on cultured species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Churro, C.I.; Carreira, C.C.; Rodrigues, F.J.; Craveiro, S.C.; Calado, A.J.; Casteleyn, G.; Lundholm, N. Diversity and abundance of potentially toxic Pseudo-nitzschia Peragallo in Aveiro coastal lagoon, Portugal and description of a new variety, P. pungens var. aveirensis var. nov. Diatom Res. 2009, 24, 35–62. [Google Scholar] [CrossRef]
- Yap-Dejeto, L.G.; Omura, T.; Nagahama, Y.; Fukuyo, Y. Observations of eleven Pseudo nitzschia species in Tokyo Bay, Japan. La mer 2010, 48, 1–16. [Google Scholar]
- Rhodes, L.L.; Adamson, J.; Scholin, C. Pseudo-nitzschia multistriata(Bacillariophyceae) in New Zealand. New Zealand J. Mar. Freshw. Res. 2000, 34, 463–467. [Google Scholar] [CrossRef]
- Rivera-Vilarelle, M.; Quijano-Scheggia, S.; Olivos-Ortiz, A.; Gaviño-Rodríguez, J.H.; Castro-Ochoa, F.; Reyes-Herrera, A. The genus Pseudo-nitzschia (Bacillariophyceae) in Manzanillo and Santiago Bays, Colima, Mexico. Bot. Mar. 2013, 56, 357–373. [Google Scholar] [CrossRef]
- Méndez, S.M.; Ferrario, M.; Cefarelli, A.O. Description of toxigenic species of the genus Pseudo-nitzschia in coastal waters of Uruguay: Morphology and distribution. Harmful Algae 2012, 19, 53–60. [Google Scholar] [CrossRef]
- Takano, H. Marine diatom Nitzschia multistriata sp. nov. common at inlets of southern Japan. Diatom 1993, 8, 39–41. [Google Scholar]
- Moschandreou, K.K.; Baxevanis, A.D.; Katikou, P.; Papaefthimiou, D.; Nikolaidis, G.; Abatzopoulos, T.J. Inter- and intra-specific diversity of Pseudo-nitzschia (Bacillariophyceae) in the northeastern Mediterranean. Eur. J. Phycol. 2012, 47, 321–339. [Google Scholar] [CrossRef]
- D’Alelio, D.; Amato, A.; Kooistra, W.H.; Procaccini, G.; Casotti, R.; Montresor, M. Internal transcribed spacer polymorphism in Pseudo-nitzschia multistriata (Bacillariophyceae) in the Gulf of Naples: Recent divergence or intraspecific hybridization? Protist 2009, 160, 9–20. [Google Scholar] [CrossRef]
- Dermastia, T.T.; Cerino, F.; Stanković, D.; Francé, J.; Ramšak, A.; Tušek, M.Ž.; Beran, A.; Natali, V.; Cabrini, M.; Mozetič, P. Ecological time series and integrative taxonomy unveil seasonality and diversity of the toxic diatom Pseudo-nitzschia H. Peragallo in the northern Adriatic Sea. Harmful Algae 2020, 93, 101773. [Google Scholar] [CrossRef] [PubMed]
- Stonik, I.V.; Orlova, T.Y.; Lundholm, N. Diversity of Pseudo-nitzschia H. Peragallo from the western North Pacific. Diatom Res. 2011, 26, 121–134. [Google Scholar] [CrossRef]
- Huang, C.X.; Dong, H.C.; Lundholm, N.; Teng, S.T.; Zheng, G.C.; Tan, Z.J.; Lim, P.T.; Li, Y. Species composition and toxicity of the genus Pseudo-nitzschia in Taiwan Strait, including P. chiniana sp. nov. and P. qiana sp. nov. Harmful Algae 2019, 84, 195–209. [Google Scholar] [CrossRef]
- Lim, H.C.; Tan, S.N.; Teng, S.T.; Lundholm, N.; Orive, E.; David, H.; Quijano-Scheggia, S.; Leong, S.C.Y.; Wolf, M.; Bates, S.S.; et al. Phylogeny and species delineation in the marine diatom Pseudo-nitzschia (Bacillariophyta) using cox1, LSU, and ITS2 rRNA genes: A perspective in character evolution. J. Phycol. 2018, 54, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Stonik, I.; Orlova, T.Y.; Propp, L.; Demchenko, N.; Skriptsova, A. An autumn bloom of diatoms of the genus Pseudo-nitzschia H. Peragallo, 1900 in Amursky Bay, the Sea of Japan. Russ. J. Mar. Biol. 2012, 38, 211–217. [Google Scholar] [CrossRef]
- Ajani, P.; Murray, S.; Hallegraeff, G.; Lundholm, N.; Gillings, M.; Brett, S.; Armand, L. The diatom genus Pseudo-nitzschia (Bacillariophyceae) in New South Wales, A ustralia: Morphotaxonomy, molecular phylogeny, toxicity, and distribution. J. Phycol. 2013, 49, 765–785. [Google Scholar] [CrossRef]
- Rhodes, L.; Jiang, W.; Knight, B.; Adamson, J.; Smith, K.; Langi, V.; Edgar, M. The genus Pseudo-nitzschia (Bacillariophyceae) in New Zealand: Analysis of the last decade’s monitoring data. New Zealand J. Mar. Freshw. Res. 2013, 47, 490–503. [Google Scholar] [CrossRef] [Green Version]
- Amato, A.; Lüdeking, A.; Kooistra, W.H. Intracellular domoic acid production in Pseudo-nitzschia multistriata isolated from the Gulf of Naples (Tyrrhenian Sea, Italy). Toxicon 2010, 55, 157–161. [Google Scholar] [CrossRef]
- Oyarzún, D.; Brierley, C.M. The future of coastal upwelling in the Humboldt current from model projections. Clim. Dyn. 2019, 52, 599–615. [Google Scholar] [CrossRef] [Green Version]
- Brink, K.; Halpern, D.; Huyer, A.; Smith, R. The physical environment of the Peruvian upwelling system. Prog. Oceanogr. 1983, 12, 285–305. [Google Scholar] [CrossRef]
- Suárez-Isla, B.A.; López, A.; Hernández, C.; Clement, A.; Guzmán, L. Impacto económico de las floraciones de microalgas nocivas en Chile y datos recientes sobre la ocurrencia de veneno amnésico de los mariscos. Floraciones Algales Nocivas en el Cono Sur Americano, Inst. Esp. Oceanogr. Madrid, España. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, Spain, 2002; pp. 257–268. [Google Scholar]
- Lopez-Rivera, A.; Pinto, M.; Insinilla, A.; Suarez Isla, B.; Uribe, E.; Alvarez, G.; Lehane, M.; Furey, A.; James, K.J. The occurrence of domoic acid linked to a toxic diatom bloom in a new potential vector: The tunicate Pyura chilensis (piure). Toxicon 2009, 54, 754–762. [Google Scholar] [CrossRef]
- Álvarez, G.; Rengel, J.; Araya, M.; Álvarez, F.; Pino, R.; Uribe, E.; Díaz, P.A.; Rossignoli, A.E.; López-Rivera, A.; Blanco, J. Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs. Toxins 2020, 12, 698. [Google Scholar] [CrossRef]
- National. Fisheries Health Organization of Perú-Organismo Nacional de Sanidad Pesquera(SANIPES). Available online: https://www.sanipes.gob.pe/web/index.php/es/fitoplancton (accessed on 15 April 2021).
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Hasle, G.R. The Inverted-Microscope Method; Sournia, A., Ed.; Phytoplankton Manual. Monographs on Oceanic Methodology; 1978; pp. 88–96. [Google Scholar]
- Lundholm, N.; Hasle, G.R.; Fryxell, G.A.; Hargraves, P.E. Morphology, phylogeny and taxonomy of species within the Pseudo-nitzschia americana complex (Bacillariophyceae) with descriptions of two new species, Pseudo-nitzschia brasiliana and Pseudo-nitzschia linea. Phycologia 2002, 41, 480–497. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pcr Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Iglesia, P.; Gimenez, G.; Diogene, J. Determination of dissolved domoic acid in seawater with reversed-phase extraction disks and rapid resolution liquid chromatography tandem mass spectrometry with head-column trapping. J. Chromatogr. A 2008, 1215, 116–124. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenorio, C.; Álvarez, G.; Quijano-Scheggia, S.; Perez-Alania, M.; Arakaki, N.; Araya, M.; Álvarez, F.; Blanco, J.; Uribe, E. First Report of Domoic Acid Production from Pseudo-nitzschia multistriata in Paracas Bay (Peru). Toxins 2021, 13, 408. https://doi.org/10.3390/toxins13060408
Tenorio C, Álvarez G, Quijano-Scheggia S, Perez-Alania M, Arakaki N, Araya M, Álvarez F, Blanco J, Uribe E. First Report of Domoic Acid Production from Pseudo-nitzschia multistriata in Paracas Bay (Peru). Toxins. 2021; 13(6):408. https://doi.org/10.3390/toxins13060408
Chicago/Turabian StyleTenorio, Cecil, Gonzalo Álvarez, Sonia Quijano-Scheggia, Melissa Perez-Alania, Natalia Arakaki, Michael Araya, Francisco Álvarez, Juan Blanco, and Eduardo Uribe. 2021. "First Report of Domoic Acid Production from Pseudo-nitzschia multistriata in Paracas Bay (Peru)" Toxins 13, no. 6: 408. https://doi.org/10.3390/toxins13060408




