Sub-Acute Feeding Study of Saxitoxin to Mice Confirms the Effectiveness of Current Regulatory Limits for Paralytic Shellfish Toxins
Abstract
:1. Introduction
2. Results
2.1. Development of Experimental Protocols
2.1.1. Development of Suitable Diet
2.1.2. Establishment of a Suitable STX Dose Rate
2.2. Results of the 21-Day Feeding Study
2.2.1. Diet Analysis
2.2.2. Dose Rates
2.2.3. Clinical Observations and Appearance
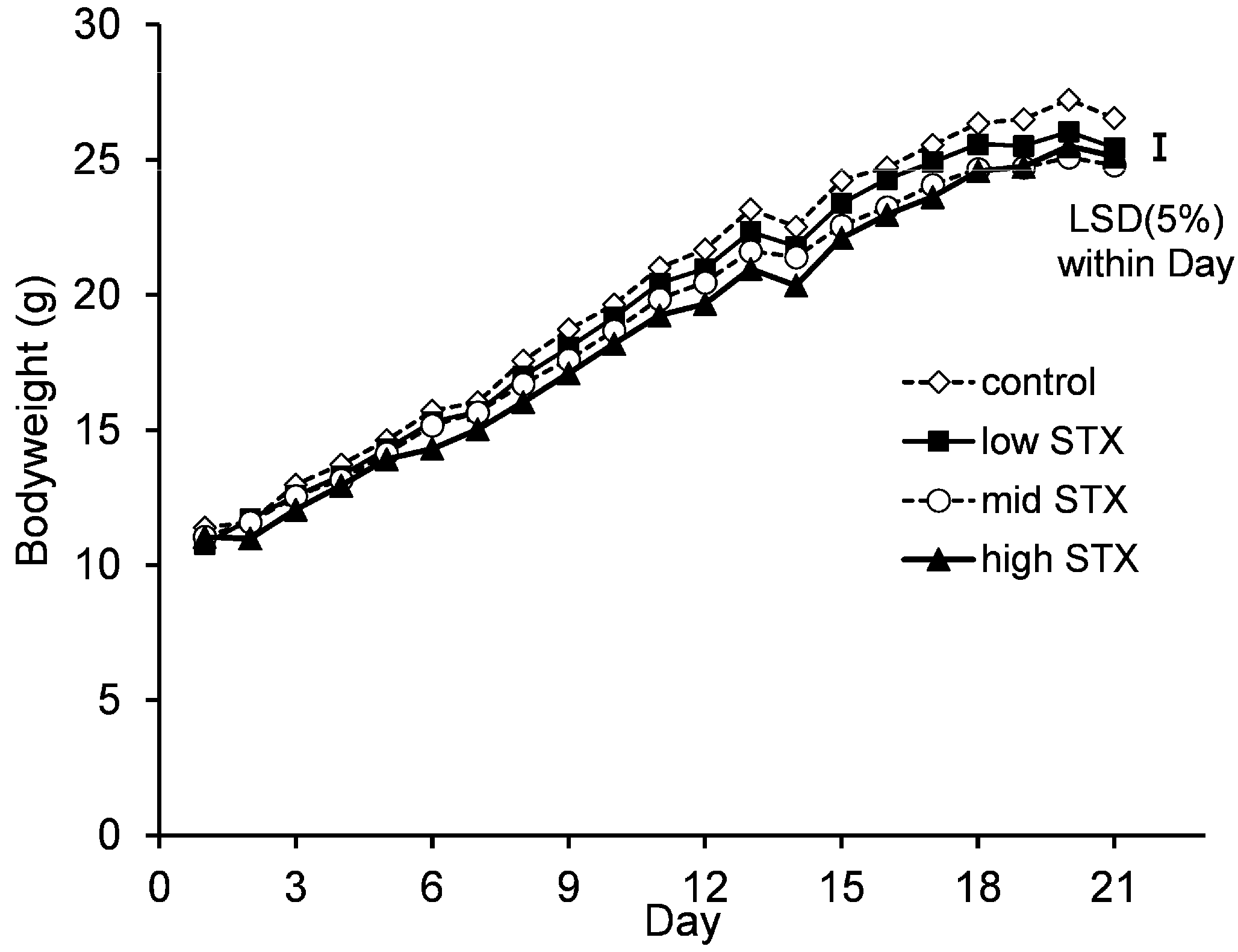
2.2.4. Bodyweight and Food Consumption
2.2.5. Motor Coordination
2.2.6. Grip Strength
2.2.7. Blood Pressure and Heart Rate
2.2.8. Haematological and Serum Biochemical Data
2.2.9. Organ Weights
2.2.10. Histological Examination
3. Discussion
4. Materials and Methods
4.1. Purity Assessment of Saxitoxin
4.2. Animals
4.3. Preparation of Mouse Diets
4.4. LC-MS Analysis
4.5. Preliminary Work and Palatability Studies
4.6. Sub-Acute 21-Day Feeding Trial
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [Green Version]
- Oshima, Y.; Blackburn, S.I.; Hallegraeff, G.M. Comparative study on paralytic shellfish toxin profiles of the dinoflagellate Gymnodinium catenatum from three different countries. Mar. Biol. 1993, 116, 471–476. [Google Scholar] [CrossRef]
- Usup, G.; Kulis, D.M.; Anderson, D.M. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures. Nat. Toxins 1994, 2, 254–262. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [Green Version]
- García, C.; Lagos, M.; Truan, D.; Lattes, K.; Véjar, O.; Chamorro, B.; Iglesias, V.; Andrinolo, D.; Lagos, N. Human intoxication with paralytic shellfish toxins: Clinical parameters and toxin analysis in plasma and urine. Biol. Res. 2005, 38, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Mons, M.N.; Van Egmond, H.P.; Speijers, G.J.A. Paralytic Shellfish Poisoning: A Review; RIVM Report 388802 005; National Institute of Public Health and the Environment: Bilthoven, The Netherlands, 1988.
- EFSA. Scientific opinion of the panel on contaminants in the food chain on a request from the european commission on marine biotoxins in shellfish—Saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- FAO/IOC/WHO. Report of the Joint FAO/IOC/WHO Ad Hoc Expert Consultation on Biotoxins in Bivalve Molluscs; Food and Agriculture Organization of the United Nations: Oslo, Norway, 2004; p. 31. [Google Scholar]
- Gessner, B.D.; Middaugh, J.P. Paralytic shellfish poisoning in Alaska: A 20-year retrospective analysis. Am. J. Epidemiol. 1995, 141, 766–770. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004, Laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, L 139/55, 151. [Google Scholar]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.K.; Clardy, J. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Shumway, S.E. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Turnbull, A.R.; Harwood, D.T.; Boundy, M.J.; Holland, P.T.; Hallegraeff, G.; Malhi, N.; Quilliam, M.A. Paralytic shellfish toxins —Call for uniform reporting units. Toxicon 2020, 178, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Tennant, A.D.; Naubert, J.; Corbeil, H.E. An outbreak of paralytic shellfish poisoning. Can. Med. Assoc. J. 1955, 72, 436–439. [Google Scholar] [PubMed]
- Wong, C.-K.; Hung, P.; Lee, K.L.H.; Mok, T.; Kam, K.-M. Effect of steam cooking on distribution of paralytic shellfish toxins in different tissue compartments of scallops Patinopecten yessoensis. Food Chem. 2009, 114, 72–80. [Google Scholar] [CrossRef]
- Medcof, J.C.; Leim, A.H.; Needler, A.B.; Needler, A.W.H.; Gibbard, J.; Naubert, J. Paralytic Shellfish Poisoning on the Canadian; Atlantic coast, Bulletin No. 75; Fisheries Research Board of Canada: Ottawa, ON, USA, 1947; p. 31.
- EFSA Marine biotoxins in shellfish—Summary on regulated marine biotoxins. EFSA J. 2009, 7, 1306.
- EFSA Scientific Committee. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 2579. [Google Scholar]
- FAO/WHO. Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO: Rome, Italy, 2016; 108p. [Google Scholar]
- Interdepartmental Group on Health Risks from Chemicals. Uncertainty Factors: Their Use in Human Health Risk Assessment by UK Government; MRC Institute for Environment and Health: Leicester, UK, 2003; p. 69. [Google Scholar]
- Finch, S.C.; Boundy, M.J.; Harwood, D.T. The acute toxicity of tetrodotoxin and tetrodotoxin–saxitoxin mixtures to mice by various routes of administration. Toxins 2018, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Harwood, D.T.; Boundy, M.; Selwood, A.I.; van Ginkel, R.; MacKenzie, L.; McNabb, P.S. Refinement and implementation of the Lawrence method (AOAC 2005.06) in a commercial laboratory: Assay performance during an Alexandrium catenella bloom event. Harmful Algae 2013, 24, 20–31. [Google Scholar] [CrossRef]
- MacKenzie, A.L. The risk to New Zealand shellfish aquaculture from paralytic shellfish poisoning (PSP) toxins. N. Z. J. Mar. Freshw. Res. 2014, 48, 430–465. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.D.; Stubbs, B.; Coates, L.; Dhanji-Rapkova, M.; Hatfield, R.G.; Lewis, A.M.; Rowland-Pilgrim, S.; O’Neil, A.; Stubbs, P.; Ross, S.; et al. Variability of paralytic shellfish toxin occurrence and profiles in bivalve molluscs from Great Britain from official control monitoring as determined by pre-column oxidation liquid chromatography and implications for applying immunochemical tests. Harmful Algae 2014, 31, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.C.; Munday, J.S.; Munday, R.; Kerby, J.W.F. Short-term toxicity studies of loline alkaloids in mice. Food Chem. Toxicol. 2016, 94, 243–249. [Google Scholar] [CrossRef]
- Finch, S.C.; Munday, J.S.; Sprosen, J.M.; Bhattarai, S. Toxicity studies of chanoclavine in mice. Toxins 2019, 11, 249. [Google Scholar] [CrossRef] [Green Version]
- Munday, R. Toxicology of Seafood Toxins: A Critical Review. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 197–290. [Google Scholar]
- Munday, R.; Thomas, K.; Gibbs, R.; Murphy, C.; Quilliam, M.A. Acute toxicities of saxitoxin, neosaxitoxin, decarbamoyl saxitoxin and gonyautoxins 14 and 23 to mice by various routes of administration. Toxicon 2013, 76, 77–83. [Google Scholar] [PubMed]
- Kao, C.Y. Paralytic shellfish poisoning. In Algal Toxins in Seafood and Drinking Water; Falconer, I.R., Ed.; Academic Press: London, UK; San Diego, CA, USA, 1993; pp. 75–86. [Google Scholar]
- Selwood, A.I.; Waugh, C.; Harwood, D.T.; Rhodes, L.L.; Reeve, J.; Sim, J.; Munday, R. Acute toxicities of the saxitoxin congeners gonyautoxin 5, gonyautoxin 6, decarbamoyl donyautoxin 23, decarbamoyl neosaxitoxin, C-12 and C-34 to mice by various routes of administration. Toxins 2017, 9, 73. [Google Scholar] [CrossRef]
- Munday, R.; Reeve, J. Risk assessment of shellfish toxins. Toxins 2013, 5, 2109–2137. [Google Scholar] [CrossRef] [Green Version]
- Craig, M.A.; Elliott, J.F. Mice fed radiolabeled protein by gavage show sporadic passage of large quantities of intact material into the blood, an artifact not associated with voluntary feeding. J. Am. Assoc. Lab. Anim. Sci. 1999, 38, 18–23. [Google Scholar]
- Ellacott, K.L.; Morton, G.J.; Woods, S.C.; Tso, P.; Schwartz, M.W. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010, 12, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Minematsu, S.; Hiruta, M.; Taki, M.; Fujii, Y.; Aburada, M. Automatic monitoring system for the measurement of body weight, food and water consumption and spontaneous activity of a mouse. J. Toxicol. Sci. 1991, 16, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Boente-Juncal, A.; Vale, C.; Cifuentes, M.; Otero, P.; Camiña, M.; Rodriguez-Vieytes, M.; Botana, L.M. Chronic in vivo effects of repeated exposure to low oral doses of tetrodotoxin: Preliminary evidence of nephrotoxicity and cardiotoxicity. Toxins 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Arnich, N.; Thébault, A. Dose-Response modelling of paralytic shellfish poisoning (PSP) in humans. Toxins 2018, 10, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anon. AOAC Official Method 959.08. Paralytic shell-fish poisoning. Biological method. In Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2005; pp. 79–82. [Google Scholar]
- Sommer, H.; Meyer, K.F. Paralytic shell-fish poisoning. Arch. Pathol. 1937, 24, 560–598. [Google Scholar]
- Boente-Juncal, A.; Otero, P.; Rodríguez, I.; Camiña, M.; Rodriguez-Vieytes, M.; Vale, C.; Botana, L.M. Oral chronic toxicity of the safe tetrodotoxin dose proposed by the European Food Safety Authority and its additive effect with saxitoxin. Toxins 2020, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Rourke, W.A.; Murphy, C.J.; Pitcher, G.; van de Riet, J.M.; Burns, B.G.; Thomas, K.M.; Quilliam, M.A. Rapid postcolumn methodology for determination of paralytic shellfish toxins in shellfish tissue. J. AOAC Int. 2019, 91, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography–mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-performance hydrophilic interaction liquid chromatography with tandem mass spectrometry method for the determination of paralytic shellfish toxins and tetrodotoxin in mussels, oysters, clams, cockles, and scallops: Collaborative study. J. AOAC Int. 2020, 103, 533–562. [Google Scholar] [CrossRef]



| Sample | Concentration (µg STX.2HCl eq/g) 1 | Recovery 2 |
|---|---|---|
| Day 1 Control | 3.30 ± 0.24 | 95% |
| Day 2 20 °C | 3.30 ± 0.48 | 95% |
| Day 2 4 °C | 3.26 ± 0.36 | 94% |
| Day 5 20 °C | 3.33 ± 0.24 | 96% |
| Day 5 4 °C | 3.26 ± 0.45 | 94% |
| Treatment | Measured Concentration (µg STX.2HCl eq/g) 1 | Theoretical Concentration (µg STX.2HCl eq/g) | Recovery 2 |
|---|---|---|---|
| Low STX | 1.12 ± 0.20 | 1.15 | 97% |
| Mid STX | 2.34 ± 0.41 | 2.27 | 103% |
| High STX | 3.19 ± 0.56 | 3.41 | 94% |
| Heart Rate 1 (BPM) | Systolic BP 1 (mmHg) | Diastolic BP 1 (mmHg) | ||||
|---|---|---|---|---|---|---|
| Day 14 | Day 21 | Day 14 | Day 21 | Day 14 | Day 21 | |
| Females | ||||||
| control | 729 ± 16.7 a | 710 ± 16.7 a | 112.6 ± 6.89 a | 119.0 ± 6.89 a | 45.8 ± 4.20 a | 52.0 ± 4.20 a |
| low | 732 ± 16.7 a | 679 ± 16.7 a | 110.2 ± 6.89 a | 118.4 ± 6.89 a | 54.4 ± 4.20 a | 57.2 ± 4.20 a |
| mid | 753 ± 16.7 a | 690 ± 16.7 a | 105.4 ± 6.89 a | 111.0 ± 6.89 a | 52.6 ± 4.20 a | 49.4 ± 4.20 a |
| high | 754 ± 16.7 a | 699 ± 16.7 a | 99.4 ± 6.89 a | 106.0 ± 6.89 a | 43.6 ± 4.20 a | 52.8 ± 4.20 a |
| Males | ||||||
| control | 709. ± 16.7 a | 704 ± 16.7 a | 108.4 ± 6.89 a | 111.8 ± 6.89 a | 48.2 ± 4.20 a | 48.0 ± 4.20 a |
| low | 701. ± 16.7 a | 692 ± 16.7 a | 105.2 ± 6.89 a | 119.4 ± 6.89 a | 41.0 ± 4.20 a | 49.2 ± 4.20 a |
| mid | 753 ± 16.7 a | 695 ± 16.7 a | 101.4 ± 6.89 a | 116.2 ± 6.89 a | 42.8 ± 4.20 a | 50.8 ± 4.20 a |
| high | 729 ± 16.7 a | 680 ± 16.7 a | 112.6 ± 6.89 a | 116.0 ± 6.89 a | 50.8 ± 4.20 a | 50.8 ± 4.20 a |
| Item | Control 1 | Low STX 1 | Mid STX 1 | High STX 1 |
|---|---|---|---|---|
| Females | ||||
| HCT (L/L) | 0.45 ± 0.01 a | 0.47 ± 0.01 a | 0.46 ± 0.01 a | 0.44 ± 0.01 a |
| HB (g/L) | 136 ± 2.9 ab | 141 ± 2.9 b | 138 ± 2.9 ab | 132 ± 2.9 a |
| RBC (×1012/L) | 8.29 ± 0.21 a | 8.75 ± 0.21 a | 8.61 ± 0.21 a | 8.31 ± 0.21 a |
| MCV (fL) | 54.5 ± 0.70 a | 53.9 ± 0.70 a | 53.9 ± 0.70 a | 53.3 ± 0.70 a |
| MCH (pg) | 16.4 ± 0.18 a | 16.2 ± 0.18 a | 16.0 ± 0.18 a | 16.0 ± 0.18 a |
| MCHC (g/L) | 303 ± 3.1 a | 299 ± 3.1 a | 297 ± 3.1 a | 299 ± 3.1 a |
| WBC (×109/L) | 6.51 ± 0.82 a | 5.51 ± 0.82 a | 4.55 ± 0.82 a | 5.25 ± 0.82 a |
| Neutrophil (%) | 9.56 ± 2.73 a | 11.24 ± 2.73 a | 6.76 ± 2.73 a | 10.56 ± 2.73 a |
| Lymphocyte (%) | 87.3 ± 3.84 a | 85.3 ± 3.84 a | 91.9 ± 3.84 a | 85.7 ± 3.84 a |
| Monocyte (%) | 1.29 ± 0.95 a | 2.71 ± 0.95 a | 0.89 ± 0.95 a | 1.89 ± 0.95 a |
| Eosinophil (%) | 0.89 ± 0.586 a | 0.91 ± 0.586 a | 0.29 ± 0.586 a | 1.29 ± 0.586 a |
| Males | ||||
| HCT (L/L) | 0.46 ± 0.01 a | 0.47 ± 0.01 a | 0.46 ± 0.01 a | 0.45 ± 0.01 a |
| HB (g/L) | 137 ± 2.9b | 136 ± 2.9 b | 135 ± 2.9 ab | 128 ± 2.9 a |
| RBC (×1012/L) | 8.58 ± 0.21 a | 8.57 ± 0.21 a | 8.57 ± 0.21 a | 8.12 ± 0.21 a |
| MCV (fL) | 54.0 ± 0.70 a | 54.7 ± 0.70 a | 53.5 ± 0.70 a | 54.8 ± 0.70 a |
| MCH (pg) | 16.0 ± 0.18 a | 16.0 ± 0.18 a | 16.0 ± 0.18 a | 16.0 ± 0.18 a |
| MCHC (g/L) | 295 ± 3.1 a | 291 ± 3.1 a | 294 ± 3.1 a | 288 ± 3.1 a |
| WBC (×109/L) | 7.36 ± 0.82 b | 4.51 ± 0.82 a | 5.25 ± 0.82 a | 5.62 ± 0.82 ab |
| Neutrophil (%) | 13.85 ± 2.73 b | 13.44 ± 2.73 b | 11.64 ± 2.73 ab | 5.95 ± 2.73 a |
| Lymphocyte (%) | 82.4 ± 3.84 a | 84.5 ± 3.84 a | 85.3 ± 3.84 a | 90.9 ± 3.84 a |
| Monocyte (%) | 2.40 ± 0.95 a | 1.31 ± 0.95 a | 1.71 ± 0.95 a | 2.41 ± 0.95 a |
| Eosinophil (%) | 1.00 ± 0.586 a | 0.91 ± 0.586 a | 1.51 ± 0.586 a | 0.78 ± 0.586 a |
| Item | Control 1 | Low STX 1 | Mid STX 1 | High STX 1 |
|---|---|---|---|---|
| Females | ||||
| log AST (log IU/L) | 5.81 ± 0.33 a | 5.27 ± 0.33 a | 5.45 ± 0.33 a | 5.46 ± 0.33 a |
| log ALT (log IU/L) | 5.25 ± 0.35 b | 3.65 ± 0.35 a | 4.01 ± 0.35 a | 3.85 ± 0.35 a |
| Urea (mmol/L) | 8.96 ± 2.12 a | 9.50 ± 2.12 a | 8.94 ± 2.12 a | 7.72 ± 2.12 a |
| TP (g/L) | 47.0 ± 1.7 a | 47.33 ± 1.7 a | 49.07 ± 1.7 a | 51.25 ± 1.7 a |
| ALB (g/L) | 28.6 ± 1.03 a | 28.0 ± 1.03 a | 29.0 ± 1.03 a | 30.6 ± 1.03 a |
| Globulin (g/L) | 19.8 ± 0.87 a | 19.5 ± 0.87 a | 20.5 ± 0.87 a | 21.3 ± 0.87 a |
| CRN (µmol/L) | 10.4 ± 1.04 b | 7.8 ± 1.04 a | 7.2 ± 1.04 a | 9.4 ± 1.04 ab |
| A/G ratio | 1.39 ± 0.03 a | 1.41 ± 0.03 a | 1.42 ± 0.03 a | 1.44 ± 0.03 a |
| Na (mmol/L) | 151 ± 1.3 a | 152 ± 1.3 a | 152 ± 1.3 a | 149 ± 1.3 a |
| K (mmol/L) | 8.07 ± 0.71 a | 7.81 ± 0.71 a | 7.53 ± 0.71 a | 8.35 ± 0.71 a |
| Cl (mmol/L) | 114.0 ± 1.02 a | 113.4 ± 1.02 a | 114.6 ± 1.02 a | 113.2 ± 1.02 a |
| Males | ||||
| log AST (log IU/L) | 4.72 ± 0.33 a | 5.27 ± 0.33 ab | 5.05 ± 0.33 ab | 5.75 ± 0.33 b |
| log ALT (log IU/L) | 3.54 ± 0.35 a | 3.58 ± 0.35 a | 3.68 ± 0.35 a | 3.79 ± 0.35 a |
| Urea (mmol/L) | 9.08 ± 2.12 a | 10.00 ± 2.12 a | 9.86 ± 2.12 a | 9.65 ± 2.12 a |
| TP (g/L) | 50.7 ± 1.7 a | 50.7 ± 1.7 a | 51.0 ± 1.7 a | 49.7 ± 1.7 a |
| ALB (g/L) | 28.2 ± 1.03 a | 28.6 ± 1.03 a | 28.8 ± 1.03 a | 28.2 ± 1.03 a |
| Globulin (g/L) | 22.5 ± 0.87 a | 22.7 ± 0.87 a | 22.3 ± 0.87 a | 21.7 ± 0.87 a |
| CRN (µmol/L) | 7.0 ± 1.04 a | 5.9 ± 1.04 a | 6.1 ± 1.04 a | 7.1 ± 1.04 a |
| A/G ratio | 1.24 ± 0.03 a | 1.26 ± 0.03 a | 1.28 ± 0.03 a | 1.30 ± 0.03 a |
| Na (mmol/L) | 152 ± 1.3 a | 151 ± 1.3 a | 151 ± 1.3 a | 152 ± 1.3 a |
| K (mmol/L) | 8.17 ± 0.71 a | 8.53 ± 0.71 a | 9.43 ± 0.71 a | 8.93 ± 0.71 a |
| Cl (mmol/L) | 111.4 ± 1.02 a | 113.0 ± 1.02 a | 112.0 ± 1.02 a | 114.0 ± 1.02 a |
| Item | Control 1 | Low STX 1 | Mid STX 1 | High STX 1 |
|---|---|---|---|---|
| Females | ||||
| brain | 1.86 ± 0.09 ab | 1.83 ± 0.09 a | 1.96 ± 0.09 ab | 2.01 ± 0.09 b |
| heart | 0.58 ± 0.03 a | 0.55 ± 0.03 a | 0.60 ± 0.03 a | 0.57 ± 0.03 a |
| kidneys | 1.34 ± 0.07 a | 1.24 ± 0.07 a | 1.30 ± 0.07 a | 1.30 ± 0.07 a |
| liver | 4.59 ± 0.10 a | 4.32 ± 0.10 a | 4.41 ± 0.10 a | 4.38 ± 0.10 a |
| spleen | 0.39 ± 0.03 a | 0.42 ± 0.03 a | 0.37 ± 0.03 a | 0.38 ± 0.03 a |
| Males | ||||
| brain | 1.73 ± 0.09 a | 1.72 ± 0.09 a | 1.74 ± 0.09 a | 1.83 ± 0.09 a |
| heart | 0.57 ± 0.03 a | 0.63 ± 0.03 b | 0.57 ± 0.03 ab | 0.55 ± 0.03 a |
| kidneys | 1.72 ± 0.07 b | 1.74 ± 0.07 b | 1.66 ± 0.07 ab | 1.59 ± 0.07 a |
| liver | 4.74 ± 0.10 a | 4.65 ± 0.10 a | 4.69 ± 0.10 a | 4.54 ± 0.10 a |
| spleen | 0.37 ± 0.03 b | 0.32 ± 0.03 ab | 0.34 ± 0.03 ab | 0.29 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finch, S.C.; Webb, N.G.; Boundy, M.J.; Harwood, D.T.; Munday, J.S.; Sprosen, J.M.; Cave, V.M.; Broadhurst, R.B.; Nicolas, J. Sub-Acute Feeding Study of Saxitoxin to Mice Confirms the Effectiveness of Current Regulatory Limits for Paralytic Shellfish Toxins. Toxins 2021, 13, 627. https://doi.org/10.3390/toxins13090627
Finch SC, Webb NG, Boundy MJ, Harwood DT, Munday JS, Sprosen JM, Cave VM, Broadhurst RB, Nicolas J. Sub-Acute Feeding Study of Saxitoxin to Mice Confirms the Effectiveness of Current Regulatory Limits for Paralytic Shellfish Toxins. Toxins. 2021; 13(9):627. https://doi.org/10.3390/toxins13090627
Chicago/Turabian StyleFinch, Sarah C., Nicola G. Webb, Michael J. Boundy, D. Tim Harwood, John S. Munday, Jan M. Sprosen, Vanessa M. Cave, Ric B. Broadhurst, and Jeane Nicolas. 2021. "Sub-Acute Feeding Study of Saxitoxin to Mice Confirms the Effectiveness of Current Regulatory Limits for Paralytic Shellfish Toxins" Toxins 13, no. 9: 627. https://doi.org/10.3390/toxins13090627





