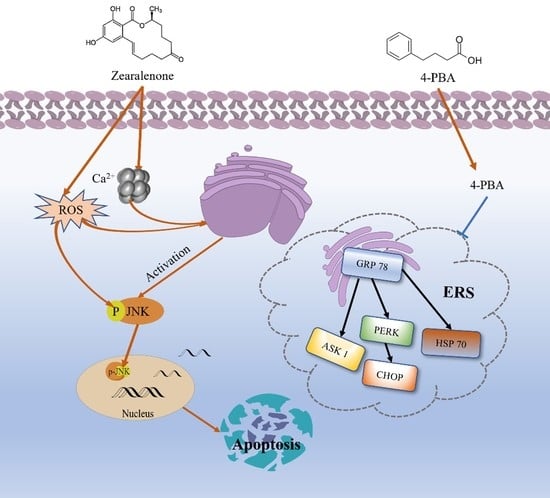
Zearalenone Induces Apoptosis in Porcine Endometrial Stromal Cells through JNK Signaling Pathway Based on Endoplasmic Reticulum Stress
Abstract
:1. Introduction
2. Results
2.1. Effects of ZEA on Cell Viability
2.2. Effects of ZEA on ESC Growth
2.3. Effect of ZEA on the Cell Ultrastructure
2.4. Effect of ZEA on Ca2+ Levels, ROS Levels, and the Apoptosis Rate
2.5. Effects of ZEA on Gene Expression in Porcine ESCs
2.6. Effects of ZEA on Protein Levels in Porcine ESCs
2.6.1. Levels of ERS-Related Proteins
2.6.2. Expression of JNK
2.6.3. Nuclear Level of p-JNK
2.6.4. Expression Levels of Apoptosis-Related Proteins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemical and Reagents
5.2. Cell Culture and Treatments
5.3. Detection of Cell Viability
5.4. Assay for Cell Growth Status
5.5. Transmission Electron Microscopy (TEM) Analysis of Cell Morphology
5.6. Detection of ROS Levels
5.7. Detection of Ca2+ Levels
5.8. Detection of the Apoptosis Rate
5.9. qRT-PCR
5.10. Western Blotting Analysis
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Edler, L.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008–3015. [Google Scholar] [CrossRef] [Green Version]
- Agnieszka, R.; Paweł, P.; Katarzyna, R.; Viorica, R.; Michał, Z.; Justyna, W.; Bogusław, B. A study of zearalenone biosorption and metabolisation by prokaryotic and eukaryotic cells. Toxicon 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Tiemann, U.; Dänicke, S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: A review. Food Addit. Contam. 2007, 24, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and Reproductive Function in Farm Animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef]
- Karen, W.; Christoph, G.; Marc, D. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology 2017, 94, 21–30. [Google Scholar] [CrossRef]
- Isin, C.; Eduardo, A.N. Endoplasmic Reticulum Stress, the Hypothalamus, and Energy Balance. Trends Endocrinol. Metab. 2019, 30, 163–176. [Google Scholar] [CrossRef]
- Mengxiong, W.; Mary, E.L.; Ronald, K.C.; Brian, K.L. The unfolded protein response as a target for anticancer therapeutics. Crit. Rev. Oncol./Hematol. 2018, 127, 66–79. [Google Scholar] [CrossRef]
- Ankita, B.; Harikrishna, T.; Timothy, S.B. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 2018, 68–69, 355–365. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, K.; Lee, H.J.; Kim, D.H.; Noh, Y.H.; Yu, K.; Jung, H.; Lee, S.H.; Lee, J.Y.; Youn, Y.C.; et al. Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS ONE 2010, 5, e10489. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Namekata, K.; Kimura, A.; Harada, C.; Harada, T. ASK1 in neurodegeneration. Adv. Biol. Regul. 2017, 66, 63–71. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hirata, Y.; Takeda, K.; Hayakawa, Y.; Sato, T.; Kinoshita, H.; Sakamoto, K.; Nakata, W.; Hikiba, Y.; Omata, M.; et al. Apoptosis signal-regulating kinase 1 inhibits hepatocarcinogenesis by controlling the tumor-suppressing function of stress-activated mitogen-activated protein kinase. Hepatology 2011, 54, 185–195. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, S.; Hu, L.; Gu, Y.; Cai, Y.; Wu, D.; Liu, W.; Jiang, C.; Kong, X.; Zhang, G. Inhibition of apoptosis signal-regulating kinase by paeoniflorin attenuates neuroinflammation and ameliorates neuropathic pain. J. Neuroinflamm. 2019, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Claire, R.W.; Roger, J.D. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007, 19, 142–149. [Google Scholar] [CrossRef]
- Shirpoor, A.; Gaderi, R.; Naderi, R. Ethanol exposure in prenatal and early postnatal induced cardiac injury in rats: Involvement of oxidative stress, Hsp70, ERK 1/2, JNK, and apoptosis in a 3-month follow-up study. Cell Stress Chaperon. 2019, 24, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, Y.; Lv, L.; Tao, Y.; Shao, M.; Zhao, C.; Xue, M.; Sun, J.; Niu, C.; Wang, Y.; et al. Acute ethanol exposure-induced autophagy-mediated cardiac injury via activation of the ROS-JNK-Bcl-2 pathway. J. Cell. Physiol. 2018, 233, 924–936. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Jedziniak, P. Mycotoxin Biomarkers in Pigs-Current State of Knowledge and Analytics. Toxins 2021, 13, 586. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.N.; Ma, J.Y.; Liu, J.C.; Wang, J.J.; Cheng, S.F.; Sun, X.F.; Li, L.; Li, B.; Nyachoti, C.M.; Shen, W. The influence of N-acetyl-l-cysteine on damage of porcine oocyte exposed to zearalenone in vitro. Toxicol. Appl. Pharmacol. 2015, 289, 341–348. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, C.; Chen, S.; Sun, S. Zearalenone exposure impairs organelle function during porcine oocyte meiotic maturation. Theriogenology 2022, 177, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carrasco, Y.; Ruiz, M.J.; Font, G.; Berrada, H. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 2013, 93, 2297–2303. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhao, J.; Cao, L.; Zhu, L.; Huang, Y.; Chen, X.; Rahman, S.U.; Feng, S.; Li, Y.; et al. Deoxynivalenol Induces Inflammatory Injury in IPEC-J2 Cells via NF-κB Signaling Pathway. Toxins 2019, 11, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, X.; Cao, L.; Zhu, L.; Zhang, Y.; Chu, X.; Zhu, D.; Rahman, S.U.; Peng, C.; Feng, S.; et al. Mechanism of deoxynivalenol-induced neurotoxicity in weaned piglets is linked to lipid peroxidation, dampened neurotransmitter levels, and interference with calcium signaling. Ecotox. Environ. Safe. 2020, 194, 110382. [Google Scholar] [CrossRef]
- Wang, X.; Chu, X.; Cao, L.; Zhao, J.; Zhu, L.; Rahman, S.U.; Hu, Z.; Zhang, Y.; Feng, S.; Li, Y.; et al. The role and regulatory mechanism of autophagy in hippocampal nerve cells of piglet damaged by deoxynivalenol. Toxicol. Vitr. 2020, 66, 104837. [Google Scholar] [CrossRef]
- Guzel, E.; Arlier, S.; Guzeloglu-Kayisli, O.; Tabak, M.S.; Ekiz, T.; Semerci, N.; Larsen, K.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology. Int. J. Mol. Sci. 2017, 18, 792. [Google Scholar] [CrossRef]
- Tao, Y.K.; Yu, P.L.; Bai, Y.P.; Yan, S.T.; Zhao, S.P.; Zhang, G.Q. Role of PERK/eIF2alpha/CHOP Endoplasmic Reticulum Stress Pathway in Oxidized Low-density Lipoprotein Mediated Induction of Endothelial Apoptosis. Biomed. Environ. Sci. 2016, 29, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lei, L.; Lv, Q.; Gong, Y.; Yang, L. Curcumin attenuates palmitic acid-induced cell apoptosis by inhibiting endoplasmic reticulum stress in H9C2 cardiomyocytes. Hum. Exp. Toxicol. 2019, 38, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Hadi-Alijanvand, H.; Sabbaghian, M.; Kiaei, M.; Khodagholi, F. Interaction of 2-APB, dantrolene, and TDMT with IP3R and RyR modulates ER stress-induced programmed cell death I and II in neuron-like PC12 cells: An experimental and computational investigation. J. Biomol. Struct. Dyn. 2014, 32, 1211–1230. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, F.; Sun, J.; Zhou, J.; Wang, X.; Wang, N.; Li, X.; Zhang, Z.; Wang, A.; Jin, Y. Mycotoxin zearalenone induces apoptosis in mouse Leydig cells via an endoplasmic reticulum stress-dependent signalling pathway. Reprod. Toxicol. 2015, 52, 71–77. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xiao, H.; Bi, M. ROS and endoplasmic reticulum stress. Chin. Pharmaco Bull. 2011, 27, 597–600. [Google Scholar] [CrossRef]
- Jaenen, V.; Fraguas, S.; Bijnens, K.; Heleven, M.; Artois, T.; Romero, R.; Smeets, K.; Cebria, F. Reactive oxygen species rescue regeneration after silencing the MAPK-ERK signaling pathway in Schmidtea mediterranea. Sci. Rep. 2021, 11, 881. [Google Scholar] [CrossRef]
- Lei, K.; Davis, R.J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 2432–2437. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.F.; Yeung, H.T.; Lam, Y.M.; Ng, R.K. Reactive oxygen species activate differentiation gene transcription of acute myeloid leukemia cells via the JNK/c-JUN signaling pathway. Leuk. Res. 2018, 68, 112–119. [Google Scholar] [CrossRef]
- Mitomo, S.; Omatsu, T.; Tsuchiaka, S.; Nagai, M.; Furuya, T.; Mizutani, T. Activation of c-Jun N-terminal kinase by Akabane virus is required for apoptosis. Res. Vet. Sci. 2016, 107, 147–151. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Mohamed, E.; Rodriguez, P.C. Unfolding anti-tumor immunity: ER stress responses sculpt tolerogenic myeloid cells in cancer. J. Immunother. Cancer 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Zhao, J.; Xu, J.; Zhu, L.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. N-acetylcysteine ameliorate cytotoxic injury in piglets sertoli cells induced by zearalenone and deoxynivalenol. Environ. Sci. Pollut. Res. Int. 2021, 28, 60276–60289. [Google Scholar] [CrossRef] [PubMed]





| Gene | Accession Number | Primer | Primer Sequences (5′–3′) | Product Size/bp |
|---|---|---|---|---|
| GAPDH | XM_021091114.1 | Forward | TGACCCCTTCATTGACCTCC | 160 |
| Reverse | CCATTTGATGTTGGCGGGAT | |||
| GRP78 | X92446.1 | Forward | GGCTCTACTCGCATCCCAAAG | 115 |
| Reverse | CCTGAACAGCAGCACCGTAA | |||
| CHOP | XM_005674378.2 | Forward | CTTCACCACTCTTGACCCTG | 170 |
| Reverse | CACTTTGTTTCCGTTTCCTG | |||
| HSP70 | NM_001123127.1 | Forward | GCACGAGGAAAGCCTTAGAG | 166 |
| Reverse | GGAGAAGATGGGACGACAAA | |||
| PERK | XM_003124925.4 | Forward | TCTTGGTAGGGTCTGATGAA | 132 |
| Reverse | GCTTGTAGTATGGCAGGTAAT | |||
| BCL2 | XM_021099602.1 | Forward | TCCAGGCAGTTTAATACATTC | 80 |
| Reverse | TCCCTTTATACACTGGGTGA | |||
| Bax | XM_003127290.5 | Forward | TGGAGCAGGTGCCTCAGGAT | 171 |
| Reverse | TGCCGTCAGCAAACATTTCG | |||
| CASP3 | NM_214131.1 | Forward | TCTAACTGGCAAACCCAAAC | 85 |
| Reverse | AGTCCCACTGTCCGTCTCAA | |||
| CASP9 | XM_013998997.2 | Forward | ACAGGACCGCCGACAGTAAC | 154 |
| Reverse | TCCCTCCAGGAGACAAACCC | |||
| JNK | XM_021073087.1 | Forward | TCAGGCAAGGGATTTGTTAT | 141 |
| Reverse | TCAGGTATCTTTGGTGGTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Hai, S.; Chen, J.; Ma, L.; Rahman, S.U.; Zhao, C.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Zearalenone Induces Apoptosis in Porcine Endometrial Stromal Cells through JNK Signaling Pathway Based on Endoplasmic Reticulum Stress. Toxins 2022, 14, 758. https://doi.org/10.3390/toxins14110758
Zhao J, Hai S, Chen J, Ma L, Rahman SU, Zhao C, Feng S, Li Y, Wu J, Wang X. Zearalenone Induces Apoptosis in Porcine Endometrial Stromal Cells through JNK Signaling Pathway Based on Endoplasmic Reticulum Stress. Toxins. 2022; 14(11):758. https://doi.org/10.3390/toxins14110758
Chicago/Turabian StyleZhao, Jie, Sirao Hai, Jiawen Chen, Li Ma, Sajid Ur Rahman, Chang Zhao, Shibin Feng, Yu Li, Jinjie Wu, and Xichun Wang. 2022. "Zearalenone Induces Apoptosis in Porcine Endometrial Stromal Cells through JNK Signaling Pathway Based on Endoplasmic Reticulum Stress" Toxins 14, no. 11: 758. https://doi.org/10.3390/toxins14110758