The Role of Plasma Membrane Pleiotropic Drug Resistance Transporters in the Killer Activity of Debaryomyces hansenii and Wickerhamomyces anomalus Toxins
Abstract
:1. Introduction
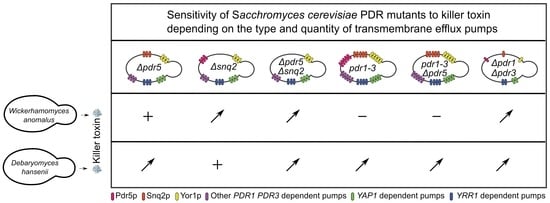
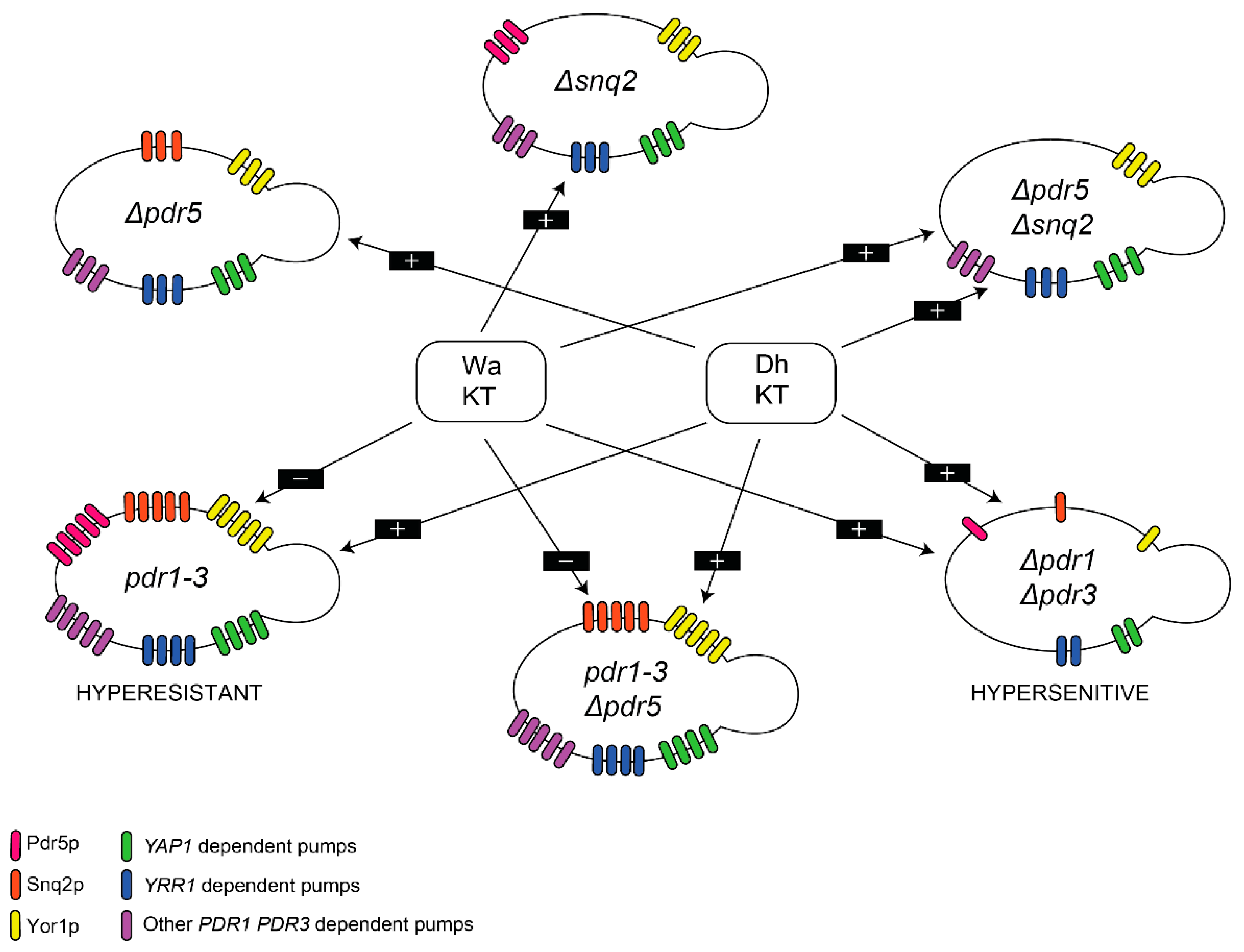
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Yeast Strains
5.2. Killer Toxin Production
5.3. Killer Toxin Diffusion Assay
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Qaysi, S.A.S.; Al-Haideri, H.; Thabit, Z.A.; Al-Kubaisy, W.H.A.A.-R.; Ibrahim, J.A.A.-R. Production, Characterization, and Antimicrobial Activity of Mycocin Produced by Debaryomyces hansenii DSMZ70238. Int. J. Microbiol. 2017, 2017, 2605382. [Google Scholar] [CrossRef] [Green Version]
- Banjara, N.; Nickerson, K.W.; Suhr, M.J.; Hallen-Adams, H.E. Killer toxin from several food-derived Debaryomyces hansenii strains effective against pathogenic Candida yeasts. Int. J. Food Microbiol. 2016, 222, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Breuer, U.; Harms, H. Debaryomyces hansenii—An extremophilic yeast with biotechnological potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef]
- Droby, S.; Chalutz, E.; Wilson, C.L.; Wisniewski, M. Characterization of the biocontrol activity of Debaryomyces hansenii in the control of Penicillium digitatum on grapefruit. Can. J. Microbiol. 1989, 35, 794–800. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Ochoa, J.L.; Troyo-Diéguez, E.; Larralde-Corona, C.P. Biocontrol of postharvest blue mold (Penicillium italicum Wehmer) on Mexican lime by marine and citrus Debaryomyces hansenii isolates. Postharvest Biol. Technol. 2010, 56, 181–187. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Gutierrez-Perez, E.D.; Murillo-Amador, B.; Vero, S.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biol. Technol. 2018, 139, 31–37. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce Penicillium digitatum postharvest decay of oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Lima, J.R.; Gondim, D.M.F.; Oliveira, J.T.A.; Oliveira, F.S.A.; Gonçalves, L.R.B.; Viana, F.M.P. Use of killer yeast in the management of postharvest papaya anthracnose. Postharvest Biol. Technol. 2013, 83, 58–64. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Platania, C.; Restuccia, C.; Muccilli, S.; Cirvilleri, G. Efficacy of killer yeasts in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis). Food Microbiol. 2012, 30, 219–225. [Google Scholar] [CrossRef]
- Prista, C.; Michan, C.; Miranda, I.; Ramos, J. The halotolerant Debaryomyces hansenii, the Cinderella of non-conventional yeasts. Yeast 2016, 33, 523–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żarowska, B.; Wojtatowicz, M.; Połomska, X.; Juszczyk, P.; Chrzanowska, J. Factors affecting killer activity of some yeast species occurring in Rokpol cheese. Folia Microbiol. 2004, 49, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Çorbacı, C.; Uçar, F.B. Purification, characterization and in vivo biocontrol efficiency of killer toxins from Debaryomyces hansenii strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef]
- Czarnecka, M.; Żarowska, B.; Połomska, X.; Restuccia, C.; Cirvilleri, G. Role of biocontrol yeasts Debaryomyces hansenii and Wickerhamomyces anomalus in plants’ defence mechanisms against Monilinia fructicola in apple fruits. Food Microbiol. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Żarowska, B.; Restuccia, C.; Cirvilleri, G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017, 61, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-J.; Ma, Y.; Xu, H.-M.; Wang, X.-H.; Chi, Z.-M. A novel killer toxin produced by the marine-derived yeast Wickerhamomyces anomalus YF07b. Antonie Van Leeuwenhoek 2013, 103, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Vitale, A.; Polizzi, G.; Restuccia, C.; Cirvilleri, G. Understanding the mechanism of biological control of postharvest phytopathogenic moulds promoted by food isolated yeasts. Acta Hortic. 2016, 1144, 93–100. [Google Scholar] [CrossRef]
- Rogers, D.; Bevan, E.A. Group Classification of Killer Yeasts Based on Cross-reactions between Strains of Different Species and Origin. J. Gen. Microbiol. 1978, 155, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Woods, D.R.; Ross, I.W.; Hendry, D.A. A New Killer Factor Produced by a Killer/Sensitive Yeast Strain. J. Gen. Microbiol. 1974, 81, 285–289. [Google Scholar] [CrossRef] [Green Version]
- El-Banna, A.A.; Malak, A.; El-Sahn; Shehata, M.G. Yeasts Producing Killer Toxins: An Overview. Alex. J. Food. Sci. Technol. 2011, 2, 41–53. [Google Scholar]
- Hodgson, V.J.; Button, D.; Walker, G.M. Anti-Candida activity of a novel killer toxin from the yeast Williopsis mrakii. Microbiology 1995, 141, 2003–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugisaki, Y.; Gunge, N.; Sakaguchi, K.; Yamasaki, M.; Tamura, G. Characterization of a novel killer toxin encoded by a double-stranded linear DNA plasmid of Kluyveromyces lactis. Eur. J. Biochem. 1984, 141, 241–245. [Google Scholar] [CrossRef]
- Woods, D.R.; Bevan, E.A. Studies on the Nature of the Killer Factor Produced by Saccharomyces cerevisiae. Microbiology 1968, 51, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Kolaczkowska, A.; Goffeau, A. Regulation of pleiotropic drug resistance in yeast. Drug Resist. Updates 1999, 2, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Golin, J.; Ambudkar, S.V. The multidrug transporter Pdr5 on the 25th anniversary of its discovery: An important model for the study of asymmetric ABC transporters. Biochem. J. 2015, 467, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Gaur, N.A.; Gaur, M.; Komath, S.S. Efflux pumps in drug resistance of Candida. Infect. Disord. Drug Targets 2006, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Balzi, E.; Chen, W.; Ulaszewskit, S.; Capieaux, E.; Goffeaust, A. The Multidrug Resistance Gene PDRl from Saccharomyces cerevisiae. J. Biol. Chem. 1987, 262, 16871–16879. [Google Scholar] [CrossRef]
- Rogers, B.; Decottignies, A.; Kolaczkowski, M.; Carvajal, E.; Balzi, E.; Goffeau, A. The pleiotropic drug ABC transporters from Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 2001, 3, 207–214. [Google Scholar]
- Delaveau, T.; Delahodde, A.; Carvajal, E.; Subik, J.; Jacq, C. PDR3, a new yeast regulatory gene, is homologous to PDR1 and controls the multidrug resistance phenomenon. Mol. Gen. Genet. MGG 1994, 244, 501–511. [Google Scholar] [CrossRef]
- Carvajal, E.; van den Hazel, H.B.; Cybularz-Kolaczkowska, A.; Balzi, E.; Goffeau, A. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol. Gen. Genet. 1997, 256, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decottignies, A.; Kolaczkowski, M.; Balzi, E.; Goffeau, A. Solubilization and characterization of the overexpressed PDR5 multidrug resistance nucleotide triphosphatase of yeast. J. Biol. Chem. 1994, 269, 12797–12803. [Google Scholar] [CrossRef]
- Decottignies, A.; Lambert, L.; Catty, P.; Degand, H.; Epping, E.A.; Moye-Rowley, W.S.; Balzi, E.; Goffeau, A. Identification and characterization of SNQ2, a new multidrug ATP binding cassette transporter of the yeast plasma membrane. J. Biol. Chem. 1995, 270, 18150–18157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahé, Y.; Lemoine, Y.; Kuchler, K. The ATP Binding Cassette Transporters Pdr5 and Snq2 of Saccharomyces cerevisiae Can Mediate Transport of Steroids In Vivo. J. Biol. Chem. 1996, 41, 25167–25172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahé, Y.; Parle-McDermott, A.; Nourani, A.; Delahodde, A.; Lamprecht, A.; Kuchler, K. The ATP-binding cassette multidrug transporter Snq2 of Saccharomyces cerevisiae: A novel target for the transcription factors Pdr1 and Pdr3. Mol. Microbiol. 1996, 20, 109–117. [Google Scholar] [CrossRef]
- de Waard, M.A.; Andrade, A.C.; Hayashi, K.; Schoonbeek, H.; Stergiopoulos, I.; Zwiers, L.-H. Impact of fungal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Manag. Sci. 2006, 62, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Hershkovitz, V.; Sela, N.; Taha-Salaime, L.; Liu, J.; Rafael, G.; Kessler, C.; Aly, R.; Levy, M.; Wisniewski, M.; Droby, S. De-novo assembly and characterization of the transcriptome of Metschnikowia fructicola reveals differences in gene expression following interaction with Penicillium digitatum and grapefruit peel. BMC Genom. 2013, 14, 168. [Google Scholar] [CrossRef] [Green Version]
- Nuruzzaman, M.; Zhang, R.; Cao, H.-Z.; Luo, Z.-Y. Plant Pleiotropic Drug Resistance Transporters: Transport Mechanism, Gene Expression, and Function. J. Integr. Plant Biol. 2014, 56, 729–740. [Google Scholar] [CrossRef]
- Izgu, F.; Altinbay, D. Isolation and Characterization of the K5-Type Yeast Killer Protein and Its Homology with an Exo-β-1,3-glucanase. Biosci. Biotechnol. Biochem. 2004, 68, 685–693. [Google Scholar] [CrossRef]
- Muccilli, S.; Wemhoff, S.; Restuccia, C.; Meinhardt, F. Exoglucanase-encoding genes from three Wickerhamomyces anomalus killer strains isolated from olive brine. Yeast 2013, 30, 33–43. [Google Scholar] [CrossRef]
- Santos, A.; Marquina, D.; Barroso, J.; Peinado, J.M. (1→6)-β-D-glucan as the cell wall binding site for Debaryomyces hansenii killer toxin. Lett. Appl. Microbiol. 2002, 34, 95–99. [Google Scholar] [CrossRef]
- Żarowska, B. Biosynthesis and Characteristics of Debaryomyces hansenii Killer Toxin, Monograph ed.; University of Environmental and Life Sciences Publishing House: Tokyo, Japan, 2012. [Google Scholar]
- Kolaczkowska, A.; Kolaczkowski, M.; Goffeau, A.; Moye-Rowley, W.S. Compensatory activation of the multidrug transporters Pdr5p, Snq2p, and Yor1p by Pdr1p in Saccharomyces cerevisiae. FEBS Lett. 2008, 582, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, A.; Hashida-Okado, T.; Endo, M.; Yoshioka, H.; Tsuruo, T.; Takesako, K.; Kato, I. Role of ABC Transporters in Aureobasidin A Resistance. Antimicrob. Agents Chemother. 1998, 42, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Takesako, K.; Ikai, K.; Haruna, F.; Endo, M.; Shimanaka, K.; Sono, E.; Nakamura, T.; Kato, I.; Yamaguchi, H. Aureobasidins, new antifungal antibiotics. Taxonomy, fermentation, isolation, and properties. J. Antibiot. 1991, 44, 919–924. [Google Scholar] [CrossRef]
- Chalutz, E. Postharvest biocontrol of green and blue mold and sour rot of citrus fruit by Debaryomyces hansenii. Plant Dis. 1990, 74, 135–137. [Google Scholar] [CrossRef]
- Santos, A.; Sánchez, A.; Marquina, D. Yeasts as biological agents to control Botrytis cinerea. Microbiol. Res. 2004, 159, 331–338. [Google Scholar] [CrossRef]
- Hua, S.S.T.; Beck, J.J.; Sarreal, S.B.L.; Gee, W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef]
- Druvefors, U.A.; Passoth, V.; Schnurer, J. Nutrient Effects on Biocontrol of Penicillium roqueforti by Pichia anomala J121 during Airtight Storage of Wheat. Appl. Environ. Microbiol. 2005, 71, 1865–1869. [Google Scholar] [CrossRef] [Green Version]
- Hazelwood, L.A.; Tai, S.L.; Boer, V.M.; de Winde, J.H.; Pronk, J.T.; Daran, J.M. A new physiological role for Pdr12p in Saccharomyces cerevisiae: Export of aromatic and branched-chain organic acids produced in amino acid catabolism. FEMS Yeast Res. 2006, 6, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Kren, A.; Mamnun, Y.M.; Bauer, B.E.; Schüller, C.; Wolfger, H.; Hatzixanthis, K.; Mollapour, M.; Gregori, C.; Piper, P.; Kuchler, K. War1p, a novel transcription factor controlling weak acid stress response in yeast. Mol. Cell. Biol. 2003, 23, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Morschhäuser, J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet. Biol. 2010, 47, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole fungicides—Understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtatowicz, M.; Chrzanowska, J.; Juszczyk, P.; Skiba, A.; Gdula, A. Identification and biochemical characteristics of yeast microflora of Rokpol cheese. Int. J. Food Microbiol. 2001, 69, 135–140. [Google Scholar] [CrossRef]
- Muccilli, S.; Caggia, C.; Randazzo, C.L.; Restuccia, C. Yeast dynamics during the fermentation of brined green olives treated in the field with kaolin and Bordeaux mixture to control the olive fruit fly. Int. J. Food Microbiol. 2011, 148, 15–22. [Google Scholar] [CrossRef]
- Bissinger, P.H.; Kuchler, K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J. Biol. Chem. 1994, 11, 4180–4186. [Google Scholar] [CrossRef]
- Kugler, G.K.; Jandric, Z.; Beyer, R.; Klopf, E.; Glaser, W.; Lemmens, M.; Shams, M.; Mayer, K.; Adam, G.; Schuller, C. Ribosome quality control is a central protection mechanism for yeast exposed to deoxynivalenol and trichothecin. BMC Genom. 2016, 17, 417. [Google Scholar] [CrossRef] [Green Version]




| Yeast Strain | Killer Phenotype |
|---|---|
| D. hansenii KI2a | K+R+ |
| D. hansenii MI1a | K+R+ |
| D. hansenii AII4b | K+R+ |
| D. hansenii CBS 767 | K+R− |
| D. hansenii CLIB 545 | K−R− |
| W. anomalus BS91 | K+R+ |
| S. cerevisiae YPH 500 | K−R−D,−W |
| S. cerevisiae FY 1679-28 | K−R−D,+W |
| S. cerevisiae YALA-B1 | K−R−D,−W |
| Strain * | Genotype | Reference |
|---|---|---|
| YPH 500 | MATα ura3-52 his3-Δ200 leu2-Δ1 trp1-Δ63 lys2-801amber ade2-101ochre | [34] |
| YKKB-13 | MATα ura3-52 his3-Δ200 leu2-Δ1 trp1-Δ63 lys2-801 ade2-101 pdr5Δ::TRP1 | [58] |
| YYM 5 | MATα ura3-52 his3-Δ200 leu2-Δ1 trp1-Δ63 lys2-801 ade2-101 snq2Δ::hisG | [34] |
| YYM 3 | MATα ura3-52 his3-Δ200 leu2-Δ1 trp1-Δ63 lys2-801 ade2-101 pdr5Δ::TRP1 snq2Δ::hisG | [35] |
| FY-1679-28C | MATa ura3-53, leu2-∆1, trp1-∆63, his3∆200, GAL2+ | [30] |
| FY Δpdr1Δpdr3 | MATa ura3-53, leu2-∆1, trp1-∆63, his3∆200, GAL2+ pdr1-∆2::TRP1 pdr3-1∆::HIS3 | [30] |
| FY-WT/Δpdr5-2 | MATa ura3-53, leu2-∆1, trp1-∆63, his3∆200, GAL2+ pdr5Δ::URA3 | [59] |
| YALA-B1 | MATa ura3-52 leu2-3,112 his3-11,115 trp1-1 | [35] |
| YALA-G4 | MATa ura3-52 leu2-3,112 his3-11,115 trp1-1 pdr1-3 | [35] |
| YZGA 278 | MATa ura3-52, leu2-3,112 his3-11,115 trp1-1 pdr1-3 pdr5∆::hisG | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka, M.; Połomska, X.; Restuccia, C.; Żarowska, B. The Role of Plasma Membrane Pleiotropic Drug Resistance Transporters in the Killer Activity of Debaryomyces hansenii and Wickerhamomyces anomalus Toxins. Toxins 2022, 14, 180. https://doi.org/10.3390/toxins14030180
Czarnecka M, Połomska X, Restuccia C, Żarowska B. The Role of Plasma Membrane Pleiotropic Drug Resistance Transporters in the Killer Activity of Debaryomyces hansenii and Wickerhamomyces anomalus Toxins. Toxins. 2022; 14(3):180. https://doi.org/10.3390/toxins14030180
Chicago/Turabian StyleCzarnecka, Monika, Xymena Połomska, Cristina Restuccia, and Barbara Żarowska. 2022. "The Role of Plasma Membrane Pleiotropic Drug Resistance Transporters in the Killer Activity of Debaryomyces hansenii and Wickerhamomyces anomalus Toxins" Toxins 14, no. 3: 180. https://doi.org/10.3390/toxins14030180