Post-Translational Modifications of Histones Are Versatile Regulators of Fungal Development and Secondary Metabolism
Abstract
:1. Introduction
2. Chromatin Dynamics to Regulate Genomic Segments in Fungi
3. Post-Translational Modifications of Histones in Fungi
3.1. Histone Acetylation, an Active Mark of Transcription
3.1.1. Molecular Events
3.1.2. Developmental Consequences
3.1.3. Production of Secondary Metabolites
3.2. Methylation, a Highly Diverse Array of Functions
3.2.1. Molecular Events
3.2.2. Developmental Events
3.2.3. Production of Secondary Metabolites
3.3. Others PTMs
3.3.1. Phosphorylation
3.3.2. Ubiquitylation
3.3.3. Sumoylation
3.3.4. Lysine Acylation
4. The Histone Code
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal Structure of the Nucleosome Core Particle at 2.8 Å Resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.D.; Poirier, M.G. Post-Translational Modifications of Histones That Influence Nucleosome Dynamics. Chem. Rev. 2015, 115, 2274–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyodorov, D.V.; Zhou, B.-R.; Skoultchi, A.I.; Bai, Y. Emerging Roles of Linker Histones in Regulating Chromatin Structure and Function. Nat. Rev. Mol. Cell Biol. 2018, 19, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Wolffe, A.P. Histone Genes. In Encyclopedia of Genetics; Elsevier: Amsterdam, The Netherlands, 2001; pp. 948–952. ISBN 978-0-12-227080-2. [Google Scholar] [CrossRef]
- Cutter, A.R.; Hayes, J.J. A Brief Review of Nucleosome Structure. FEBS Lett. 2015, 589, 2914–2922. [Google Scholar] [CrossRef] [Green Version]
- Allan, J.; Harborne, N.; Rau, D.C.; Gould, H. Participation of Core Histone" Tails" in the Stabilization of the Chromatin Solenoid. J. Cell Biol. 1982, 93, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Garcia, B.A.; Shabanowitz, J.; Hunt, D.F. Characterization of Histones and Their Post-Translational Modifications by Mass Spectrometry. Curr. Opin. Chem. Biol. 2007, 11, 66–73. [Google Scholar] [CrossRef]
- Lu, C.; Coradin, M.; Porter, E.G.; Garcia, B.A. Accelerating the Field of Epigenetic Histone Modification Through Mass Spectrometry–Based Approaches. Mol. Cell. Proteom. 2021, 20, 100006. [Google Scholar] [CrossRef]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41. [Google Scholar] [CrossRef]
- Biswas, S.; Rao, C.M. Epigenetic Tools (The Writers, The Readers and The Erasers) and Their Implications in Cancer Therapy. Eur. J. Pharmacol. 2018, 837, 8–24. [Google Scholar] [CrossRef]
- Botstein, D.; Chervitz, S.A.; Cherry, M. Yeast as a Model Organism. Science 1997, 277, 1259–1260. [Google Scholar] [CrossRef] [Green Version]
- Galagan, J.E.; Calvo, S.E.; Borkovich, K.A.; Selker, E.U.; Read, N.D.; Jaffe, D.; FitzHugh, W.; Ma, L.-J.; Smirnov, S.; Purcell, S.; et al. The Genome Sequence of the Filamentous Fungus Neurospora Crassa. Nature 2003, 422, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium Head Blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Fumonisin B1 Induces Oxidative Stress in Oesophageal (SNO) Cancer Cells. Toxicon 2018, 141, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.M.; Keller, N.P. Secondary Metabolism in Fungi: Does Chromosomal Location Matter? Curr. Opin. Microbiol. 2010, 13, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Keller, N.P. Fungal Secondary Metabolism: Regulation, Function and Drug Discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Keller, N.P.; Hohn, T.M. Metabolic Pathway Gene Clusters in Filamentous Fungi. Fungal Genet. Biol. 1997, 21, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Brakhage, A.A. Regulation of Fungal Secondary Metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Bok, J.W.; Chiang, Y.-M.; Szewczyk, E.; Reyes-Dominguez, Y.; Davidson, A.D.; Sanchez, J.F.; Lo, H.-C.; Watanabe, K.; Strauss, J.; Oakley, B.R.; et al. Chromatin-Level Regulation of Biosynthetic Gene Clusters. Nat. Chem. Biol. 2009, 5, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Gacek, A.; Strauss, J. The Chromatin Code of Fungal Secondary Metabolite Gene Clusters. Appl. Microbiol. Biotechnol. 2012, 95, 1389–1404. [Google Scholar] [CrossRef] [Green Version]
- Pfannenstiel, B.T.; Keller, N.P. On Top of Biosynthetic Gene Clusters: How Epigenetic Machinery Influences Secondary Metabolism in Fungi. Biotechnol. Adv. 2019, 37, 107345. [Google Scholar] [CrossRef]
- Strauss, J.; Reyes-Dominguez, Y. Regulation of Secondary Metabolism by Chromatin Structure and Epigenetic Codes. Fungal Genet. Biol. 2011, 48, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitag, M. Histone Methylation by SET Domain Proteins in Fungi. Annu. Rev. Microbiol. 2017, 71, 413–439. [Google Scholar] [CrossRef] [PubMed]
- Karch, K.R.; Sidoli, S.; Garcia, B.A. Identification and Quantification of Histone PTMs Using High-Resolution Mass Spectrometry. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 574, pp. 3–29. ISBN 978-0-12-805381-2. [Google Scholar] [CrossRef] [Green Version]
- Minshull, T.C.; Dickman, M.J. Mass Spectrometry Analysis of Histone Post Translational Modifications. Drug Discov. Today Dis. Models 2014, 12, 41–48. [Google Scholar] [CrossRef]
- Fenley, A.T.; Anandakrishnan, R.; Kidane, Y.H.; Onufriev, A.V. Modulation of Nucleosomal DNA Accessibility via Charge-Altering Post-Translational Modifications in Histone Core. Epigenetics Chromatin 2018, 11, 11. [Google Scholar] [CrossRef]
- Martin, C.; Zhang, Y. The Diverse Functions of Histone Lysine Methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef]
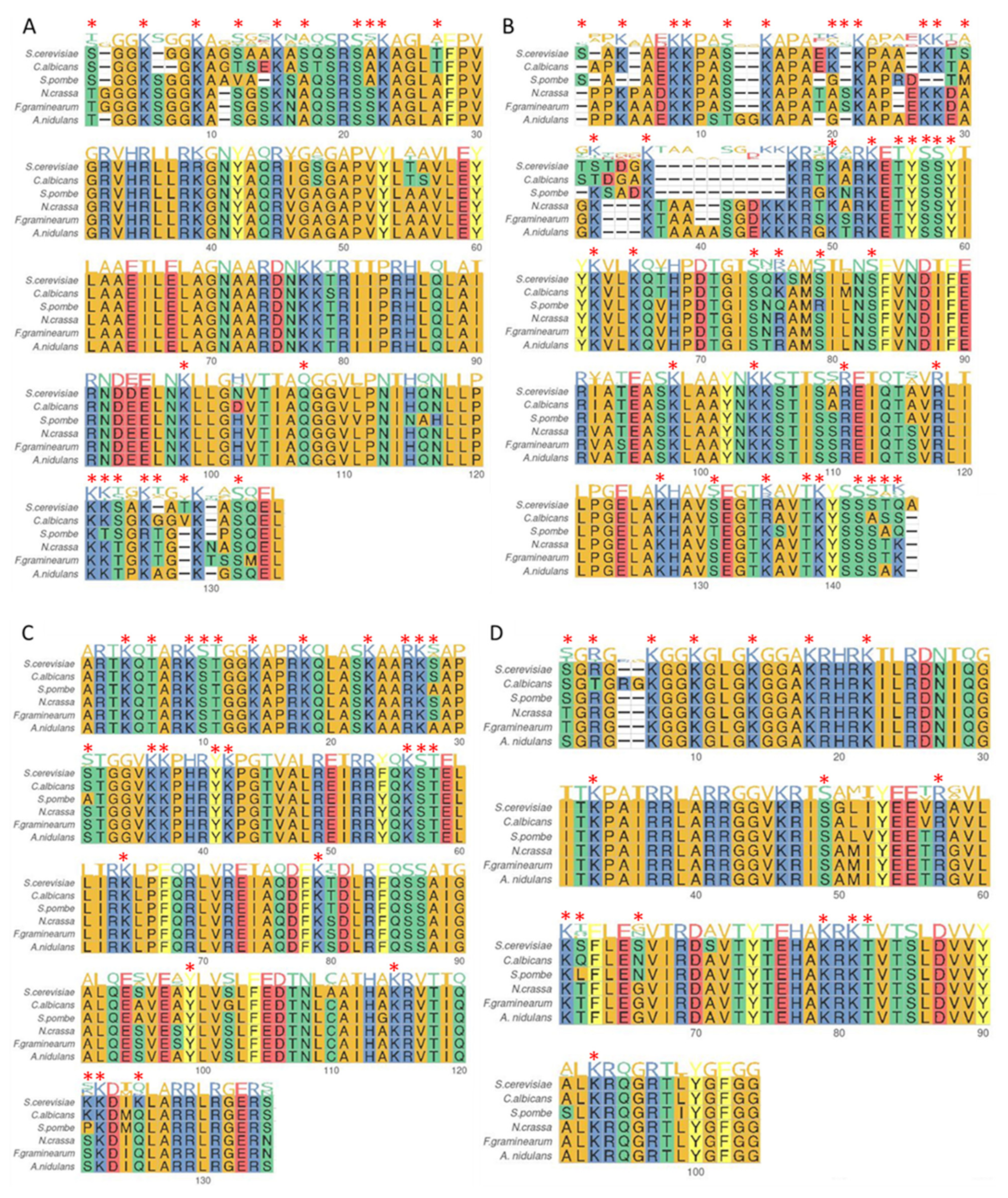
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-Based Alignment Tool for Multiple Protein Sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Zhou, L.; Huang, H. Ggmsa: Plot Multiple Sequence Alignment Using “Ggplot2”. 2020. Available online: https://CRAN.R-project.org/package=ggmsa (accessed on 29 March 2022).
- Clayton, A.L.; Hazzalin, C.A.; Mahadevan, L.C. Enhanced Histone Acetylation and Transcription: A Dynamic Perspective. Mol. Cell 2006, 23, 289–296. [Google Scholar] [CrossRef]
- Marmorstein, R.; Zhou, M.-M. Writers and Readers of Histone Acetylation: Structure, Mechanism, and Inhibition. Cold Spring Harb. Perspect. Biol. 2014, 6, a018762. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.-H.; Allis, C.D. Roles of Histone Acetyltransferases and Deacetylases in Gene Regulation. BioEssays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Fan, J.; Krautkramer, K.A.; Feldman, J.L.; Denu, J.M. Metabolic Regulation of Histone Post-Translational Modifications. ACS Chem. Biol. 2015, 10, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Thorne, A.W.; Kmiciek, D.; Mitchelson, K.; Sautiere, P.; Crane-Robinson, C. Patterns of Histone Acetylation. Eur. J. Biochem. 1990, 193, 701–713. [Google Scholar] [CrossRef]
- Jeon, J.; Kwon, S.; Lee, Y.-H. Histone Acetylation in Fungal Pathogens of Plants. Plant Pathol. J. 2014, 30, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gallinari, P.; Marco, S.D.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, Histone Deacetylation and Gene Transcription: From Molecular Biology to Cancer Therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef]
- Li, X.; Pan, L.; Wang, B.; Pan, L. The Histone Deacetylases HosA and HdaA Affect the Phenotype and Transcriptomic and Metabolic Profiles of Aspergillus Niger. Toxins 2019, 11, 520. [Google Scholar] [CrossRef] [Green Version]
- Grant, P.A.; Eberharter, A.; John, S.; Cook, R.G.; Turner, B.M.; Workman, J.L. Expanded Lysine Acetylation Specificity of Gcn5 in Native Complexes. J. Biol. Chem. 1999, 274, 5895–5900. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bone, J.R.; Edmondson, D.G.; Turner, B.M.; Roth, S.Y. Essential and Redundant Functions of Histone Acetylation Revealed by Mutation of Target Lysines and Loss of the Gcn5p Acetyltransferase. EMBO J. 1998, 17, 3155–3167. [Google Scholar] [CrossRef]
- Howe, L.; Auston, D.; Grant, P.; John, S.; Cook, R.G.; Workman, J.L.; Pillus, L. Histone H3 Specific Acetyltransferases Are Essential for Cell Cycle Progression. Genes Dev. 2001, 15, 3144–3154. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, T.; Liu, C.L.; Erkmann, J.A.; Holik, J.; Grunstein, M.; Kaufman, P.D.; Friedman, N.; Rando, O.J. Cell Cycle- and Chaperone-Mediated Regulation of H3K56ac Incorporation in Yeast. PLoS Genet. 2008, 4, e1000270. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Hay, C.; Price, M.S.; Giles, S.; Alspaugh, J.A. Cryptococcus Neoformans Histone Acetyltransferase Gcn5 Regulates Fungal Adaptation to the Host. Eukaryot. Cell 2010, 9, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Soto, D.; González-Prieto, J.M.; Ruiz-Herrera, J. Transcriptomic Analysis of the GCN5 Gene Reveals Mechanisms of the Epigenetic Regulation of Virulence and Morphogenesis in Ustilago Maydis. FEMS Yeast Res. 2015, 15, fov055. [Google Scholar] [CrossRef] [Green Version]
- González-Prieto, J.M.; Rosas-Quijano, R.; Domínguez, A.; Ruiz-Herrera, J. The UmGcn5 Gene Encoding Histone Acetyltransferase from Ustilago Maydis Is Involved in Dimorphism and Virulence. Fungal Genet. Biol. 2014, 71, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; van Diepeningen, A.D.; van der Lee, T.A.J.; Waalwijk, C.; Xu, J.; Xu, J.; Zhang, H.; Chen, W.; Feng, J. The Fusarium Graminearum Histone Acetyltransferases Are Important for Morphogenesis, DON Biosynthesis, and Pathogenicity. Front. Microbiol. 2018, 9, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; Wang, J.-J.; Shao, W.; Ying, S.-H.; Feng, M.-G. Rtt109-Dependent Histone H3 K56 Acetylation and Gene Activity Are Essential for the Biological Control Potential of Beauveria Bassiana: Role of Rtt109 in Sustaining Pest Control Potential of Beauveria Bassiana. Pest Manag. Sci. 2018, 74, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wen, M.; Wu, L.; Lan, H.; Yuan, J.; Wang, S. The Fungi-Specific Histone Acetyltransferase Rtt109 Mediates Morphogenesis, Aflatoxin Synthesis and Pathogenicity in Aspergillus Flavus by Acetylating H3K9. IMA Fungus 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, B.; Coiro, P.; Filetici, P.; Berge, E.; Dobosy, J.R.; Freitag, M.; Selker, E.U.; Ballario, P. The Neurospora Crassa White Collar-1 Dependent Blue Light Response Requires Acetylation of Histone H3 Lysine 14 by NGF-1. Mol. Biol. Cell 2006, 17, 4576–4583. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.; Lee, J.; Kwon, S.; Lee, Y.; Jeon, J. A MYST Family Histone Acetyltransferase, MoSAS3, Is Required for Development and Pathogenicity in the Rice Blast Fungus. Mol. Plant Pathol. 2019, 20, 1491–1505. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-L.; Liu, W.; Iliuk, A.; Ribot, C.; Vallet, J.; Tao, A.; Wang, Y.; Lebrun, M.-H.; Xu, J.-R. The Tig1 Histone Deacetylase Complex Regulates Infectious Growth in the Rice Blast Fungus Magnaporthe Oryzae. Plant Cell 2010, 22, 2495–2508. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Xu, Y.; Chen, J.; Luo, Y.; Lv, Y.; Su, J.; Kershaw, M.J.; Li, W.; Wang, J.; Yin, J.; et al. MoSnt2-Dependent Deacetylation of Histone H3 Mediates MoTor-Dependent Autophagy and Plant Infection by the Rice Blast Fungus Magnaporthe Oryzae. Autophagy 2018, 14, 1543–1561. [Google Scholar] [CrossRef] [Green Version]
- Lopes da Rosa, J.; Boyartchuk, V.L.; Zhu, L.J.; Kaufman, P.D. Histone Acetyltransferase Rtt109 Is Required for Candida Albicans Pathogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 1594–1599. [Google Scholar] [CrossRef] [Green Version]
- Baidyaroy, D.; Brosch, G.; Ahn, J.; Graessle, S.; Wegener, S.; Tonukari, N.J.; Caballero, O.; Loidl, P.; Walton, J.D. A Gene Related to Yeast HOS2 Histone Deacetylase Affects Extracellular Depolymerase Expression and Virulence in a Plant Pathogenic Fungus. Plant Cell 2001, 13, 1609–1624. [Google Scholar] [CrossRef] [Green Version]
- Elías-Villalobos, A.; Fernández-Álvarez, A.; Moreno-Sánchez, I.; Helmlinger, D.; Ibeas, J.I. The Hos2 Histone Deacetylase Controls Ustilago Maydis Virulence through Direct Regulation of Mating-Type Genes. PLOS Pathog. 2015, 11, e1005134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwab, E.K.; Bok, J.W.; Tribus, M.; Galehr, J.; Graessle, S.; Keller, N.P. Histone Deacetylase Activity Regulates Chemical Diversity in Aspergillus. Eukaryot. Cell 2007, 6, 1656–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutzmann, H.-W.; Reyes-Dominguez, Y.; Scherlach, K.; Schroeckh, V.; Horn, F.; Gacek, A.; Schumann, J.; Hertweck, C.; Strauss, J.; Brakhage, A.A. Bacteria-Induced Natural Product Formation in the Fungus Aspergillus Nidulans Requires Saga/Ada-Mediated Histone Acetylation. Proc. Natl. Acad. Sci. USA 2011, 108, 14282–14287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Dominguez, Y.; Bok, J.W.; Berger, H.; Shwab, E.K.; Basheer, A.; Gallmetzer, A.; Scazzocchio, C.; Keller, N.; Strauss, J. Heterochromatic Marks Are Associated with the Repression of Secondary Metabolism Clusters in Aspergillus Nidulans: Heterochromatin Regulation of Secondary Metabolism. Mol. Microbiol. 2010, 76, 1376–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandão, F.A.; Derengowski, L.S.; Albuquerque, P.; Nicola, A.M.; Silva-Pereira, I.; Poças-Fonseca, M.J. Histone Deacetylases Inhibitors Effects on Cryptococcus Neoformans Major Virulence Phenotypes. Virulence 2015, 6, 618–630. [Google Scholar] [CrossRef] [Green Version]
- Tribus, M.; Galehr, J.; Trojer, P.; Brosch, G.; Loidl, P.; Marx, F.; Haas, H.; Graessle, S. HdaA, a Major Class 2 Histone Deacetylase of Aspergillus Nidulans, Affects Growth under Conditions of Oxidative Stress. Eukaryot. Cell 2005, 4, 1736–1745. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Oh, J.-H.; Keats Shwab, E.; Dagenais, T.R.T.; Andes, D.; Keller, N.P. HdaA, a Class 2 Histone Deacetylase of Aspergillus Fumigatus, Affects Germination and Secondary Metabolite Production. Fungal Genet. Biol. 2009, 46, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Roze, L.V.; Arthur, A.E.; Hong, S.-Y.; Chanda, A.; Linz, J.E. The Initiation and Pattern of Spread of Histone H4 Acetylation Parallel the Order of Transcriptional Activation of Genes in the Aflatoxin Cluster. Mol. Microbiol. 2007, 66, 713–726. [Google Scholar] [CrossRef]
- Hueza, I.; Raspantini, P.; Raspantini, L.; Latorre, A.; Górniak, S. Zearalenone, an Estrogenic Mycotoxin, Is an Immunotoxic Compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [Green Version]
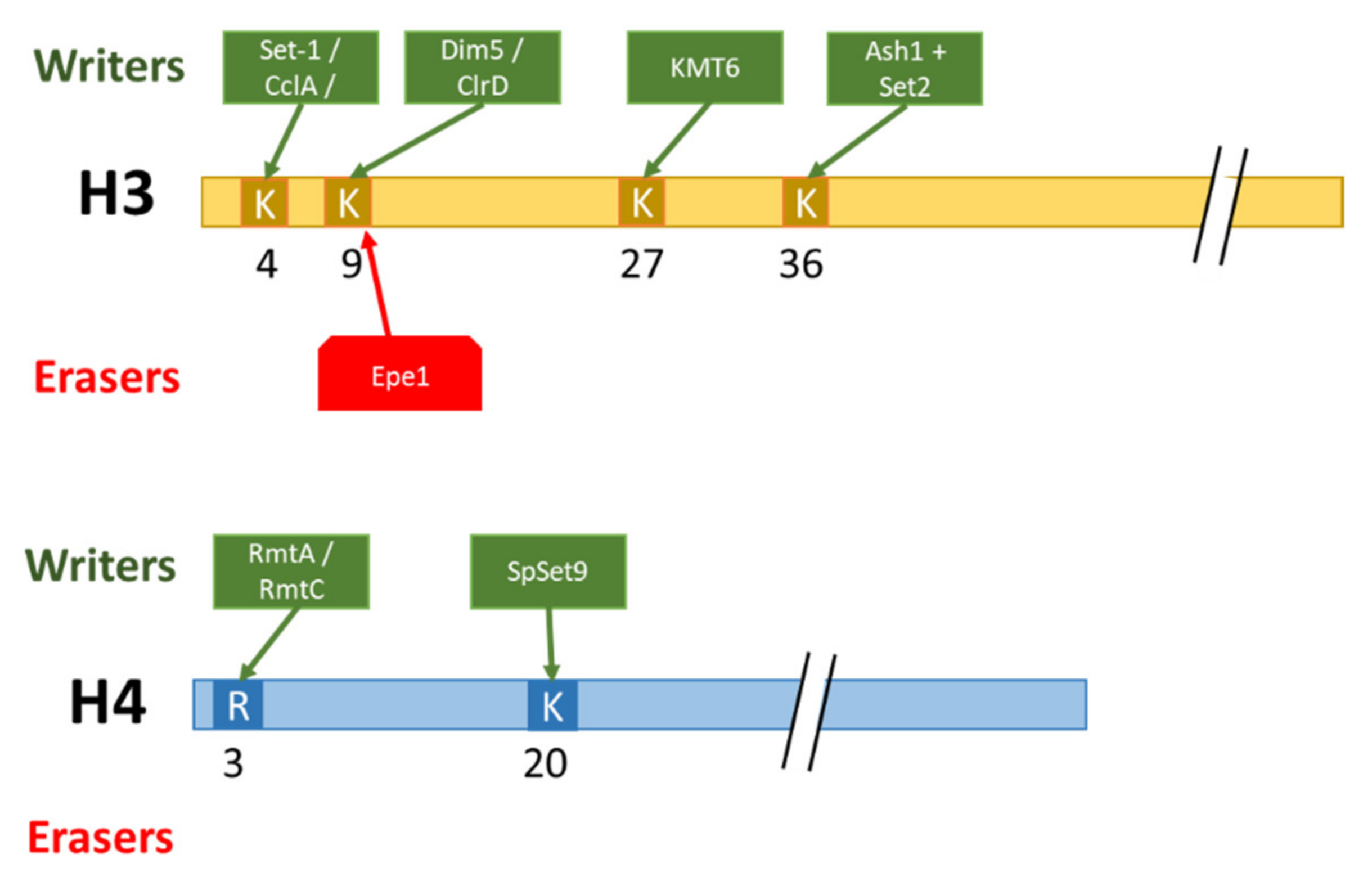
- Connolly, L.R.; Smith, K.M.; Freitag, M. The Fusarium Graminearum Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters. PLoS Genet. 2013, 9, e1003916. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, J.F.; Mi, L.-Z.; Chruszcz, M.; Cymborowski, M.; Clines, K.L.; Kim, Y.; Minor, W.; Rastinejad, F.; Khorasanizadeh, S. Double Chromodomains Cooperate to Recognize the Methylated Histone H3 Tail. Nature 2005, 438, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Binda, O. On Your Histone Mark, SET, Methylate! Epigenetics 2013, 8, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannister, A.J.; Schneider, R.; Kouzarides, T. Histone Methylation. Cell 2002, 109, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, N.; Yin, Y.; Chen, Y.; Jiang, J.; Ma, Z. Histone H3K4 Methylation Regulates Hyphal Growth, Secondary Metabolism and Multiple Stress Responses in F Usarium Graminearum: Functions of H3K4me in F. Graminearum. Environ. Microbiol. 2015, 17, 4615–4630. [Google Scholar] [CrossRef] [PubMed]
- Torres-Garcia, S.; Yaseen, I.; Shukla, M.; Audergon, P.N.C.B.; White, S.A.; Pidoux, A.L.; Allshire, R.C. Epigenetic Gene Silencing by Heterochromatin Primes Fungal Resistance. Nature 2020, 585, 453–458. [Google Scholar] [CrossRef]
- Wiles, E.T.; Selker, E.U. H3K27 Methylation: A Promiscuous Repressive Chromatin Mark. Curr. Opin. Genet. Dev. 2017, 43, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.G.; Tsukada, Y.-I. The Discovery of Histone Demethylases. Cold Spring Harb. Perspect. Biol. 2013, 5, a017947. [Google Scholar] [CrossRef] [Green Version]
- Janevska, S.; Baumann, L.; Sieber, C.M.K.; Münsterkötter, M.; Ulrich, J.; Kämper, J.; Güldener, U.; Tudzynski, B. Elucidation of the Two H3K36me3 Histone Methyltransferases Set2 and Ash1 in Fusarium Fujikuroi Unravels Their Different Chromosomal Targets and a Major Impact of Ash1 on Genome Stability. Genetics 2018, 208, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Reddy, B.; Thompson, J.; Wang, H.; Noma, K.; Yates, J.R.; Jia, S. Regulation of Set9-Mediated H4K20 Methylation by a PWWP Domain Protein. Mol. Cell 2009, 33, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Tamaru, H.; Selker, E.U. A Histone H3 Methyltransferase Controls DNA Methylation in Neurospora Crassa. Nature 2001, 414, 277. [Google Scholar] [CrossRef] [PubMed]
- Gessaman, J.D.; Selker, E.U. Induction of H3K9me3 and DNA Methylation by Tethered Heterochromatin Factors in Neurospora Crassa. Proc. Natl. Acad. Sci. USA 2017, 114, E9598–E9607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maison, C.; Almouzni, G. HP1 and the Dynamics of Heterochromatin Maintenance. Nat. Rev. Mol. Cell Biol. 2004, 5, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Dominguez, Y.; Boedi, S.; Sulyok, M.; Wiesenberger, G.; Stoppacher, N.; Krska, R.; Strauss, J. Heterochromatin Influences the Secondary Metabolite Profile in the Plant Pathogen Fusarium Graminearum. Fungal Genet. Biol. 2012, 49, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Wang, X.; Moazed, D. Epigenetic Inheritance Mediated by Coupling of RNAi and Histone H3K9 Methylation. Nature 2018, 558, 615–619. [Google Scholar] [CrossRef]
- Dallery, J.; Adelin, É.; Le Goff, G.; Pigné, S.; Auger, A.; Ouazzani, J.; O’Connell, R.J. H3K4 Trimethylation by CclA Regulates Pathogenicity and the Production of Three Families of Terpenoid Secondary Metabolites in Colletotrichum Higginsianum. Mol. Plant Pathol. 2019, 20, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Raduwan, H.; Isola, A.L.; Belden, W.J. Methylation of Histone H3 on Lysine 4 by the Lysine Methyltransferase SET1 Protein Is Needed for Normal Clock Gene Expression. J. Biol. Chem. 2013, 288, 8380–8390. [Google Scholar] [CrossRef] [Green Version]
- Proietto, M.; Bianchi, M.; Ballario, P.; Brenna, A. Epigenetic and Posttranslational Modifications in Light Signal Transduction and the Circadian Clock in Neurospora Crassa. Int. J. Mol. Sci. 2015, 16, 15347–15383. [Google Scholar] [CrossRef] [Green Version]
- Kronholm, I.; Ketola, T. Effects of Acclimation Time and Epigenetic Mechanisms on Growth of Neurospora in Fluctuating Environments. Heredity 2018, 121, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Zhou, Z.; Liu, X.; Gai, K.; Liu, Q.; Cha, J.; Kaleri, F.N.; Wang, Y.; He, Q. Suppression of WHITE COLLAR-Independent Frequency Transcription by Histone H3 Lysine 36 Methyltransferase SET-2 Is Necessary for Clock Function in Neurospora. J. Biol. Chem. 2016, 291, 11055–11063. [Google Scholar] [CrossRef] [Green Version]
- Adhvaryu, K.K.; Morris, S.A.; Strahl, B.D.; Selker, E.U. Methylation of Histone H3 Lysine 36 Is Required for Normal Development in Neurospora Crassa. Eukaryot. Cell 2005, 4, 1455–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Q.; Ji, T.; Sun, X.; Huang, H.; Zhang, H.; Lu, X.; Wu, L.; Huo, R.; Wu, H.; Gao, X. Histone H3 Lysine 9 Methyltransferase FvDim5 Regulates Fungal Development, Pathogenicity and Osmotic Stress Responses in Fusarium Verticillioides. FEMS Microbiol. Lett. 2017, 364, 364. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Rösler, S.M.; Burkhardt, I.; Arndt, B.; Freitag, M.; Humpf, H.-U.; Dickschat, J.S.; Tudzynski, B. Knock-down of the Methyltransferase Kmt6 Relieves H3K27me3 and Results in Induction of Cryptic and Otherwise Silent Secondary Metabolite Gene Clusters in Fusarium Fujikuroi: Relief of H3K27me3 Induces Silent SM Gene Clusters. Environ. Microbiol. 2016, 18, 4037–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Huang, J.; Cook, D.E. Histone Modification Dynamics at H3K27 Are Associated with Altered Transcription of in Planta Induced Genes in Magnaporthe Oryzae. PLoS Genet. 2021, 17, e1009376. [Google Scholar] [CrossRef]
- Trojer, P.; Dangl, M.; Bauer, I.; Graessle, S.; Loidl, P.; Brosch, G. Histone Methyltransferases in Aspergillus Nidulans: Evidence for a Novel Enzyme with a Unique Substrate Specificity †. Biochemistry 2004, 43, 10834–10843. [Google Scholar] [CrossRef]
- Bauer, I.; Graessle, S.; Loidl, P.; Hohenstein, K.; Brosch, G. Novel Insights into the Functional Role of Three Protein Arginine Methyltransferases in Aspergillus Nidulans. Fungal Genet. Biol. 2010, 47, 551–561. [Google Scholar] [CrossRef]
- Studt, L.; Janevska, S.; Arndt, B.; Boedi, S.; Sulyok, M.; Humpf, H.-U.; Tudzynski, B.; Strauss, J. Lack of the COMPASS Component Ccl1 Reduces H3K4 Trimethylation Levels and Affects Transcription of Secondary Metabolite Genes in Two Plant–Pathogenic Fusarium Species. Front. Microbiol. 2017, 7, 2144. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Tahir, H.; Zhang, H.; Huang, H.; Ji, T.; Sun, X.; Wu, L.; Wu, H.; Gao, X. Involvement of FvSet1 in Fumonisin B1 Biosynthesis, Vegetative Growth, Fungal Virulence, and Environmental Stress Responses in Fusarium Verticillioides. Toxins 2017, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Lukito, Y.; Chujo, T.; Hale, T.K.; Mace, W.; Johnson, L.J.; Scott, B. Regulation of Subtelomeric Fungal Secondary Metabolite Genes by H3K4me3 Regulators CclA and KdmB. Mol. Microbiol. 2019, 112, 837–853. [Google Scholar] [CrossRef]
- Shinohara, Y.; Kawatani, M.; Futamura, Y.; Osada, H.; Koyama, Y. An Overproduction of Astellolides Induced by Genetic Disruption of Chromatin-Remodeling Factors in Aspergillus Oryzae. J. Antibiot. 2016, 69, 4–8. [Google Scholar] [CrossRef]
- Palmer, J.M.; Bok, J.W.; Lee, S.; Dagenais, T.R.T.; Andes, D.R.; Kontoyiannis, D.P.; Keller, N.P. Loss of CclA, Required for Histone 3 Lysine 4 Methylation, Decreases Growth but Increases Secondary Metabolite Production in Aspergillus Fumigatus. Peer J. 2013, 1, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gacek-Matthews, A.; Berger, H.; Sasaki, T.; Wittstein, K.; Gruber, C.; Lewis, Z.A.; Strauss, J. KdmB, a Jumonji Histone H3 Demethylase, Regulates Genome-Wide H3K4 Trimethylation and Is Required for Normal Induction of Secondary Metabolism in Aspergillus Nidulans. PLoS Genet. 2016, 12, e1006222. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; Scott, B. Histone H3K9 and H3K27 Methylation Regulates Fungal Alkaloid Biosynthesis in a Fungal Endophyte-Plant Symbiosis: K9 and K27 Methylation Regulates Symbiosis. Mol. Microbiol. 2014, 92, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Brehove, M.; Wang, T.; North, J.; Luo, Y.; Dreher, S.J.; Shimko, J.C.; Ottesen, J.J.; Luger, K.; Poirier, M.G. Histone Core Phosphorylation Regulates DNA Accessibility. J. Biol. Chem. 2015, 290, 22612–22621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, W.-S.; Duggan, L.; Tolga, N.C.; Emre; Belotserkovskya, R.; Lane, W.S.; Shiekhattar, R.; Berger, S.L. Snf1—A Histone Kinase That Works in Concert with the Histone Acetyltransferase Gcn5 to Regulate Transcription. Science 2001, 293, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Tan, H.L.; Nguyen, T.T.T.; Sayed, A.M.M.; Li, Y.; Mok, Y.-K.; Yang, H.; Chen, E.S. Regulation of Transcriptional Silencing and Chromodomain Protein Localization at Centromeric Heterochromatin by Histone H3 Tyrosine 41 Phosphorylation in Fission Yeast. Nucleic Acids Res. 2018, 46, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Govin, J.; Schug, J.; Krishnamoorthy, T.; Dorsey, J.; Khochbin, S.; Berger, S.L. Genome-Wide Mapping of Histone H4 Serine-1 Phosphorylation during Sporulation in Saccharomyces Cerevisiae. Nucleic Acids Res. 2010, 38, 4599–4606. [Google Scholar] [CrossRef]
- Krishnamoorthy, T. Phosphorylation of Histone H4 Ser1 Regulates Sporulation in Yeast and Is Conserved in Fly and Mouse Spermatogenesis. Genes Dev. 2006, 20, 2580–2592. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.C.; Jackson, S.P.; Downs, J.A. Saccharomyces Cerevisiae Histone H2A Ser122 Facilitates DNA Repair. Genetics 2005, 170, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Redon, C.; Pilch, D.R.; Rogakou, E.P.; Orr, A.H.; Lowndes, N.F.; Bonner, W.M. Yeast Histone 2A Serine 129 Is Essential for the Efficient Repair of Checkpoint-Blind DNA Damage. EMBO Rep. 2003, 4, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Cheung, W.L.; Turner, F.B.; Krishnamoorthy, T.; Wolner, B.; Ahn, S.-H.; Foley, M.; Dorsey, J.A.; Peterson, C.L.; Berger, S.L.; Allis, C.D. Phosphorylation of Histone H4 Serine 1 during DNA Damage Requires Casein Kinase II in S. Cerevisiae. Curr. Biol. 2005, 15, 656–660. [Google Scholar] [CrossRef] [Green Version]
- Millan-Zambrano, G.; Santos-Rosa, H.; Puddu, F.; Robson, S.C.; Jackson, S.P.; Kouzarides, T. Phosphorylation of Histone H4T80 Triggers DNA Damage Checkpoint Recovery. Mol. Cell 2018, 72, 625–635.e4. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, S.A.; Yamagishi, Y.; Honda, T.; Ishiguro, K.; Watanabe, Y. Phosphorylation of H2A by Bub1 Prevents Chromosomal Instability Through Localizing Shugoshin. Science 2010, 327, 172–177. [Google Scholar] [CrossRef]
- Brimacombe, C.A.; Burke, J.E.; Parsa, J.-Y.; Catania, S.; O’Meara, T.R.; Witchley, J.N.; Burrack, L.S.; Madhani, H.D.; Noble, S.M. A Natural Histone H2A Variant Lacking the Bub1 Phosphorylation Site and Regulated Depletion of Centromeric Histone CENP-A Foster Evolvability in Candida Albicans. PLoS Biol. 2019, 17, e3000331. [Google Scholar] [CrossRef]
- Rossetto, D.; Avvakumov, N.; Côté, J. Histone Phosphorylation: A Chromatin Modification Involved in Diverse Nuclear Events. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.P.; Phillips, J.; Anderson, S.; Qiu, Q.; Shabanowitz, J.; Smith, M.M.; Yates, J.R.; Hunt, D.F.; Grant, P.A. Histone H3 Thr 45 Phosphorylation Is a Replication-Associated Post-Translational Modification in S. Cerevisiae. Nat. Cell Biol. 2010, 12, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.-Y.; Sun, Z.-W.; Li, X.; Reuben, M.; Tatchell, K.; Bishop, D.K.; Grushcow, J.M.; Brame, C.J.; Caldwell, J.A.; Hunt, D.F.; et al. Mitotic Phosphorylation of Histone H3 Is Governed by Ipl1/Aurora Kinase and Glc7/PP1 Phosphatase in Budding Yeast and Nematodes. Cell 2000, 102, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Kalashnikova, A.A.; Porter-Goff, M.E.; Muthurajan, U.M.; Luger, K.; Hansen, J.C. The Role of the Nucleosome Acidic Patch in Modulating Higher Order Chromatin Structure. J. R. Soc. Interface 2013, 10, 20121022. [Google Scholar] [CrossRef] [Green Version]
- Rampitsch, C.; Tinker, N.A.; Subramaniam, R.; Barkow-Oesterreicher, S.; Laczko, E. Phosphoproteome Profile of Fusarium Graminearum Grown in Vitro under Nonlimiting Conditions. Proteomics 2012, 12, 1002–1005. [Google Scholar] [CrossRef]
- Viéitez, C.; Martínez-Cebrián, G.; Solé, C.; Böttcher, R.; Potel, C.M.; Savitski, M.M.; Onnebo, S.; Fabregat, M.; Shilatifard, A.; Posas, F.; et al. A Genetic Analysis Reveals Novel Histone Residues Required for Transcriptional Reprogramming upon Stress. Nucleic Acids Res. 2020, 48, 3455–3475. [Google Scholar] [CrossRef] [Green Version]
- Swatek, K.N.; Komander, D. Ubiquitin Modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [Green Version]
- Cucinotta, C.E.; Young, A.N.; Klucevsek, K.M.; Arndt, K.M. The Nucleosome Acidic Patch Regulates the H2B K123 Monoubiquitylation Cascade and Transcription Elongation in Saccharomyces Cerevisiae. PLoS Genet. 2015, 11, e1005420. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cao, C.; Wang, F.; Zhao, J.; Li, W. H2B Ubiquitination: Conserved Molecular Mechanism, Diverse Physiologic Functions of the E3 Ligase during Meiosis. Nucleus 2017, 8, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Wood, A.; Krogan, N.J.; Dover, J.; Schneider, J.; Heidt, J.; Boateng, M.A.; Dean, K.; Golshani, A.; Zhang, Y.; Greenblatt, J.F.; et al. Bre1, an E3 Ubiquitin Ligase Required for Recruitment and Substrate Selection of Rad6 at a Promoter. Mol. Cell 2003, 11, 267–274. [Google Scholar] [CrossRef]
- Hwang, W.W.; Venkatasubrahmanyam, S.; Ianculescu, A.G.; Tong, A.; Boone, C.; Madhani, H.D. A Conserved RING Finger Protein Required for Histone H2B Monoubiquitination and Cell Size Control. Mol. Cell 2003, 11, 261–266. [Google Scholar] [CrossRef]
- Henry, K.W. Transcriptional Activation via Sequential Histone H2B Ubiquitylation and Deubiquitylation, Mediated by SAGA-Associated Ubp8. Genes Dev. 2003, 17, 2648–2663. [Google Scholar] [CrossRef] [Green Version]
- Batta, K.; Zhang, Z.; Yen, K.; Goffman, D.B.; Pugh, B.F. Genome-Wide Function of H2B Ubiquitylation in Promoter and Genic Regions. Genes Dev. 2011, 25, 2254–2265. [Google Scholar] [CrossRef] [Green Version]
- Tanny, J.C.; Erdjument-Bromage, H.; Tempst, P.; Allis, C.D. Ubiquitylation of Histone H2B Controls RNA Polymerase II Transcription Elongation Independently of Histone H3 Methylation. Genes Dev. 2007, 21, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.-F.; Hillyer, C.; Tsukuda, T.; Henry, K.; Berger, S.; Osley, M.A. Rad6 Plays a Role in Transcriptional Activation through Ubiquitylation of Histone H2B. Genes Dev. 2004, 18, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Daniel, J.A.; Torok, M.S.; Sun, Z.-W.; Schieltz, D.; Allis, C.D.; Yates, J.R.; Grant, P.A. Deubiquitination of Histone H2B by a Yeast Acetyltransferase Complex Regulates Transcription. J. Biol. Chem. 2004, 279, 1867–1871. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.-W.; Allis, C.D. Ubiquitination of Histone H2B Regulates H3 Methylation and Gene Silencing in Yeast. Nature 2002, 418, 104. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, M.-H.; Zhang, L.; Liu, J.; Zhang, Q.-D.; Zhou, J.-Q. Rad6-Bre1 Mediated Histone H2Bub1 Protects Uncapped Telomeres from Exonuclease Exo1 in Saccharomyces Cerevisiae. DNA Repair 2018, 72, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Garrett, A.S.; Yen, K.; Takahashi, Y.-H.; Hu, D.; Jackson, J.; Seidel, C.; Pugh, B.F.; Shilatifard, A. Codependency of H2B Monoubiquitination and Nucleosome Reassembly on Chd1. Genes Dev. 2012, 26, 914–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekharan, M.B.; Huang, F.; Sun, Z.-W. Ubiquitination of Histone H2B Regulates Chromatin Dynamics by Enhancing Nucleosome Stability. Proc. Natl. Acad. Sci. USA 2009, 106, 16686–16691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo, K.M.; Osley, M.A. A Role for H2B Ubiquitylation in DNA Replication. Mol. Cell 2012, 48, 734–746. [Google Scholar] [CrossRef] [Green Version]
- Robzyk, K. Rad6-Dependent Ubiquitination of Histone H2B in Yeast. Science 2000, 287, 501–504. [Google Scholar] [CrossRef]
- Zhao, Y.; Upadhyay, S.; Lin, X. PAS Domain Protein Pas3 Interacts with the Chromatin Modifier Bre1 in Regulating Cryptococcal Morphogenesis. mBio 2018, 9, e02135-18. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xin, J.; Liu, L.; Song, A.; Liao, Y.; Guan, Z.; Fang, W.; Chen, F. Ubiquitin E3 Ligase AaBre1 Responsible for H2B Monoubiquitination Is Involved in Hyphal Growth, Conidiation and Pathogenicity in Alternaria Alternata. Genes 2020, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Garapati, H.S.; Kakumanu, A.V.S.; Shukla, R.; Mishra, K. SUMOylation in Fungi: A Potential Target for Intervention. Comput. Struct. Biotechnol. J. 2020, 18, 3484–3493. [Google Scholar] [CrossRef]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and Regulation of SUMO Proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.-Y.; Hochstrasser, M. Histone Sumoylation and Chromatin Dynamics. Nucleic Acids Res. 2021, 49, 6043–6052. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.; Ingvarsdottir, K.; Sterner, D.E.; Bylebyl, G.R.; Dokmanovic, M.; Dorsey, J.A.; Whelan, K.A.; Krsmanovic, M.; Lane, W.S.; Meluh, P.B.; et al. Histone Sumoylation Is a Negative Regulator in Saccharomyces Cerevisiae and Shows Dynamic Interplay with Positive-Acting Histone Modifications. Genes Dev. 2006, 20, 966–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, H.; Su, D.; Wilson-Eisele, N.R.; Zhao, D.; López-Giráldez, F.; Hochstrasser, M. The Ulp2 SUMO Protease Promotes Transcription Elongation through Regulation of Histone Sumoylation. EMBO J. 2019, 38, e102003. [Google Scholar] [CrossRef] [PubMed]
- Brahma, S.; Henikoff, S. RSC-Associated Subnucleosomes Define MNase-Sensitive Promoters in Yeast. Mol. Cell 2019, 73, 238–249.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Zuo, X.; Zhuo, Y.; Zhu, M.; Danziger, S.A.; Zhou, Z. The Functional Role of SUMO E3 Ligase Mms21p in the Maintenance of Subtelomeric Silencing in Budding Yeast. Biochem. Biophys. Res. Commun. 2013, 438, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Dhall, A.; Weller, C.E.; Chu, A.; Shelton, P.M.M.; Chatterjee, C. Chemically Sumoylated Histone H4 Stimulates Intranucleosomal Demethylation by the LSD1–CoREST Complex. ACS Chem. Biol. 2017, 12, 2275–2280. [Google Scholar] [CrossRef]
- Ryu, H.-Y.; Zhao, D.; Li, J.; Su, D.; Hochstrasser, M. Histone Sumoylation Promotes Set3 Histone-Deacetylase Complex-Mediated Transcriptional Regulation. Nucleic Acids Res. 2020, 48, 12151–12168. [Google Scholar] [CrossRef]
- Komaniecki, G.; Lin, H. Lysine Fatty Acylation: Regulatory Enzymes, Research Tools, and Biological Function. Front. Cell Dev. Biol. 2021, 9, 717503. [Google Scholar] [CrossRef]
- Nitsch, S.; Zorro Shahidian, L.; Schneider, R. Histone Acylations and Chromatin Dynamics: Concepts, Challenges, and Links to Metabolism. EMBO Rep. 2021, 22, e52774. [Google Scholar] [CrossRef]
- Kollenstart, L.; de Groot, A.J.L.; Janssen, G.M.C.; Cheng, X.; Vreeken, K.; Martino, F.; Côté, J.; van Veelen, P.A.; van Attikum, H. Gcn5 and Esa1 Function as Histone Crotonyltransferases to Regulate Crotonylation-Dependent Transcription. J. Biol. Chem. 2019, 294, 20122–20134. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Dai, J.; Dai, L.; Tan, M.; Cheng, Z.; Wu, Y.; Boeke, J.D.; Zhao, Y. Lysine Succinylation and Lysine Malonylation in Histones. Mol. Cell. Proteomics 2012, 11, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Shangguan, Y.; Tang, D.; Dai, Y. Histone Succinylation and Its Function on the Nucleosome. J. Cell. Mol. Med. 2021, 25, 7101–7109. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Ding, D.; Tian, G.; Kwan, K.C.J.; Liu, Z.; Ishibashi, T.; Li, X.D. Semisynthesis of Site-Specifically Succinylated Histone Reveals That Succinylation Regulates Nucleosome Unwrapping Rate and DNA Accessibility. Nucleic Acids Res. 2020, 48, 9538–9549. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Luo, Z.; Ying, W.; Cao, Q.; Huang, H.; Dong, J.; Wu, Q.; Zhao, Y.; Qian, X.; Dai, J. 2-Hydroxyisobutyrylation on Histone H4K8 Is Regulated by Glucose Homeostasis in Saccharomyces Cerevisiae. Proc. Natl. Acad. Sci. USA 2017, 114, 8782–8787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, H.; Lin, H. Sirtuins in Epigenetic Regulation. Chem. Rev. 2015, 115, 2350–2375. [Google Scholar] [CrossRef] [Green Version]
- Kawauchi, M.; Nishiura, M.; Iwashita, K. Fungus-Specific Sirtuin HstD Coordinates Secondary Metabolism and Development through Control of LaeA. Eukaryot. Cell 2013, 12, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Itoh, E.; Odakura, R.; Oinuma, K.-I.; Shimizu, M.; Masuo, S.; Takaya, N. Sirtuin E Is a Fungal Global Transcriptional Regulator That Determines the Transition from the Primary Growth to the Stationary Phase. J. Biol. Chem. 2017, 292, 11043–11054. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, J.; Marroquin-Guzman, M.; Nandakumar, R.; Shijo, S.; Cornwell, K.M.; Li, G.; Wilson, R.A. Plant Defence Suppression Is Mediated by a Fungal Sirtuin during Rice Infection by M Agnaporthe Oryzae: The Role of MoSir2 in Rice Blast Disease. Mol. Microbiol. 2014, 94, 70–88. [Google Scholar] [CrossRef]
- Zhao, G.; Rusche, L.N. Genetic Analysis of Sirtuin Deacetylases in Hyphal Growth of Candida Albicans. mSphere 2021, 6, e00053-21. [Google Scholar] [CrossRef]
- Abmayr, S.M.; Workman, J.L. Histone Lysine De-β-Hydroxybutyrylation by SIRT3. Cell Res. 2019, 29, 694–695. [Google Scholar] [CrossRef]
- Prakash, K.; Fournier, D. Evidence for the Implication of the Histone Code in Building the Genome Structure. Biosystems 2018, 164, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.A.; Hake, S.B.; Diaz, R.L.; Kauer, M.; Morris, S.A.; Recht, J.; Shabanowitz, J.; Mishra, N.; Strahl, B.D.; Allis, C.D.; et al. Organismal Differences in Post-Translational Modifications in Histones H3 and H4. J. Biol. Chem. 2007, 282, 7641–7655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merhej, J.; Richard-Forget, F.; Barreau, C. Regulation of Trichothecene Biosynthesis in Fusarium: Recent Advances and New Insights. Appl. Microbiol. Biotechnol. 2011, 91, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Britton, L.-M.P.; Gonzales-Cope, M.; Zee, B.M.; Garcia, B.A. Breaking the Histone Code with Quantitative Mass Spectrometry. Expert Rev. Proteom. 2011, 8, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Sidoli, S.; Cheng, L.; Jensen, O.N. Proteomics in Chromatin Biology and Epigenetics: Elucidation of Post-Translational Modifications of Histone Proteins by Mass Spectrometry. J. Proteom. 2012, 75, 3419–3433. [Google Scholar] [CrossRef]
- Cristobal, A.; Marino, F.; Post, H.; van den Toorn, H.W.P.; Mohammed, S.; Heck, A.J.R. Toward an Optimized Workflow for Middle-Down Proteomics. Anal. Chem. 2017, 89, 3318–3325. [Google Scholar] [CrossRef] [Green Version]
- Eliuk, S.M.; Maltby, D.; Panning, B.; Burlingame, A.L. High Resolution Electron Transfer Dissociation Studies of Unfractionated Intact Histones from Murine Embryonic Stem Cells Using On-Line Capillary LC Separation. Mol. Cell. Proteom. 2010, 9, 824–837. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.; Papasotiriou, D.G.; Karas, M. 100% Protein Sequence Coverage: A Modern Form of Surrealism in Proteomics. Amino Acids 2011, 41, 291–310. [Google Scholar] [CrossRef]
- Rommelfanger, S.R.; Zhou, M.; Shaghasi, H.; Tzeng, S.-C.; Evans, B.S.; Paša-Tolić, L.; Umen, J.G.; Pesavento, J.J. An Improved Top-Down Mass Spectrometry Characterization of Chlamydomonas Reinhardtii Histones and Their Post-Translational Modifications. J. Am. Soc. Mass Spectrom. 2021, 32, 1671–1688. [Google Scholar] [CrossRef]
- Janssen, K.A.; Sidoli, S.; Garcia, B.A. Recent Achievements in Characterizing the Histone Code and Approaches to Integrating Epigenomics and Systems Biology. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 586, pp. 359–378. ISBN 978-0-12-809743-4. [Google Scholar] [CrossRef] [Green Version]
- Fischle, W.; Wang, Y.; Allis, C.D. Histone and Chromatin Cross-Talk. Curr. Opin. Cell Biol. 2003, 15, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Oya, E.; Nakagawa, R.; Yoshimura, Y.; Tanaka, M.; Nishibuchi, G.; Machida, S.; Shirai, A.; Ekwall, K.; Kurumizaka, H.; Tagami, H.; et al. H3K14 Ubiquitylation Promotes H3K9 Methylation for Heterochromatin Assembly. EMBO Rep. 2019, 20, e48111. [Google Scholar] [CrossRef] [PubMed]
- Fingerman, I.M.; Du, H.-N.; Briggs, S.D. Controlling Histone Methylation via Trans-Histone Pathways. Epigenetics 2008, 3, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Welsem, T.; Korthout, T.; Ekkebus, R.; Morais, D.; Molenaar, T.M.; van Harten, K.; Poramba-Liyanage, D.W.; Sun, S.M.; Lenstra, T.L.; Srivas, R.; et al. Dot1 Promotes H2B Ubiquitination by a Methyltransferase-Independent Mechanism. Nucleic Acids Res. 2018, 46, 11251–11261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Li, D.; Lu, Z.; Liu, G.; Wang, M.; Xing, P.; Wang, M.; Dong, Y.; Wang, X.; Li, J.; et al. Bre1-Dependent H2B Ubiquitination Promotes Homologous Recombination by Stimulating Histone Eviction at DNA Breaks. Nucleic Acids Res. 2018, 46, 11326–11339. [Google Scholar] [CrossRef] [PubMed]
- Sidoli, S.; Garcia, B.A. Middle-down Proteomics: A Still Unexploited Resource for Chromatin Biology. Expert Rev. Proteom. 2017, 14, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchler, K.; Jenull, S.; Shivarathri, R.; Chauhan, N. Fungal KATs/KDACs: A New Highway to Better Antifungal Drugs? PLoS Pathog. 2016, 12, e1005938. [Google Scholar] [CrossRef]
- Medina, Á.; Rodríguez, A.; Magan, N. Climate Change and Mycotoxigenic Fungi: Impacts on Mycotoxin Production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Chang, Z.; Yadav, V.; Lee, S.C.; Heitman, J. Epigenetic Mechanisms of Drug Resistance in Fungi. Fungal Genet. Biol. 2019, 132, 103253. [Google Scholar] [CrossRef]
- Tscherner; Kuchler A Histone Acetyltransferase Inhibitor with Antifungal Activity against CTG Clade Candida Species. Microorganisms 2019, 7, 201. [CrossRef] [Green Version]
- Chimenti, F.; Bizzarri, B.; Maccioni, E.; Secci, D.; Bolasco, A.; Chimenti, P.; Fioravanti, R.; Granese, A.; Carradori, S.; Tosi, F.; et al. A Novel Histone Acetyltransferase Inhibitor Modulating Gcn5 Network: Cyclopentylidene-[4-(4′-Chlorophenyl)Thiazol-2-Yl)Hydrazone. J. Med. Chem. 2009, 52, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Garnaud, C.; Champleboux, M.; Maubon, D.; Cornet, M.; Govin, J. Histone Deacetylases and Their Inhibition in Candida Species. Front. Microbiol. 2016, 7, 1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamoth, F.; Juvvadi, P.R.; Steinbach, W.J. Histone Deacetylase Inhibition as an Alternative Strategy against Invasive Aspergillosis. Front. Microbiol. 2015, 6, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izawa, M.; Takekawa, O.; Arie, T.; Teraoka, T.; Yoshida, M.; Kimura, M.; Kamakura, T. Inhibition of Histone Deacetylase Causes Reduction of Appressorium Formation in the Rice Blast Fungus Magnaporthe Oryzae. J. Gen. Appl. Microbiol. 2009, 55, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Messer, S.A.; Georgopapadakou, N.; Martell, L.A.; Besterman, J.M.; Diekema, D.J. Activity of MGCD290, a Hos2 Histone Deacetylase Inhibitor, in Combination with Azole Antifungals against Opportunistic Fungal Pathogens. J. Clin. Microbiol. 2009, 47, 3797–3804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.M.; Hoda, S.; Saha, D.; Georgescu, L.; Serratore, N.D.; Zhang, Y.; Lanman, N.A.; Briggs, S.D. Set1-Mediated Histone H3K4 Methylation Is Required for Azole Induction of the Ergosterol Biosynthesis Genes and Antifungal Drug Resistance in Candida Glabrata. bioRxiv 2021. [Google Scholar] [CrossRef]
- South, P.F.; Harmeyer, K.M.; Serratore, N.D.; Briggs, S.D. H3K4 Methyltransferase Set1 Is Involved in Maintenance of Ergosterol Homeostasis and Resistance to Brefeldin A. Proc. Natl. Acad. Sci. USA 2013, 110, E1016–E1025. [Google Scholar] [CrossRef] [Green Version]
- Nirello, V.D.; Rodrigues de Paula, D.; Araújo, N.V.P.; Varga-Weisz, P.D. Does Chromatin Function as a Metabolite Reservoir? Trends Biochem. Sci. 2022. [Google Scholar] [CrossRef]
- Ye, C.; Tu, B.P. Sink into the Epigenome: Histones as Repositories That Influence Cellular Metabolism. Trends Endocrinol. Metab. 2018, 29, 626–637. [Google Scholar] [CrossRef]
- Trefely, S.; Lovell, C.D.; Snyder, N.W.; Wellen, K.E. Compartmentalised Acyl-CoA Metabolism and Roles in Chromatin Regulation. Mol. Metab. 2020, 38, 100941. [Google Scholar] [CrossRef]
- Galdieri, L.; Vancura, A. Acetyl-CoA Carboxylase Regulates Global Histone Acetylation. J. Biol. Chem. 2012, 287, 23865–23876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Galdieri, L.; Vancura, A. The Yeast AMPK Homolog SNF1 Regulates Acetyl Coenzyme A Homeostasis and Histone Acetylation. Mol. Cell. Biol. 2013, 33, 4701–4717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, A.B.; García, R.; Pavón-Vergés, M.; Rodríguez-Peña, J.M.; Arroyo, J. Control of Gene Expression via the Yeast CWI Pathway. Int. J. Mol. Sci. 2022, 23, 1791. [Google Scholar] [CrossRef]
- Song, O.; Wang, X.; Waterborg, J.H.; Sternglanz, R. An N α-Acetyltransferase Responsible for Acetylation of the N-Terminal Residues of Histones H4 and H2A. J. Biol. Chem. 2003, 278, 38109–38112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polevoda, B.; Hoskins, J.; Sherman, F. Properties of Nat4, an N α -Acetyltransferase of Saccharomyces Cerevisiae That Modifies N Termini of Histones H2A and H4. Mol. Cell. Biol. 2009, 29, 2913–2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, F.; Olson, D.K.; Christiano, R.; Farese, R.V.; Walther, T.C. Proteomic and Phosphoproteomic Analyses of Yeast Reveal the Global Cellular Response to Sphingolipid Depletion. Proteomics 2016, 16, 2759–2763. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.S.; Lowell, J.E.; Jacobson, S.J.; Pillus, L. Esa1p Is an Essential Histone Acetyltransferase Required for Cell Cycle Progression. Mol. Cell. Biol. 1999, 19, 2515–2526. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Wang, Y. Mapping Post-Translational Modifications of Histones H2A, H2B and H4 in Schizosaccharomyces Pombe. Int. J. Mass Spectrom. 2011, 301, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Xia, A.; Ye, M.; Ren, J.; Li, D.; Liu, H.; Wang, Q.; Lu, P.; Wu, C.; Xu, J.-R.; et al. Opposing Functions of Fng1 and the Rpd3 HDAC Complex in H4 Acetylation in Fusarium Graminearum. PLOS Genet. 2020, 16, e1009185. [Google Scholar] [CrossRef]
- Suka, N.; Suka, Y.; Carmen, A.A.; Wu, J.; Grunstein, M. Highly Specific Antibodies Determine Histone Acetylation Site Usage in Yeast Heterochromatin and Euchromatin. Mol. Cell 2001, 8, 473–479. [Google Scholar] [CrossRef]
- Xiong, L.; Adhvaryu, K.K.; Selker, E.U.; Wang, Y. Mapping of Lysine Methylation and Acetylation in Core Histones of Neurospora Crassa. Biochemistry 2010, 49, 5236–5243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downey, M.; Johnson, J.R.; Davey, N.E.; Newton, B.W.; Johnson, T.L.; Galaang, S.; Seller, C.A.; Krogan, N.; Toczyski, D.P. Acetylome Profiling Reveals Overlap in the Regulation of Diverse Processes by Sirtuins, Gcn5, and Esa1. Mol. Cell. Proteomics 2015, 14, 162–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Côté, V.; Côté, J. DNA Damage-Induced Phosphorylation of Histone H2A at Serine 15 Is Linked to DNA End Resection. Mol. Cell. Biol. 2021, 41, e00056-21. [Google Scholar] [CrossRef] [PubMed]
- Willger, S.D.; Liu, Z.; Olarte, R.A.; Adamo, M.E.; Stajich, J.E.; Myers, L.C.; Kettenbach, A.N.; Hogan, D.A. Analysis of the Candida Albicans Phosphoproteome. Eukaryot. Cell 2015, 14, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Dephoure, N.; Gygi, S.P. A Solid Phase Extraction-Based Platform for Rapid Phosphoproteomic Analysis. Methods 2011, 54, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Kettenbach, A.N.; Deng, L.; Wu, Y.; Baldissard, S.; Adamo, M.E.; Gerber, S.A.; Moseley, J.B. Quantitative Phosphoproteomics Reveals Pathways for Coordination of Cell Growth and Division by the Conserved Fission Yeast Kinase Pom1*. Mol. Cell. Proteomics 2015, 14, 1275–1287. [Google Scholar] [CrossRef] [Green Version]
- Swaney, D.L.; Beltrao, P.; Starita, L.; Guo, A.; Rush, J.; Fields, S.; Krogan, N.J.; Villén, J. Global Analysis of Phosphorylation and Ubiquitylation Cross-Talk in Protein Degradation. Nat. Methods 2013, 10, 676–682. [Google Scholar] [CrossRef]
- Xiong, Y.; Coradetti, S.T.; Li, X.; Gritsenko, M.A.; Clauss, T.; Petyuk, V.; Camp, D.; Smith, R.; Cate, J.H.D.; Yang, F.; et al. The Proteome and Phosphoproteome of Neurospora Crassa in Response to Cellulose, Sucrose and Carbon Starvation. Fungal Genet. Biol. 2014, 72, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Suhandynata, R.T.; Wan, L.; Zhou, H.; Hollingsworth, N.M. Identification of Putative Mek1 Substrates during Meiosis in Saccharomyces Cerevisiae Using Quantitative Phosphoproteomics. PLoS ONE 2016, 11, e0155931. [Google Scholar] [CrossRef] [Green Version]
- Weinert, B.T.; Iesmantavicius, V.; Moustafa, T.; Schölz, C.; Wagner, S.A.; Magnes, C.; Zechner, R.; Choudhary, C. Acetylation Dynamics and Stoichiometry in S Accharomyces Cerevisiae. Mol. Syst. Biol. 2014, 10, 716. [Google Scholar] [CrossRef]
- Tessarz, P.; Santos-Rosa, H.; Robson, S.C.; Sylvestersen, K.B.; Nelson, C.J.; Nielsen, M.L.; Kouzarides, T. Glutamine Methylation in Histone H2A Is an RNA-Polymerase-I-Dedicated Modification. Nature 2014, 505, 564–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, T.; Tanabe, K.; Kobayashi, Y.; Mizumoto, S.; Kanai, M.; Kawashima, S.A. Malonylation of Histone H2A at Lysine 119 Inhibits Bub1-Dependent H2A Phosphorylation and Chromosomal Localization of Shugoshin Proteins. Sci. Rep. 2018, 8, 7671. [Google Scholar] [CrossRef] [PubMed]
- Swaffer, M.P.; Jones, A.W.; Flynn, H.R.; Snijders, A.P.; Nurse, P. Quantitative Phosphoproteomics Reveals the Signaling Dynamics of Cell-Cycle Kinases in the Fission Yeast Schizosaccharomyces Pombe. Cell Rep. 2018, 24, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, L.; Huard, S.; Cotrut, M.; Pourhanifeh-Lemeri, R.; Steunou, A.-L.; Hamza, A.; Lambert, J.-P.; Zhou, H.; Ning, Z.; Basu, A.; et al. MChIP-KAT-MS, a Method to Map Protein Interactions and Acetylation Sites for Lysine Acetyltransferases. Proc. Natl. Acad. Sci. USA 2013, 110, E1641–E1650. [Google Scholar] [CrossRef] [Green Version]
- Reiter, W.; Anrather, D.; Dohnal, I.; Pichler, P.; Veis, J.; Grøtli, M.; Posas, F.; Ammerer, G. Validation of Regulated Protein Phosphorylation Events in Yeast by Quantitative Mass Spectrometry Analysis of Purified Proteins. Proteomics 2012, 12, 3030–3043. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, W.; Leeder, A.C.; Ansong, C.; Wang, Y.; Yang, F.; Starr, T.L.; Camp, D.G.; Smith, R.D.; Glass, N.L. HAM-5 Functions As a MAP Kinase Scaffold during Cell Fusion in Neurospora Crassa. PLoS Genet. 2014, 10, e1004783. [Google Scholar] [CrossRef] [Green Version]
- Ficarro, S.B.; McCleland, M.L.; Stukenberg, P.T.; Burke, D.J.; Ross, M.M.; Shabanowitz, J.; Hunt, D.F.; White, F.M. Phosphoproteome Analysis by Mass Spectrometry and Its Application to Saccharomyces Cerevisiae. Nat. Biotechnol. 2002, 20, 301–305. [Google Scholar] [CrossRef]
- Nakamura, T.M.; Du, L.-L.; Redon, C.; Russell, P. Histone H2A Phosphorylation Controls Crb2 Recruitment at DNA Breaks, Maintains Checkpoint Arrest, and Influences DNA Repair in Fission Yeast. Mol. Cell. Biol. 2004, 24, 6215–6230. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.C.; Green, G.R.; Smith, K.; Selker, E.U. Extensive and Varied Modifications in Histone H2B of Wild-Type and Histone Deacetylase 1 Mutant Neurospora Crassa. Biochemistry 2010, 49, 5244–5257. [Google Scholar] [CrossRef] [Green Version]
- Capriotti, A.L.; Cavaliere, C.; Ferraris, F.; Gianotti, V.; Laus, M.; Piovesana, S.; Sparnacci, K.; Zenezini Chiozzi, R.; Laganà, A. New Ti-IMAC Magnetic Polymeric Nanoparticles for Phosphopeptide Enrichment from Complex Real Samples. Talanta 2018, 178, 274–281. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Cheung, W.L.; Hsu, J.-Y.; Diaz, R.L.; Smith, M.M.; Allis, C.D. Sterile 20 Kinase Phosphorylates Histone H2B at Serine 10 during Hydrogen Peroxide-Induced Apoptosis in S. Cerevisiae. Cell 2005, 120, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.-H.; Henderson, K.A.; Keeney, S.; Allis, C.D. H2B (Ser10) Phosphorylation Is Induced during Apoptosis and Meiosis in S. Cerevisiae. Cell Cycle 2005, 4, 780–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurdistani, S.K.; Tavazoie, S.; Grunstein, M. Mapping Global Histone Acetylation Patterns to Gene Expression. Cell 2004, 117, 721–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterborg, J.H. Steady-State Levels of Histone Acetylation in Saccharomyces Cerevisiae. J. Biol. Chem. 2000, 275, 13007–13011. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Chen, Y.; Zhang, Z.; Zhao, Y. Identification and Verification of Lysine Propionylation and Butyrylation in Yeast Core Histones Using PTMap Software. J. Proteome Res. 2009, 8, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Rødkær, S.V.; Pultz, D.; Brusch, M.; Bennetzen, M.V.; Falkenby, L.G.; Andersen, J.S.; Færgeman, N.J. Quantitative Proteomics Identifies Unanticipated Regulators of Nitrogen- and Glucose Starvation. Mol BioSyst 2014, 10, 2176–2188. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Liu, Z.; Tian, G.; Bao, X.; Ishibashi, T.; Li, X.D. Site-Specific Installation of Succinyl Lysine Analog into Histones Reveals the Effect of H2BK34 Succinylation on Nucleosome Dynamics. Cell Chem. Biol. 2018, 25, 166–174.e7. [Google Scholar] [CrossRef] [Green Version]
- Chi, A.; Huttenhower, C.; Geer, L.Y.; Coon, J.J.; Syka, J.E.P.; Bai, D.L.; Shabanowitz, J.; Burke, D.J.; Troyanskaya, O.G.; Hunt, D.F. Analysis of Phosphorylation Sites on Proteins from Saccharomyces Cerevisiae by Electron Transfer Dissociation (ETD) Mass Spectrometry. Proc. Natl. Acad. Sci. USA 2007, 104, 2193–2198. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, M.; Haas, W.; Beausoleil, S.A.; Rush, J.; Gygi, S.P. Gas-Phase Rearrangements Do Not Affect Site Localization Reliability in Phosphoproteomics Data Sets. J. Proteome Res. 2010, 9, 3103–3107. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, C.P.; Smolka, M.B.; Payne, S.H.; Bafna, V.; Eng, J.; Zhou, H. A Multidimensional Chromatography Technology for In-Depth Phosphoproteome Analysis. Mol. Cell. Proteomics 2008, 7, 1389–1396. [Google Scholar] [CrossRef] [Green Version]
- Lao, J.P.; Ulrich, K.M.; Johnson, J.R.; Newton, B.W.; Vashisht, A.A.; Wohlschlegel, J.A.; Krogan, N.J.; Toczyski, D.P. The Yeast DNA Damage Checkpoint Kinase Rad53 Targets the Exoribonuclease, Xrn1. G3 GenesGenomesGenetics 2018, 8, 3931–3944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsubramaniam, N.; Harris, S.D.; Marten, M.R. The Phosphoproteome of Aspergillus Nidulans Reveals Functional Association with Cellular Processes Involved in Morphology and Secretion. Proteomics 2014, 14, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Nakamura, T.; Hayashi, T.; Yanagida, M. Histone H2B Mutations in Inner Region Affect Ubiquitination, Centromere Function, Silencing and Chromosome Segregation. EMBO J. 2006, 25, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.K.; Jedrychowski, M.P.; McAlister, G.C.; Everley, R.A.; Kunz, R.; Gygi, S.P. Evaluating Multiplexed Quantitative Phosphopeptide Analysis on a Hybrid Quadrupole Mass Filter/Linear Ion Trap/Orbitrap Mass Spectrometer. Anal. Chem. 2015, 87, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Roguev, A. The Saccharomyces Cerevisiae Set1 Complex Includes an Ash2 Homologue and Methylates Histone 3 Lysine 4. EMBO J. 2001, 20, 7137–7148. [Google Scholar] [CrossRef]
- Liu, C.L.; Kaplan, T.; Kim, M.; Buratowski, S.; Schreiber, S.L.; Friedman, N.; Rando, O.J. Single-Nucleosome Mapping of Histone Modifications in S. Cerevisiae. PLoS Biol. 2005, 3, e328. [Google Scholar] [CrossRef] [Green Version]
- Briggs, S.D.; Xiao, T.; Sun, Z.-W.; Caldwell, J.A.; Shabanowitz, J.; Hunt, D.F.; Allis, C.D.; Strahl, B.D. Trans-Histone Regulatory Pathway in Chromatin. Nature 2002, 418, 498. [Google Scholar] [CrossRef]
- Santos-Rosa, H.; Schneider, R.; Bannister, A.J.; Sherriff, J.; Bernstein, B.E.; Emre, N.C.T.; Schreiber, S.L.; Mellor, J.; Kouzarides, T. Active Genes Are Tri-Methylated at K4 of Histone H3. Nature 2002, 419, 407–411. [Google Scholar] [CrossRef]
- Briggs, S.D.; Bryk, M.; Strahl, B.D.; Cheung, W.L.; Davie, J.K.; Dent, S.Y.R.; Winston, F.; Allis, C.D. Histone H3 Lysine 4 Methylation Is Mediated by Set1 and Required for Cell Growth and RDNA Silencing in Saccharomyces Cerevisiae. Genes Dev. 2001, 15, 3286–3295. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Klose, R.J.; Gardner, K.E.; Zhang, Y. Yeast Jhd2p Is a Histone H3 Lys4 Trimethyl Demethylase. Nat. Struct. Mol. Biol. 2007, 14, 243–245. [Google Scholar] [CrossRef]
- Lee, K.Y.; Chen, Z.; Jiang, R.; Meneghini, M.D. H3K4 Methylation Dependent and Independent Chromatin Regulation by JHD2 and SET1 in Budding Yeast. G3 GenesGenomesGenetics 2018, 8, 1829–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, F.; Zaratiegui, M.; Villén, J.; Vaughn, M.W.; Verdel, A.; Huarte, M.; Shi, Y.; Gygi, S.P.; Moazed, D.; Martienssen, R.A.; et al. S. Pombe LSD1 Homologs Regulate Heterochromatin Propagation and Euchromatic Gene Transcription. Mol. Cell 2007, 26, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional Silencing and Longevity Protein Sir2 Is an NAD-Dependent Histone Deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Andrews, F.H.; Shinsky, S.A.; Shanle, E.K.; Bridgers, J.B.; Gest, A.; Tsun, I.K.; Krajewski, K.; Shi, X.; Strahl, B.D.; Kutateladze, T.G. The Taf14 YEATS Domain Is a Reader of Histone Crotonylation. Nat. Chem. Biol. 2016, 12, 396–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuscu, C.; Zaratiegui, M.; Kim, H.S.; Wah, D.A.; Martienssen, R.A.; Schalch, T.; Joshua-Tor, L. CRL4-like Clr4 Complex in Schizosaccharomyces Pombe Depends on an Exposed Surface of Dos1 for Heterochromatin Silencing. Proc. Natl. Acad. Sci. USA 2014, 111, 1795–1800. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, J.; Rice, J.C.; Strahl, B.D.; Allis, C.D.; Grewal, S.I.S. Role of Histone H3 Lysine 9 Methylation in Epigenetic Control of Heterochromatin Assembly. Science 2001, 292, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Qian, G.; Yi, X.; Li, X.; Liu, W. Systematic Analysis of the Lysine Acetylome in Candida Albicans. J. Proteome Res. 2016, 15, 2525–2536. [Google Scholar] [CrossRef]
- Tamaru, H.; Zhang, X.; McMillen, D.; Singh, P.B.; Nakayama, J.; Grewal, S.I.; Allis, C.D.; Cheng, X.; Selker, E.U. Trimethylated Lysine 9 of Histone H3 Is a Mark for DNA Methylation in Neurospora Crassa. Nat. Genet. 2003, 34, 75–79. [Google Scholar] [CrossRef]
- Lo, W.-S.; Trievel, R.C.; Rojas, J.R.; Duggan, L.; Hsu, J.-Y.; Allis, C.D.; Marmorstein, R.; Berger, S.L. Phosphorylation of Serine 10 in Histone H3 Is Functionally Linked In Vitro and In Vivo to Gcn5-Mediated Acetylation at Lysine 14. Mol. Cell 2000, 5, 917–926. [Google Scholar] [CrossRef]
- Lo, W.-S.; Gamache, E.R.; Henry, K.W.; Yang, D.; Pillus, L.; Berger, S.L. Histone H3 Phosphorylation Can Promote TBP Recruitment through Distinct Promoter-Specific Mechanisms. EMBO J. 2005, 24, 997–1008. [Google Scholar] [CrossRef] [Green Version]
- Carpy, A.; Krug, K.; Graf, S.; Koch, A.; Popic, S.; Hauf, S.; Macek, B. Absolute Proteome and Phosphoproteome Dynamics during the Cell Cycle of Schizosaccharomyces Pombe (Fission Yeast). Mol. Cell. Proteomics 2014, 13, 1925–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudarzi, A.; Zhang, D.; Huang, H.; Barral, S.; Kwon, O.K.; Qi, S.; Tang, Z.; Buchou, T.; Vitte, A.-L.; He, T.; et al. Dynamic Competing Histone H4 K5K8 Acetylation and Butyrylation Are Hallmarks of Highly Active Gene Promoters. Mol. Cell 2016, 62, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, K.; Rountree, M.R.; Lewis, Z.A.; Stajich, J.E.; Selker, E.U. Regional Control of Histone H3 Lysine 27 Methylation in Neurospora. Proc. Natl. Acad. Sci. USA 2013, 110, 6027–6032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, S.A.; Rao, B.; Garcia, B.A.; Hake, S.B.; Diaz, R.L.; Shabanowitz, J.; Hunt, D.F.; Allis, C.D.; Lieb, J.D.; Strahl, B.D. Identification of Histone H3 Lysine 36 Acetylation as a Highly Conserved Histone Modification. J. Biol. Chem. 2007, 282, 7632–7640. [Google Scholar] [CrossRef] [Green Version]
- Strahl, B.D.; Grant, P.A.; Briggs, S.D.; Sun, Z.-W.; Bone, J.R.; Caldwell, J.A.; Mollah, S.; Cook, R.G.; Shabanowitz, J.; Hunt, D.F.; et al. Set2 Is a Nucleosomal Histone H3-Selective Methyltransferase That Mediates Transcriptional Repression. Mol. Cell. Biol. 2002, 22, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Klose, R.J.; Gardner, K.E.; Liang, G.; Erdjument-Bromage, H.; Tempst, P.; Zhang, Y. Demethylation of Histone H3K36 and H3K9 by Rph1: A Vestige of an H3K9 Methylation System in Saccharomyces Cerevisiae? Mol. Cell. Biol. 2007, 27, 3951–3961. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.A.; Shibata, Y.; Noma, K.; Tsukamoto, Y.; Warren, E.; Temple, B.; Grewal, S.I.S.; Strahl, B.D. Histone H3 K36 Methylation Is Associated with Transcription Elongation in Schizosaccharomyces Pombe. Eukaryot. Cell 2005, 4, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Zhou, M.-M. SET Domain Protein Lysine Methyltransferases: Structure, Specificity and Catalysis. Cell. Mol. Life Sci. 2006, 63, 2755–2763. [Google Scholar] [CrossRef]
- KdmA, a Histone H3 Demethylase with Bipartite Function, Differentially Regulates Primary and Secondary Metabolism in Aspergillus Nidulans. Mol. Microbiol. 2015, 97, 606. [CrossRef] [Green Version]
- Shen, Y.; Mevius, D.E.H.F.; Caliandro, R.; Carrozzini, B.; Roh, Y.; Kim, J.; Kim, S.; Ha, S.C.; Morishita, M.; di Luccio, E. Set7 Is a H3K37 Methyltransferase in Schizosaccharomyces Pombe and Is Required for Proper Gametogenesis. Structure 2019, 27, 631–638.e8. [Google Scholar] [CrossRef] [Green Version]
- Beltrao, P.; Albanèse, V.; Kenner, L.R.; Swaney, D.L.; Burlingame, A.; Villén, J.; Lim, W.A.; Fraser, J.S.; Frydman, J.; Krogan, N.J. Systematic Functional Prioritization of Protein Posttranslational Modifications. Cell 2012, 150, 413–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubota, T.; Berndsen, C.E.; Erkmann, J.A.; Smith, C.L.; Yang, L.; Freitas, M.A.; Denu, J.M.; Kaufman, P.D. Histone H3-K56 Acetylation Is Catalyzed by Histone Chaperone-Dependent Complexes. Mol. Cell 2007, 25, 703–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyland, E.M.; Cosgrove, M.S.; Molina, H.; Wang, D.; Pandey, A.; Cottee, R.J.; Boeke, J.D. Insights into the Role of Histone H3 and Histone H4 Core Modifiable Residues in Saccharomyces Cerevisiae. Mol. Cell. Biol. 2005, 25, 10060–10070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir, A.; Spicuglia, S.; Lasonder, E.; Vermeulen, M.; Campsteijn, C.; Stunnenberg, H.G.; Logie, C. Characterization of Lysine 56 of Histone H3 as an Acetylation Site in Saccharomyces Cerevisiae. J. Biol. Chem. 2005, 280, 25949–25952. [Google Scholar] [CrossRef] [Green Version]
- Masumoto, H.; Hawke, D.; Kobayashi, R.; Verreault, A. A Role for Cell-Cycle-Regulated Histone H3 Lysine 56 Acetylation in the DNA Damage Response. Nature 2005, 436, 294–298. [Google Scholar] [CrossRef]
- Xhemalce, B.; Miller, K.M.; Driscoll, R.; Masumoto, H.; Jackson, S.P.; Kouzarides, T.; Verreault, A.; Arcangioli, B. Regulation of Histone H3 Lysine 56 Acetylation in Schizosaccharomyces Pombe. J. Biol. Chem. 2007, 282, 15040–15047. [Google Scholar] [CrossRef] [Green Version]
- Mayor, T.; Lipford, J.R.; Graumann, J.; Smith, G.T.; Deshaies, R.J. Analysis of Polyubiquitin Conjugates Reveals That the Rpn10 Substrate Receptor Contributes to the Turnover of Multiple Proteasome Targets. Mol. Cell. Proteomics 2005, 4, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.H.; Feng, Q.; Wang, H.; Erdjument-Bromage, H.; Tempst, P.; Zhang, Y.; Struhl, K. Lysine Methylation within the Globular Domain of Histone H3 by Dot1 Is Important for Telomeric Silencing and Sir Protein Association. Genes Dev. 2002, 16, 1518–1527. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.H.; Ciccone, D.N.; Morshead, K.B.; Oettinger, M.A.; Struhl, K. Lysine-79 of Histone H3 Is Hypomethylated at Silenced Loci in Yeast and Mammalian Cells: A Potential Mechanism for Position-Effect Variegation. Proc. Natl. Acad. Sci. USA 2003, 100, 1820–1825. [Google Scholar] [CrossRef] [Green Version]
- Lacoste, N.; Utley, R.T.; Hunter, J.M.; Poirier, G.G.; Côté, J. Disruptor of Telomeric Silencing-1 Is a Chromatin-Specific Histone H3 Methyltransferase. J. Biol. Chem. 2002, 277, 30421–30424. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Kabbaj, M.-H.M.; Paik, J.; Gunjan, A. Histone Levels Are Regulated by Phosphorylation and Ubiquitylation-Dependent Proteolysis. Nat. Cell Biol. 2009, 11, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayor, T.; Graumann, J.; Bryan, J.; MacCoss, M.J.; Deshaies, R.J. Quantitative Profiling of Ubiquitylated Proteins Reveals Proteasome Substrates and the Substrate Repertoire Influenced by the Rpn10 Receptor Pathway. Mol. Cell. Proteomics 2007, 6, 1885–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, A.; Umehara, T.; Horikoshi, M. Chromosomal Gradient of Histone Acetylation Established by Sas2p and Sir2p Functions as a Shield against Gene Silencing. Nat. Genet. 2002, 32, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.S.; Tobe, B.T.D.; Huh, J.H.; Kron, S.J. Yng2p-Dependent NuA4 Histone H4 Acetylation Activity Is Required for Mitotic and Meiotic Progression. J. Biol. Chem. 2001, 276, 43653–43662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-Q.; Li, Y.; Pan, X.; Lei, B.-K.; Chang, C.; Liu, Z.-X.; Lu, H. The Fission Yeast Inhibitor of Growth (ING) Protein Png1p Functions in Response to DNA Damage. J. Biol. Chem. 2010, 285, 15786–15793. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chang, P.; Ding, J.; Chen, J. Distinct and Redundant Roles of the Two MYST Histone Acetyltransferases Esa1 and Sas2 in Cell Growth and Morphogenesis of Candida Albicans. Eukaryot. Cell 2013, 12, 438–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmen, A.A.; Milne, L.; Grunstein, M. Acetylation of the Yeast Histone H4 N Terminus Regulates Its Binding to Heterochromatin Protein SIR3. J. Biol. Chem. 2002, 277, 4778–4781. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.O.; Wang, S.-S.; Zhang, Y.; Fu, X.-H.; Dang, W.; Lenzmeier, B.A.; Zhou, J.-Q. Histone H4 Lysine 12 Acetylation Regulates Telomeric Heterochromatin Plasticity in Saccharomyces Cerevisiae. PLoS Genet. 2011, 7, e1001272. [Google Scholar] [CrossRef] [Green Version]
- Verrier, L.; Taglini, F.; Barrales, R.R.; Webb, S.; Urano, T.; Braun, S.; Bayne, E.H. Global Regulation of Heterochromatin Spreading by Leo1. Open Biol. 2015, 5, 150045. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.R.; Dang, W.; Berger, S.L. Histone H4 Lysine 20 of Saccharomyces Cerevisiae Is Monomethylated and Functions in Subtelomeric Silencing. Biochemistry 2011, 50, 10473–10483. [Google Scholar] [CrossRef] [Green Version]
- Sanders, S.L.; Portoso, M.; Mata, J.; Bähler, J.; Allshire, R.C.; Kouzarides, T. Methylation of Histone H4 Lysine 20 Controls Recruitment of Crb2 to Sites of DNA Damage. Cell 2004, 119, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Bachleitner, S.; Sulyok, M.; Sørensen, J.L.; Strauss, J.; Studt, L. The H4K20 Methyltransferase Kmt5 Is Involved in Secondary Metabolism and Stress Response in Phytopathogenic Fusarium Species. Fungal Genet. Biol. 2021, 155, 103602. [Google Scholar] [CrossRef]
- Wilson-Grady, J.T.; Villén, J.; Gygi, S.P. Phosphoproteome Analysis of Fission Yeast. J. Proteome Res. 2008, 7, 1088–1097. [Google Scholar] [CrossRef]
- Holt, L.J.; Tuch, B.B.; Villén, J.; Johnson, A.D.; Gygi, S.P.; Morgan, D.O. Global Analysis of Cdk1 Substrate Phosphorylation Sites Provides Insights into Evolution. Science 2009, 325, 1682–1686. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, M.S.; Bever, K.; Avalos, J.L.; Muhammad, S.; Zhang, X.; Wolberger, C. The Structural Basis of Sirtuin Substrate Affinity. Biochemistry 2006, 45, 7511–7521. [Google Scholar] [CrossRef]
- Bao, X.; Liu, Z.; Zhang, W.; Gladysz, K.; Fung, Y.M.E.; Tian, G.; Xiong, Y.; Wong, J.W.H.; Yuen, K.W.Y.; Li, X.D. Glutarylation of Histone H4 Lysine 91 Regulates Chromatin Dynamics. Mol. Cell 2019, 76, 660–675.e9. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etier, A.; Dumetz, F.; Chéreau, S.; Ponts, N. Post-Translational Modifications of Histones Are Versatile Regulators of Fungal Development and Secondary Metabolism. Toxins 2022, 14, 317. https://doi.org/10.3390/toxins14050317
Etier A, Dumetz F, Chéreau S, Ponts N. Post-Translational Modifications of Histones Are Versatile Regulators of Fungal Development and Secondary Metabolism. Toxins. 2022; 14(5):317. https://doi.org/10.3390/toxins14050317
Chicago/Turabian StyleEtier, Aurelie, Fabien Dumetz, Sylvain Chéreau, and Nadia Ponts. 2022. "Post-Translational Modifications of Histones Are Versatile Regulators of Fungal Development and Secondary Metabolism" Toxins 14, no. 5: 317. https://doi.org/10.3390/toxins14050317




