Research Progress of Safety of Zearalenone: A Review
Abstract
:1. Introduction
2. Toxicokinetics of Zearalenone
2.1. Absorption and Distribution
2.2. Metabolism and Excretion
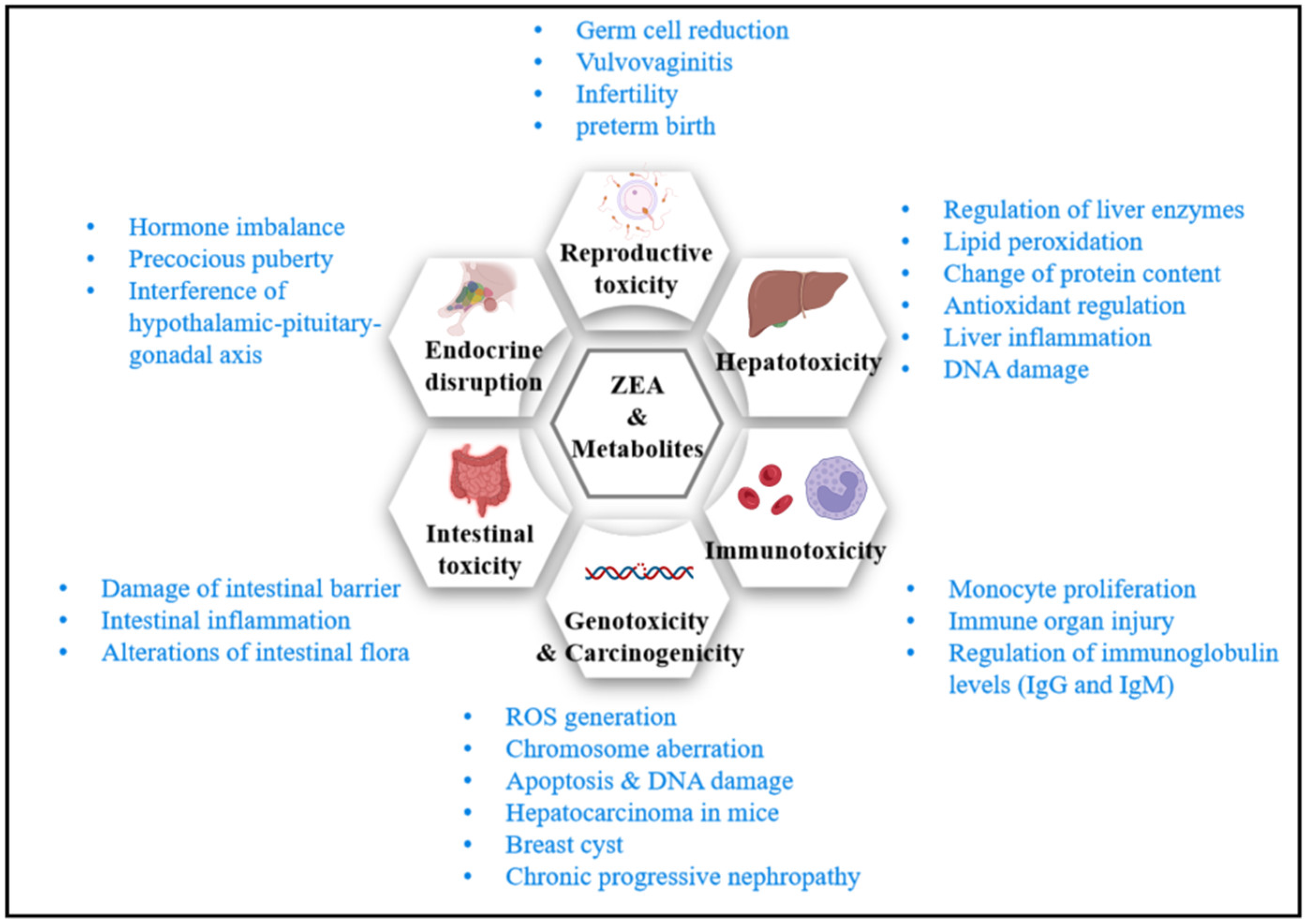
3. Research Progress on the Toxicity of Zearalenone
3.1. Reproductive Toxicity
3.2. Hepatotoxicity
3.3. Immunotoxicity
3.4. Genotoxicity
3.5. Carcinogenicity
3.6. Gastrointestinal Health
3.7. Endocrine-Disrupting Effects
4. ZEA Exposure and Risk Assessment
4.1. ZEA Generations
4.1.1. Temperature
4.1.2. Humidity (Water Activity)
4.1.3. CO2
4.1.4. Climate Change
4.1.5. Other Factors
4.2. Exposure and Allowable Limits
4.3. Disintoxication and Detoxification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Urry, W.H.; Wehrmeister, H.L.; Hodge, E.B.; Hidy, P.H. The structure of zearalenone. Tetrahedron Lett. 1966, 7, 3109–3114. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Env. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 16 February 2022).
- Takemura, H.; Shim, J.-Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J. Steroid Biochem. 2007, 103, 170–177. [Google Scholar] [CrossRef]
- Hasunuma, H.; Takagi, M.; Kawamura, O.; Taniguchi, C.; Nakamura, M.; Chuma, T.; Uno, S.; Kokushi, E.; Matsumoto, D.; Tshering, C.; et al. Natural contamination of dietary rice straw with zearalenone and urinary zearalenone oncentrations in a cattle herd. J. Anim. Sci. 2012, 90, 1610–1616. [Google Scholar] [CrossRef] [Green Version]
- Yazar, S.; Omurtag, G.Z. Fumonisins, Trichothecenes and Zearalenone in Cereals. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar] [CrossRef]
- Prelusky, D.B.; Scott, P.M.; Trenholm, H.L.; Lawrence, G.A. Minimal transmission of zearalenone to milk of dairy cows. J. Environ. Sci. Health Part B 1990, 25, 87–103. [Google Scholar] [CrossRef]
- Gimenez, I.; Herrera, M.; Escobar, J.; Ferruz, E.; Loran, S.; Herrera, A.; Arino, A. Distribution of deoxynivalenol and zearalenone in milled germ during wheat milling and analysis of toxin levels in wheat germ and wheat germ oil. Food Control 2013, 34, 268–273. [Google Scholar] [CrossRef]
- Minervini, F.; Dell’aquila, M.E. Zearalenone and Reproductive Function in Farm Animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [Green Version]
- Kuiper-Goodman, T.; Scott, P.; Watanabe, H. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharm. 1987, 7, 253–306. [Google Scholar] [CrossRef]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef]
- Fitzpatrick, D.W.; Arbuckle, L.D.; Hassen, A.M. Zearalenone metabolism and excretion in the rat: Effect of different doses. J. Environ. Sci. Health Part B 1988, 23, 343–354. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Pathre, S.V.; Robison, T.S. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet. Toxicol. 1981, 19, 25–30. [Google Scholar] [CrossRef]
- Dailey, R.E.; Reese, R.E.; Brouwer, E.A. Metabolism of [14C]zearalenone in laying hens. J. Agric. Food Chem. 1980, 28, 286–291. [Google Scholar] [CrossRef]
- Biehl, M.L.; Prelusky, D.B.; Koritz, G.D.; Hartin, K.E.; Buck, W.B.; Trenholm, H.L. Biliary Excretion and Enterohepatic Cycling of Zearalenone in Immature Pigs. Toxicol. Appl. Pharmacol. 1993, 121, 152–159. [Google Scholar] [CrossRef]
- Danicke, S.; Swiech, E.; Buraczewska, L.; Ueberschar, K.H. Kinetics and metabolism of zearalenone in young female pigs. J. Anim. Physiol. Anim. Nutr. 2005, 89, 268–276. [Google Scholar] [CrossRef]
- Devreese, M.; Antonissen, G.; Broekaert, N.; De Baere, S.; Vanhaecke, L.; De Backer, P.; Croubels, S. Comparative Toxicokinetics, Absolute Oral Bioavailability, and Biotransformation of Zearalenone in Different Poultry Species. J. Agric. Food Chem. 2015, 63, 5092–5098. [Google Scholar] [CrossRef]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef]
- Dong, M.; He, X.J.; Tulayakul, P.; Li, J.Y.; Dong, K.S.; Manabe, N.; Nakayama, H.; Kumagai, S. The toxic effects and fate of intravenously administered zearalenone in goats. Toxicon 2010, 55, 523–530. [Google Scholar] [CrossRef]
- Li, C.; Deng, C.; Zhou, S.; Zhao, Y.; Wang, D.; Wang, X.; Gong, Y.Y.; Wu, Y. High-throughput and sensitive determination of urinary zearalenone and metabolites by UPLC-MS/MS and its application to a human exposure study. Anal. Bioanal. Chem. 2018, 410, 5301–5312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, B.S.; Hong, S.H.; Bulitta, J.B.; Lee, J.B.; Hwang, S.W.; Kim, H.J.; Yang, S.D.; Yoon, H.-S.; Kim, D.J.; Lee, B.M.; et al. Physiologically Based Pharmacokinetics of Zearalenone. J. Toxicol. Environ. Health A 2009, 72, 1395–1405. [Google Scholar] [CrossRef]
- Dänicke, S.; Ueberschär, K.H.; Halle, I.; Valenta, H.; Flachowsky, G. Excretion kinetics and metabolism of zearalenone in broilers in dependence on a detoxifying agent. Arch. Tierernaehr. 2001, 55, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.C.; Churchwell, M.I.; Doerge, D.R. Metabolism and pharmacokinetics of zearalenone following oral and intravenous administration in juvenile female pigs. Food Chem. Toxicol. 2017, 106, 193–201. [Google Scholar] [CrossRef]
- Collins, S.L.; Walsh, J.P.; Renaud, J.B.; Mcmillan, A.; Rulisa, S.; Miller, J.D.; Reid, G.; Sumarah, M.W. Improved methods for biomarker analysis of the big five mycotoxins enables reliable exposure characterization in a population of childbearing age women in Rwanda. Food Chem. Toxicol. 2021, 147, 111854. [Google Scholar] [CrossRef]
- FAO/WHO. Safety Evaluation of Certain Contaminants in Food; Prepared by the Sixty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); FAO Food and Nutrition Paper; FAO: Rome, Italy, 2006; Volume 82, pp. 1–778. [Google Scholar]
- Chang, H.; Kim, W.; Park, J.-H.; Kim, D.; Kim, C.-R.; Chung, S.; Lee, C. The Occurrence of Zearalenone in South Korean Feedstuffs between 2009 and 2016. Toxins 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Yang, L.; Chen, Y.; Sun, T.; Wang, N.; Chen, X.; Yang, Z.; Ge, J.; Jiang, S. Comparative study of stress response, growth and development of uteri in post-weaning gilts challenged with zearalenone and estradiol benzoate. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1885–1894. [Google Scholar] [CrossRef]
- Abid-Essefi, S.; Baudrimont, I.; Hassen, W.; Ouanes, Z.; Mobio, T.A.; Anane, R.; Creppy, E.E.; Bacha, H. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: Prevention by Vitamin E. Toxicology 2003, 192, 237–248. [Google Scholar] [CrossRef]
- Abid-Essefi, S.; Ouanes, Z.; Hassen, W.; Baudrimont, I.; Creppy, E.; Bacha, H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol. In Vitro 2004, 18, 467–474. [Google Scholar] [CrossRef]
- Hassen, W.; Ayed-Boussema, I.; Oscoz, A.A.; De Cerain Lopez, A.; Bacha, H. The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: Oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicology 2007, 232, 294–302. [Google Scholar] [CrossRef]
- Abbès, S.; Ouanes, Z.; Salah-Abbès, J.B.; Houas, Z.; Oueslati, R.; Bacha, H.; Othman, O. The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by Zearalenone in mice. Toxicon 2006, 47, 567–574. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Kongtawelert, P.; Khantamat, O.; Srisomsap, C.; Chokchaichamnankit, D.; Subhasitanont, P.; Svasti, J. Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone-induced apoptosis of human leukemic cells. J. Hematol. Oncol. 2010, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Li, Q.; Zhang, Z.; Lin, P.; Lei, L.; Wang, A.; Jin, Y. Endoplasmic Reticulum Stress Cooperates in Zearalenone-Induced Cell Death of RAW 264.7 Macrophages. Int. J. Mol. Sci. 2015, 16, 19780–19795. [Google Scholar] [CrossRef] [Green Version]
- Farb, R.M.; Mego, J.L.; Hayes, A.W. Effect of mycotoxins on uptake and degradation of [125I]albumin in mouse liver and kidney lysosomes. J. Toxicol. Environ. Health 1976, 1, 985–990. [Google Scholar] [CrossRef]
- Becci, P.J.; Voss, K.A.; Hess, F.G.; Gallo, M.A.; Parent, R.A.; Stevens, K.R.; Taylor, J.M. Long-term carcinogenicity and toxicity study of zearalenone in the rat. J. Appl. Toxicol. 1982, 2, 247–254. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Jiao, R.; Peng, Z.; Li, Y.; Bai, W. Toxic effects of zearalenone on gametogenesis and embryonic development: A molecular point of review. Food Chem. Toxicol. 2018, 119, 24–30. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, G.X.; Liu, J.L.; Fan, J.J.; Cui, S. Toxic effects of zearalenone and its derivatives α-zearalenol on male reproductive system in mice. Reprod. Toxicol. 2007, 24, 381–387. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, C.C.; Leung, L.K. Effect of zeranol on expression of apoptotic and cell cycle proteins in murine placentae. Toxicology 2013, 314, 148–154. [Google Scholar] [CrossRef]
- Su, Y.; Sun, Y.; Ju, D.; Chang, S.; Shi, B.; Shan, A. The detoxification effect of vitamin C on zearalenone toxicity in piglets. Ecotoxicol. Environ. Saf. 2018, 158, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Čonková, E.; Laciaková, A.; Pástorová, B.; Seidel, H.; Kováč, G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol. Lett. 2001, 121, 145–149. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Yin, S.; Jia, Z.; Shan, A. Biochemical changes and oxidative stress induced by zearalenone in the liver of pregnant rats. Hum. Exp. Toxicol. 2015, 34, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Boussabbeh, M.; Helali, S.; Abid-Essefi, S.; Bacha, H. Protective effect of Crocin against zearalenone-induced oxidative stress in liver and kidney of Balb/c mice. Environ. Sci. Pollut. Res. 2015, 22, 19069–19076. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Bulgaru, C.V.; Taranu, I. Cytotoxic and inflammatory effects of individual and combined exposure of HepG2 cells to zearalenone and its metabolites. Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 937–947. [Google Scholar] [CrossRef]
- Ayed-Boussema, I.; Pascussi, J.M.; Maurel, P.; Bacha, H.; Hassen, W. Zearalenone activates pregnane X receptor, constitutive androstane receptor and aryl hydrocarbon receptor and corresponding phase I target genes mRNA in primary cultures of human hepatocytes. Environ. Toxicol. Pharmacol. 2011, 31, 79–87. [Google Scholar] [CrossRef]
- Yoon, J.E.; Lee, K.Y.; Seok, J.S.; Cheng, W.N.; Kwon, H.C.; Jeong, C.H.; Han, S.G. Zearalenone Induces Endoplasmic Reticulum Stress and Modulates the Expression of Phase I/II Enzymes in Human Liver Cells. Toxins 2019, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Karaman, E.F.; Zeybel, M.; Ozden, S. Evaluation of the epigenetic alterations and gene expression levels of HepG2 cells exposed to zearalenone and alpha-zearalenol. Toxicol. Lett. 2020, 326, 52–60. [Google Scholar] [CrossRef]
- Islam, M.R.; Kim, J.W.; Roh, Y.-S.; Kim, J.-H.; Han, K.M.; Kwon, H.-J.; Lim, C.W.; Kim, B. Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017, 14, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Hueza, I.; Raspantini, P.; Raspantini, L.; Latorre, A.; Górniak, S. Zearalenone, an Estrogenic Mycotoxin, is an Immunotoxic Compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Sun, K.; Xia, S.; Feng, Z.; Zou, H.; Gu, J.; Yuan, Y.; Zhu, J.; Liu, Z.; Bian, J. Decrease in immune function and the role of mitogen-activated protein kinase (MAPK) overactivation in apoptosis during T lymphocytes activation induced by zearalenone, deoxynivalenol, and their combinations. Chemosphere 2020, 255, 126999. [Google Scholar] [CrossRef]
- Cai, G.; Pan, S.; Feng, N.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone inhibits T cell chemotaxis by inhibiting cell adhesion and migration related proteins. Ecotoxicol. Environ. Saf. 2019, 175, 263–271. [Google Scholar] [CrossRef]
- Lioi, M.B.; Santoro, A.; Barbieri, R.; Salzano, S.; Ursini, M.V. Ochratoxin A and zearalenone: A comparative study on genotoxic effects and cell death induced in bovine lymphocytes. Mutat. Res./Genet. Toxicol. Environ. Mutagenes. 2004, 557, 19–27. [Google Scholar] [CrossRef]
- Grosse, Y.; Chekir-Ghedira, L.; Huc, A.; Obrecht-Pflumio, S.; Dirheimer, G.; Bacha, H.; Pfohl-Leszkowicz, A. Retinol, ascorbic acid and α-tocopherol prevent DNA adduct formation in mice treated with the mycotoxins ochratoxin A and zearalenone. Cancer Lett. 1997, 114, 225–229. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Fang, H.; Zhao, Y.; Jin, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; Wang, J.; et al. Transcriptional profiling of zearalenone-induced inhibition of IPEC-J2 cell proliferation. Toxicon 2019, 172, 8–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, B.; Wang, M.; Tong, J.; Pan, J.; Wang, N.; Gong, P.; Long, M. Selenium Protects against Zearalenone-Induced Oxidative Stress and Apoptosis in the Mouse Kidney by Inhibiting Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2020, 2020, 6059058. [Google Scholar] [CrossRef]
- Ben Salah-Abbès, J.; Belgacem, H.; Ezzdini, K.; Abdel-Wahhab, M.A.; Abbès, S. Zearalenone nephrotoxicity: DNA fragmentation, apoptotic gene expression and oxidative stress protected by Lactobacillus plantarum MON03. Toxicon 2020, 175, 28–35. [Google Scholar] [CrossRef]
- Zheng, W.L.; Wang, B.J.; Wang, L.; Shan, Y.P.; Zou, H.; Song, R.L.; Wang, T.; Gu, J.H.; Yuan, Y.; Liu, X.Z.; et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wang, B.; Si, M.; Zou, H.; Song, R.; Gu, J.; Yuan, Y.; Liu, X.; Zhu, G.; Bai, J.; et al. Zearalenone altered the cytoskeletal structure via ER stress- autophagy- oxidative stress pathway in mouse TM4 Sertoli cells. Sci. Rep. 2018, 8, 3320. [Google Scholar] [CrossRef]
- Ahamed, S.; Foster, J.S.; Bukovsky, A.; Wimalasena, J. Signal transduction through the ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 cells. Mol. Carcinog. 2001, 30, 88–98. [Google Scholar] [CrossRef]
- Zhang, R.-Q.; Sun, X.-F.; Wu, R.-Y.; Cheng, S.-F.; Zhang, G.-L.; Zhai, Q.-Y.; Liu, X.-L.; Zhao, Y.; Shen, W.; Li, L. Zearalenone exposure elevated the expression of tumorigenesis genes in mouse ovarian granulosa cells. Toxicol. Appl. Pharmacol. 2018, 356, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Wang, J.; Shan, A.; Xu, L. Changes in intestinal barrier functions and gut microbiota in rats exposed to zearalenone. Ecotoxicol. Environ. Saf. 2020, 204, 111072. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lv, Y.; Ren, S.; Shao, M.; Shen, T.; Huang, K.; Zhou, J.; Yan, L.; Song, S. Zearalenone (ZEA)-induced intestinal inflammation is mediated by the NLRP3 inflammasome. Chemosphere 2018, 190, 272–279. [Google Scholar] [CrossRef]
- Gu, A.; Yang, L.; Wang, J.; Li, J.; Shan, A. Protective effect of glutamine and alanyl-glutamine against zearalenone-induced intestinal epithelial barrier dysfunction in IPEC-J2 cells. Res. Vet. Sci. 2021, 137, 48–55. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.; Feng, Q.; Huang, L.; Zhang, G.; Liu, F.; Jiang, S.; Yang, Z. Effects of purified zearalenone on selected immunological measurements of blood in post-weaning gilts. Anim. Nutr. 2016, 2, 142–148. [Google Scholar] [CrossRef]
- Milano, G.D.; Becuvillalobos, D.; Tapia, M.O. Effects of long-term zearalenone administration on spermatogenesis and serum luteinizing hormone, follicle-stimulating hormone, and prolactin values in male rats. Am. J. Vet. Res. 1995, 56, 954–958. [Google Scholar]
- Lijie, Y.; Min, Z.; Libo, H.; Weiren, Y.; Zaibin, Y.; Shuzhen, J.; Ge, J. Zearalenone promotes follicle growth through modulation of Wnt-1/β-catenin signaling pathway and expression of estrogen receptor genes in ovaries of post-weaning piglets. J. Agric. Food Chem. 2018, 66, 7899–7906. [Google Scholar]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic Effects of Maternal Zearalenone Exposure on Intestinal Oxidative Stress, Barrier Function, Immunological and Morphological Changes in Rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Models Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- González-Alvarez, M.E.; Mcguire, B.C.; Keating, A.F. Obesity alters the ovarian proteomic response to zearalenone exposure†. Biol. Reprod. 2021, 105, 278–289. [Google Scholar] [CrossRef]
- Chen, S.; Yang, S.; Wang, M.; Chen, J.; Huang, S.; Wei, Z.; Cheng, Z.; Wang, H.; Long, M.; Li, P. Curcumin inhibits zearalenone-induced apoptosis and oxidative stress in Leydig cells via modulation of the PTEN/Nrf2/Bip signaling pathway. Food Chem. Toxicol. 2020, 141, 111385. [Google Scholar] [CrossRef]
- Wang, B.-J.; Zheng, W.-L.; Feng, N.-N.; Wang, T.; Zou, H.; Gu, J.-H.; Yuan, Y.; Liu, X.-Z.; Liu, Z.-P.; Bian, J.-C. The Effects of Autophagy and PI3K/AKT/m-TOR Signaling Pathway on the Cell-Cycle Arrest of Rats Primary Sertoli Cells Induced by Zearalenone. Toxins 2018, 10, 398. [Google Scholar] [CrossRef]
- Cai, G.; Sun, K.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Mechanism and effects of Zearalenone on mouse T lymphocytes activation in vitro. Ecotoxicol. Environ. Saf. 2018, 162, 208–217. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Shi, L.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; Zhang, H.; et al. Zearalenone induces NLRP3-dependent pyroptosis via activation of NF-κB modulated by autophagy in INS-1 cells. Toxicology 2019, 428, 152304. [Google Scholar] [CrossRef]
- Wang, M.; Wu, W.; Li, L.; He, J.; Huang, S.; Chen, S.; Chen, J.; Long, M.; Yang, S.; Li, P. Analysis of the miRNA Expression Profiles in the Zearalenone-Exposed TM3 Leydig Cell Line. Int. J. Mol. Sci. 2019, 20, 635. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Wang, Y.-M.; Zhang, L.; Zhao, Z.-M.; Zhao, J.; Peng, S.-Q. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signaling. Mol. Cell Endocrinol. 2016, 437, 62–74. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.L.; Martins, H.M. Influence of water activity, temperature and incubation time on the simultaneous production of deoxynivalenol and zearalenone in corn (Zea mays) by Fusarium graminearum. Food Chem. 2002, 79, 315–318. [Google Scholar] [CrossRef]
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef]
- Jiménez, M.; Máñez, M.; Hernández, E. Influence of water activity and temperature on the production of zearalenone in corn by three Fusarium species. Int. J. Food Microbiol. 1996, 29, 417–421. [Google Scholar] [CrossRef]
- Manstretta, V.; Rossi, V.; Brakhage, A.A. Effects of Temperature and Moisture on Development of Fusarium graminearum Perithecia in Maize Stalk Residues. Appl. Environ. Microbiol. 2016, 82, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panwar, V.; Aggarwal, A.; Paul, S.; Singh, V.; Singh, P.; Sharma, D.; Saharan, M. Effect of temperature and pH on the growth of Fusarium spp. causing Fusarium head blight (FHB) in wheat. South Asian J. Exp. Biol. 2016, 6, 186–193. [Google Scholar] [CrossRef]
- Lahouar, A.; Marin, S.; Crespo-Sempere, A.; Saïd, S.; Sanchis, V. Influence of temperature, water activity and incubation time on fungal growth and production of ochratoxin A and zearalenone by toxigenic Aspergillus tubingensis and Fusarium incarnatum isolates in sorghum seeds. Int. J. Food Microbiol. 2017, 242, 53–60. [Google Scholar] [CrossRef]
- Van Asselt, E.D.; Booij, C.J.H.; Van Der Fels-Klerx, H.J. Modelling mycotoxin formation by Fusarium graminearum in maize in The Netherlands. Food Addit. Contam. Part A 2012, 29, 1572–1580. [Google Scholar] [CrossRef]
- Edwards, S.G. Zearalenone risk in European wheat. World Mycotoxin J. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Food Safety, Climate Change, and the Role of WHO. Available online: https://apo.who.int/publications/i/item/food-safety-climate-change-and-the-role-of-who (accessed on 15 February 2022).
- Váry, Z.; Mullins, E.; Mcelwain, J.C.; Doohan, F.M. The severity of wheat diseases increases when plants and pathogens are acclimatized to elevated carbon dioxide. Glob. Chang. Biol. 2015, 21, 2661–2669. [Google Scholar] [CrossRef]
- Peter Mshelia, L.; Selamat, J.; Iskandar Putra Samsudin, N.; Rafii, M.Y.; Abdul Mutalib, N.-A.; Nordin, N.; Berthiller, F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins 2020, 12, 478. [Google Scholar] [CrossRef]
- Marroquín-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef]
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef]
- Dowd, P.F. Insect management to facilitate preharvest mycotoxin management. J. Toxicol.-Toxin Rev. 2003, 22, 327–350. [Google Scholar] [CrossRef]
- Liu, C.; Van Der Fels-Klerx, H.J. Quantitative Modeling of Climate Change Impacts on Mycotoxins in Cereals: A Review. Toxins 2021, 13, 276. [Google Scholar] [CrossRef]
- Annunziata, L.; Schirone, M.; Visciano, P.; Campana, G.; De Massis, M.R.; Migliorati, G. Determination of aflatoxins, deoxynivalenol, ochratoxin A and zearalenone in organic wheat flour under different storage conditions. Int. J. Food Sci. Technol. 2021, 56, 4139–4148. [Google Scholar] [CrossRef]
- Bresler, G.; Vaamonde, G.; Degrossi, C.; Fernandez Pinto, V. Amaranth grain as substrate for aflatoxin and zearalenone production at different water activity levels. Int. J. Food Microbiol. 1998, 42, 57–61. [Google Scholar] [CrossRef]
- Gromadzka, K.; Waśkiewicz, A.; Świetlik, J.; Bocianowski, J.; Goliński, P. The role of wastewater treatment in reducing pollution of surface waters with zearalenone/Uloga pročišćavanja otpadnih voda u smanjenju onečišćenja površinskih voda zearalenonom. Arch. Ind. Hyg. Toxicol. 2015, 66, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Waśkiewicz, A.; Gromadzka, K.; Bocianowski, J.; Pluta, P.; Goliński, P. Zearalenone Contamination of the Aquatic Environment as a Result of its Presence in Crops / Pojava Mikotoksina U Vodenom Okolišu Zbog Njihove Prisutnosti U Usjevima. Arch. Ind. Hyg. Toxicol. 2012, 63, 429–435. [Google Scholar] [CrossRef]
- Gajecka, M.; Zielonka, L.; Dabrowski, M.; Gajecki, M. Threats resulting from the presence of zearalenone in water. Med. Weter.-Vet. Med.-Sci. Pract. 2011, 67, 643–646. [Google Scholar]
- Laranjeiro, C.S.M.; Da Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. The mycoestrogen zearalenone in Portuguese flowing waters and its potential environmental impact. Mycotoxin Res. 2018, 34, 77–83. [Google Scholar] [CrossRef]
- Gromadzka, K.; Waśkiewicz, A.; Goliński, P.; Świetlik, J. Occurrence of estrogenic mycotoxin—Zearalenone in aqueous environmental samples with various NOM content. Water Res. 2009, 43, 1051–1059. [Google Scholar] [CrossRef]
- Schwartz, P.; Thorpe, K.L.; Bucheli, T.D.; Wettstein, F.E.; Burkhardt-Holm, P. Short-term exposure to the environmentally relevant estrogenic mycotoxin zearalenone impairs reproduction in fish. Sci. Total Environ. 2010, 409, 326–333. [Google Scholar] [CrossRef]
- Yu, S.; Jia, B.; Li, K.; Zhou, H.; Lai, W.; Tang, Y.; Yan, Z.; Sun, W.; Liu, N.; Yu, D.; et al. Pre-warning of abiotic factors in maize required for potential contamination of fusarium mycotoxins via response surface analysis. Food Control 2021, 121, 107570. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Ou, W.; Zhang, Z.; Wang, Y.; Xu, Q.; Huang, H. Recent advances in detoxification strategies for zearalenone contamination in food and feed. Chin. J. Chem. Eng. 2021, 30, 168–177. [Google Scholar] [CrossRef]
- Horky, P.; Venusova, E.; Aulichova, T.; Ridoskova, A.; Skladanka, J.; Skalickova, S. Usability of graphene oxide as a mycotoxin binder: In vitro study. PLoS ONE 2020, 15, e0239479. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lian, C.; Li, C.; Zheng, S. Investigations on organo-montmorillonites modified by binary nonionic/zwitterionic surfactant mixtures for simultaneous adsorption of aflatoxin B1 and zearalenone. J. Colloid Interface Sci. 2020, 565, 11–22. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; De Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Pallaroni, L.; Björklund, E.; Von Holst, C. Alternative extraction methods for Zearalenone: Microwave assisted extraction and accelerated solvent extraction. Mycotoxin Res. 2002, 18, 74–77. [Google Scholar] [CrossRef]
- Leibetseder, J. Decontamination and detoxification of mycotoxins. Biol. Grow. Anim. 2006, 4, 439–465. [Google Scholar]
- Gbashi, S.; Madala, N.E.; De Saeger, S.; De Boevre, M.; Njobeh, P.B. Numerical optimization of temperature-time degradation of multiple mycotoxins. Food Chem. Toxicol. 2019, 125, 289–304. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Tavčar-Kalcher, G.; Babič, J.; Walsh, J.L.; Cvelbar, U. Mycotoxin decontamination efficacy of atmospheric pressure air plasma. Toxins 2019, 11, 219. [Google Scholar] [CrossRef] [Green Version]
- Wielogorska, E.; Ahmed, Y.; Meneely, J.; Graham, W.G.; Elliott, C.T.; Gilmore, B.F. A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem. 2019, 301, 125281. [Google Scholar] [CrossRef]
- Alexandre, A.P.S.; Castanha, N.; Costa, N.S.; Santos, A.S.; Badiale-Furlong, E.; Augusto, P.E.D.; Calori-Domingues, M.A. Ozone technology to reduce zearalenone contamination in whole maize flour: Degradation kinetics and impact on quality. J. Sci. Food Agric. 2019, 99, 6814–6821. [Google Scholar] [CrossRef]
- Vega, M.F.; Dieguez, S.N.; Riccio, B.; Aranguren, S.; Giordano, A.; Denzoin, L.; Soraci, A.L.; Tapia, M.O.; Ross, R.; Apás, A. Zearalenone adsorption capacity of lactic acid bacteria isolated from pigs. Braz. J. Microbiol. 2017, 48, 715–723. [Google Scholar] [CrossRef]
- Wall-Martínez, H.A.; Pascari, X.; Bigordà, A.; Ramos, A.J.; Marín, S.; Sanchis, V. The fate of Fusarium mycotoxins (deoxynivalenol and zearalenone) through wort fermenting by Saccharomyces yeasts (S. cerevisiae and S. pastorianus). Food Res. Int. 2019, 126, 108587. [Google Scholar] [CrossRef]
- Wang, N.; Wu, W.; Pan, J.; Long, M. Detoxification strategies for zearalenone using microorganisms: A review. Microorganisms 2019, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Tinyiro, S.E.; Yao, W.; Yu, H.; Guo, Y.; Qian, H.; Xie, Y. The ability of Bacillus subtilis and Bacillus natto to degrade zearalenone and its application in food. J. Food Processing Preserv. 2019, 43, e14122. [Google Scholar] [CrossRef]
- Wang, G.; Miao, Y.; Sun, Z.; Zheng, S. Simultaneous adsorption of aflatoxin B1 and zearalenone by mono-and di-alkyl cationic surfactants modified montmorillonites. J. Colloid Interface Sci. 2018, 511, 67–76. [Google Scholar] [CrossRef]
- Vega, M.F.; Diéguez, S.N.; Riccio, B.; Tapia, M.O.; González, S.N. Zearalenone adsorbent based on a lyophilized indigenous bacterial lactobacillus plantarum strain as feed additive for pigs: A preliminary study in vivo. Curr. Microbiol. 2021, 78, 1807–1812. [Google Scholar] [CrossRef]
- Yang, S.; Gong, P.; Pan, J.; Wang, N.; Tong, J.; Wang, M.; Long, M.; Li, P.; He, J. Pediococcus pentosaceus xy46 can absorb zearalenone and alleviate its toxicity to the reproductive systems of male mice. Microorganisms 2019, 7, 266. [Google Scholar] [CrossRef] [Green Version]
- Döll, S.; Dänicke, S.; Valenta, H.; Flachowsky, G. In vitro studies on the evaluation of mycotoxin detoxifying agents for their efficacy on deoxynivalenol and zearalenone. Arch. Anim. Nutr. 2004, 58, 311–324. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Z.-H.; Liu, M.; Zhang, N.-Y.; Khalil, M.M.; Gu, C.-Q.; Qi, D.-S.; Sun, L.-H. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J. Nutr. 2018, 148, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Zhao, J.; Xu, J.; Zhu, L.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. N-acetylcysteine ameliorate cytotoxic injury in piglets sertoli cells induced by zearalenone and deoxynivalenol. Environ. Sci. Pollut. Res. 2021, 28, 60276–60289. [Google Scholar] [CrossRef]
- Huang, W.; Chang, J.; Wang, P.; Liu, C.; Yin, Q.; Song, A.; Gao, T.; Dang, X.; Lu, F. Effect of compound probiotics and mycotoxin degradation enzymes on alleviating cytotoxicity of swine jejunal epithelial cells induced by aflatoxin B1 and zearalenone. Toxins 2019, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, K.-H.; Sun, M.-H.; Lan, M.; Wan, X.; Zhang, Y.; Sun, S.-C. Protective effects of melatonin against zearalenone toxicity on porcine embryos in vitro. Front. Pharmacol. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Khalifa, E.; Diab, A.M.; Khallaf, M.A.; Abdel-Razek, N.; Khalil, R.H. Dietary garlic and chitosan alleviated zearalenone toxic effects on performance, immunity, and challenge of European sea bass, Dicentrarchus labrax, to Vibrio alginolyticus infection. Aquac. Int. 2020, 28, 493–510. [Google Scholar] [CrossRef]
- Yi, Y.; Wan, S.; Wang, S.; Khan, A.; Guo, J.; Zheng, X.; Li, H.; Sun, N. Scutellarin protects mouse ovarian granulosa cells from injury induced by the toxin zearalenone. Food Funct. 2021, 12, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Virk, P.; Al-Mukhaizeem, N.a.R.; Morebah, S.H.B.; Fouad, D.; Elobeid, M. Protective effect of resveratrol against toxicity induced by the mycotoxin, zearalenone in a rat model. Food Chem. Toxicol. 2020, 146, 111840. [Google Scholar] [CrossRef]
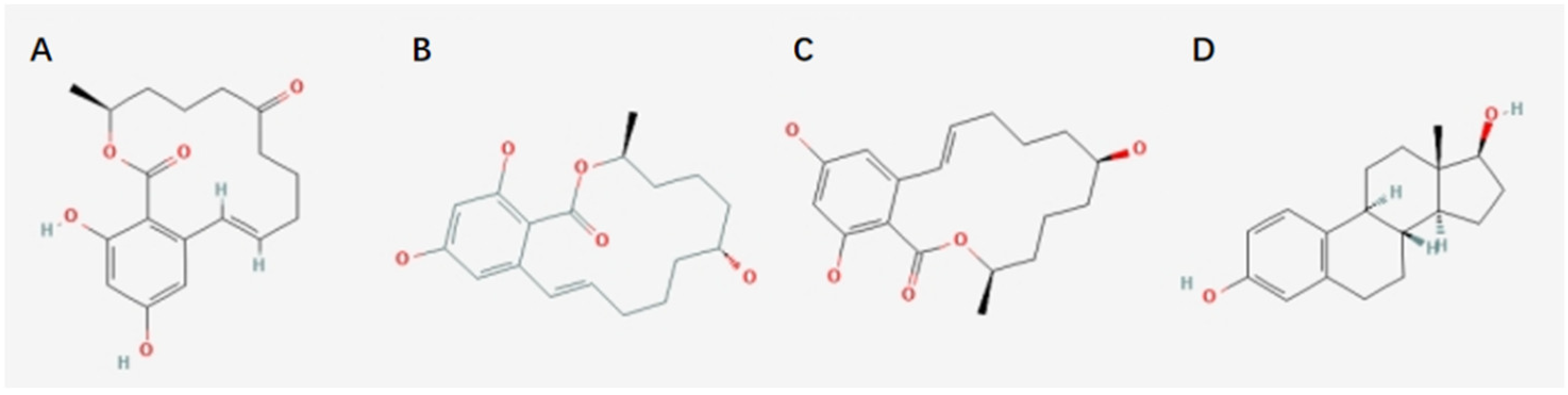

| Toxic Type | Animal/Cell Model | Dose | Exposure Time | Phenotypic Modulation | Pathway | Reference |
|---|---|---|---|---|---|---|
| Reproductive toxicity | Postweaning piglets | 3 mg/kg bw | 28 d | Vulvar malformation, decreased immune response, and disorder of the level of serum hormones. | Hypothalamic–pituitary–ovarian axis pathway. | [43] |
| TM4 cells | 0~30 μM | 24 h | TM4 cell autophagy, oxidative stress, and cytoskeletal structure destruction. | PI3K/Akt/mTOR and MAPK signaling pathway. | [62] | |
| TM3 cells | 50 μM | 24 h | Decreased cell viability and testosterone concentration, increased LDH, and cell apoptosis. | PI3K/Akt, PTEN/Nrf 2/Bip, and ER-stress signaling pathway. | [75] | |
| Sertoli cells (SCs) | 0~80 μM | 24 h | Cell cycle arrest, inhibited SCs proliferation, and cell morphological autophagy. | PI3K/Akt/mTOR signaling pathway. | [76] | |
| Hepatotoxicity | Balb/c mice | 40 mg/kg bw | 24 h | Increased MDA level, protein carbonylation, SOD activity, CAT activity, and the expression level of HSP70. | Oxidative stress pathway. | [46] |
| HepG2 cells | 0~100 μM | 72 h | Decreased cell viability and the expression of liver inflammation-related factors. | Inhibit inflammatory response and liver immunity. | [47] | |
| HepG2 cells | 0~40 μM | 24 h | Decreased cell viability, increased production of ROS, and regulated phase-I/II metabolism, resulting in autophagy and apoptosis. | Oxidative stress, ER-stress, and PERK/eIF2α pathway. | [49] | |
| Immunotoxicity | T lymphocytes | 0~40 μM | 24 h | Decreased cell viability, damaged cell surface and intracellular ultrastructure of T lymphocytes, and decreased secretion of cytokines, resulting in cell apoptosis. | MAPK signaling pathway, TNF-α-independent JNK signaling pathway. | [53] |
| HL-60, U937, PBMCs | 0~50 μM | 24 h | Decreased cell viability, increased production of ROS, and cell apoptosis. | The death receptor pathway with direct involvement of caspase-8, the mitochondrial pathway, and ER-stress pathway. | [35] | |
| T lymphocytes | 0~40 μM | 48 h | Surface and intracellular ultrastructural damage of T lymphocytes. | Chemokines MIP-1α and RANTES secreted by T lymphocytes and chemokine receptor CCR2 and CCR7. | [54] | |
| T lymphocytes | 0~40 μM | 24 h | Decreased cell viability and the expression of different activation signals in T cells inhibited the secretion of cytokines. | Co-stimulatory signal and PI3K/Akt/mTOR signaling pathway. | [77,78] | |
| Genotoxicity | Kunming mice | 40 mg/kg bw | 28 d | Damaged kidney resulting in oxidative stress and renal cell apoptosis. | Bip, CHOP, caspase-12, and JNK signaling pathway | [59] |
| TM4 cells | 0~100 μM | 24 h | Inhibited cell proliferation, cell cycle arrest, and cell apoptosis. | ROS and ER stress, ATP/AMPK pathway. | [61] | |
| Carcinogenicity | INS-1 cells | 0~800 μM | 24 h | NLRP3 inflammasome activation, decreased cell viability, cell autophagy, and pyroptosis. | NF-κB p65 activation and nuclear translocation. | [79] |
| Mouse granulosa cells | 10&30 μM | 72 h | Changed cell morphology, cell cycle arrest, and increased expression of genes related to tumorigenesis. | Hippo signaling pathway. | [64] | |
| TM3 cells | 0~90 μM | 24 h | Decreased cell viability. | Ras, Rap1, PI3K/AKT, Foxo, and AMPK signaling pathway. | [80] | |
| Gastrointestinal health | IPEC-J2 cells | 0~80 μM | 24 h | Decreased the cell viability and increased LDH activity, and inhibited cell proliferation, resulting in cell cycle arrest. | Pathways involved in the cell cycle G2 phase. | [58] |
| SD mice | 0~5.0 mg/kg bw | 28 d | Impaired intestinal barrier, increased permeability and imbalance of intestinal microbiota, and increased systematic intestinal inflammation. | RhoA/ROCK signal pathway. | [65] | |
| Balb/c mice | 4.5 mg/kg bw | 7 d | NLRP3 inflammasome activation and intestine inflammatory. | NLRP3 signaling pathway. | [66] | |
| Endocrine-disrupting effects | Postweaning piglets | 0~3.2 mg/kg bw | 18 d | Inhibited LH secretion. | Kisspeptin–Gpr54–GnRH Pathway. | [68] |
| Postweaning piglets | 0~1.5 mg/kg bw | 35 d | Inhibited follicles maturation and ovarian development. | ERs/GSK-3β-dependent Wnt-1/β-catenin signaling pathway. | [70] | |
| SD mice | 0~5 mg/kg bw | 5 d | Released gonadotropin early—resulted from the advancement of vaginal opening and enlargement of the uterus at the periphery. | Hypothalamic kisspeptin–GPR54 signaling pathway. | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. https://doi.org/10.3390/toxins14060386
Han X, Huangfu B, Xu T, Xu W, Asakiya C, Huang K, He X. Research Progress of Safety of Zearalenone: A Review. Toxins. 2022; 14(6):386. https://doi.org/10.3390/toxins14060386
Chicago/Turabian StyleHan, Xiao, Bingxin Huangfu, Tongxiao Xu, Wentao Xu, Charles Asakiya, Kunlun Huang, and Xiaoyun He. 2022. "Research Progress of Safety of Zearalenone: A Review" Toxins 14, no. 6: 386. https://doi.org/10.3390/toxins14060386





