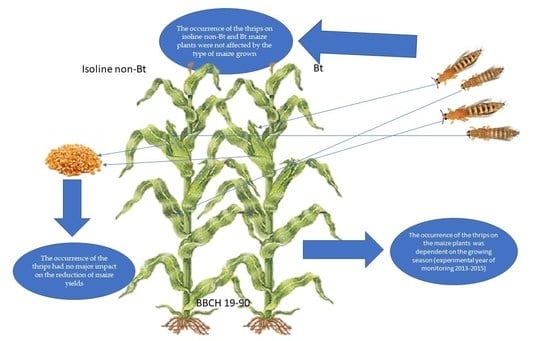
Could the Presence of Thrips AFFECT the Yield Potential of Genetically Modified and Conventional Maize?
Abstract
:1. Introduction
2. Results
2.1. Occurrence of the Thrips in Maize Plots
2.2. The Impact of Weather and Maize Physiological Phase on Thrips Occurrence
2.3. The Impact of the Occurrence of Thrips on the Yield of Maize
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Site and TRIAL Design
5.2. Data Analysis and Thrips Sampling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasconcelos, M.J.V.; Carneiro, A.A.; Valicente, F.H. Estudo de caso em milho Bt. In Plantas Geneticamente Modificadas: Desafios e Oportunidades Para Regiões Tropicais; Borém, A., Almeida, G.D., Eds.; Universidade Federal de Viçosa: Viçosa, Brazil, 2011; pp. 311–332. [Google Scholar]
- Resende, D.C.; Mendes, S.M.; Marucci, R.C.; Silva, A.C.; Campanha, M.M.; Waquil, J.M. Does Bt maize cultivation affect the non-target insect community in the agro ecosystem? Rev. Bras. Entomol. 2016, 60, 82–93. [Google Scholar] [CrossRef]
- Muñóz-Garay, C.; Portugal, L.; Padro-López, L.; Jimenéz-Juárez, N.; Arenas, I.; Gómez, I.; Sánchez-López, R.; Arroyo, R.; Holzenburg, A.; Sawa, C.G.; et al. Characterization of the mechanism of action of the genetically modified Cry1AbMod toxin that is active against Cry1Ab-resistant insects. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2229–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Václavík, L.; Ovesná, J.; Kučera, L.; Hodek, J.; Demnerová, K.; Hajšlová, J. Application of Ultra-high Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS) Metabolomic Fingerprinting to Characterise GM and Conventional Maize Varieties. Czech J. Food Sci. 2013, 31, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.; Sahid, A.A.; Samiullah, T.R.; Rao, A.Q.; Khan, M.A.U.; Tahir, S.; Mirza, S.A.; Husnain, T. Risk assessment of Bt crops on the non-target plant-associated insects and soil organisms. J. Sci. Food Agric. 2016, 96, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Romeis, J.; Naranjo, S.E.; Meissle, M.; Shelton, A.M. Genetically engineered crops help support conservation biological control. Biol. Control. 2019, 130, 136–154. [Google Scholar] [CrossRef]
- Bernal, J.S.; Griset, J.G.; Gillogly, P.O. Impacts of developing on Bt maize-intoxicated hosts on fitness parameters of a stemborer parasitoid. J. Entomol. Sci. 2002, 37, 27–34. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Y.; Cao, K.; Qin, Z.; Zhao, X.; Dong, X.; Shi, W. Impact assessment of Bt maize expressing the Cry1Ab and Cry2Ab protein simultaneously on non-target arthropods. Environ. Sci. Pollut. Res. Int. 2020, 27, 21552–21559. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.Z.; Collins, H.L.; Earle, E.D.; Cao, J.; Shelton, A.M. A critical assessment of the effects of Bt transgenic pants on parasitoids. PLoS ONE 2008, 3, e2284. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Almazán, C.; Zavala, L.E.; Muñoz-Garay, C.; Jiménez-Juárez, N.; Pacheco, S.; Masson, L.; Soberón, M.; Bravo, A. Dominant Negative Mutants of Bacillus thuringiensis Cry1Ab Toxin Function as Anti-Toxins: Demonstration of the Role of Oligomerization in Toxicity. PLoS ONE 2009, 4, e5545, Correction PLoS ONE 2013, 8. https://doi.org/10.1371/annotation/0f267db9-6773-449d-b3c3-8f6c50e637ec. [Google Scholar] [CrossRef]
- Schmitz, G.; Bartsch, D. Biozoenotische Untersuchungen in Maisfeldern bei Bonn und Aachen. Mitt. Der Dtsch. Ges. Für Allg. Und Angew. Entomol. 2001, 13, 615–618. [Google Scholar]
- Steffey, K.L.; Rice, M.E.; All, J.; Andow, D.A.; Gray, M.E.; Van Duyn, J.W. Handbook of Corn Insects; Entomological Society of America: Lanham, MD, USA, 1999; p. 164. [Google Scholar]
- Ramkat, R.C.; Wangai, A.W.; Ouma, J.P.; Rapando, P.N.; Lelgut, D.K. Cropping system influences Tomato spotted wilt virus disease development, thrips population and yield of tomato (Lycopersicon esculentum). Ann. Appl. Biol. 2008, 153, 373–380. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Relationships of immature and adult thrips with silk-cut, fusarium ear rot and fumonisin B1 contamination of corn in California and Ha-waii. Plant Pathol. 2010, 59, 1099–1106. [Google Scholar] [CrossRef]
- Bereś, P.K.; Kucharczyk, H.; Górski, D. Effects of Insecticides Used against the European Corn Borer on Thrips Abundance on Maize. Plant Protect. Sci. 2017, 53, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Lisowicz, F. The occurrence of economically important maize pests in south-eastern Poland. J. Plant Prot. Res. 2001, 41, 250–255. [Google Scholar]
- Dutton, A.; Obrist, L.; D’Aleksandro, M.; Diener, L.; Müller, M.; Romeis, J.; Bigler, F. Tracking Bt-toxin in transgenic maize to assess the risks on non-target arthropods. IOBC/WPRS Bull. 2004, 27, 57–63. [Google Scholar]
- Górecka, J. The Evaluation of Unintended Effects of Growing Genetically Modified Varieties on Chosed Non-Target Arthropod Species and Tri-Trophic Relations. Ph.D. Thesis, Faculty of Horticulture and Landscape Architecture, Warsaw University of Life Sciences, Warsaw, Poland, 2010; 170p. [Google Scholar]
- Montano, J.D.; Fuchs, F.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): A global pest of increasing concern in Onion. J. Econ. Entomol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Syed, T.S.; Khanzada, M.S.; Khanzada, S.R.; Abro, G.H.; Salman, M.; Sarwar, M.; Dayo, S.H.; Anwar, S.; Su, W. Population Dynamics of Thrips, Whiteflies and Their Natural Enemies on Mustard (Brassica campestris L.) Crop in Different Localities of Sindh, Pakista. J. Entomol. Zool. Stud. 2016, 4, 7–16. Available online: https://www.entomoljournal.com/archives/2016/vol4issue1/PartA/3-5-26.pdf (accessed on 10 February 2021).
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected shifts in Fusarium species’ composition on cereal grain in North-ern Europe due to climatic change. Food Addit. Cont. Part A 2012, 29, 1543–1555. [Google Scholar] [CrossRef]
- Lewis, T. Thrips, Their Biology, Ecology, and Economic Importance; Academic Press: London, UK; New York, NY, USA, 1973; 349p, ISBN 0124471609. [Google Scholar]
- Nault, B.A.; Shelton, A.M.; Gangloff-Kaufmann, J.L.; Clark, M.E.; Werren, J.L.; Cabrera-La Rosa, J.C. Reproductive modes in onion thrips (Thysanoptera: Thripidae) population from New York onion fields. Environ. Entomol. 2006, 35, 1264–1271. [Google Scholar] [CrossRef]
- Rőth, F.; Gall, Z.; Tóth, M.; Fail, J.; Jenser, G. The Hypothesized Visual System of Thrips tabaci (Lindeman) and Frankliniella occidentalis (Pergande) Based on Different Coloured Traps’ Catches. North-West. J. Zool. 2016, 12, 40–49. Available online: http://real.mtak.hu/42607/1/2016_NWJZ.pdf (accessed on 22 February 2021).
- He, Z.; Guo, J.F.; Reitz, S.R.; Lei, Z.R.; Wu, S.Y. A global invasion by the thrips, Frankliniella occidentalis: Current virus vector status and its management. Insect Sci. 2020, 27, 626–645. [Google Scholar] [CrossRef] [PubMed]
- Šefrová, H. Třásněnkovití (Thysanoptera: Thripidae) škodící na řepě. Listy Cukrov. Řepař 2015, 131, 142–144. Available online: http://www.cukr-listy.cz/on_line/2015/PDF/142-144.pdf (accessed on 3 March 2021).
- Larsson, H. Aphids and Thrips: The Dynamics and Bio-Economics of Cereal Pests. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2005; 42p. [Google Scholar]
- Bergant, K.; Trdan, S.; Žnidarčič, D.; Črepinšek, Z.; Kajfež-Bogataj, L. Impact of Climate Change on Developmental Dynamics of Thrips tabaci (Thysanoptera: Thripidae): Can It Be Quantified? Environ. Entomol. 2005, 34, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Pinto-Zevallos, D.M.; Vänninen, I. Yellow sticky traps for decision-making in whitefly management: What has been achieved? Crop Prot. 2013, 47, 74–84. [Google Scholar] [CrossRef]
- Abdullah, Z.S.; Greenfield, B.P.J.; Ficken, K.J.; Taylor, J.W.D.; Wood, M.; Butt, T.M. A new attractant for monitoring western flower thrips, Frankliniella occidentalis in protected crops. Springer Plus 2015, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Lisowicz, F. Theoretical and practical bases for applications of integrated pest control protecting corn from damages. Pract. Nauk. Inst. Ochr. Roślin 1996, 36, 5–46. [Google Scholar]
- Kucharczyk, H.; Bereś, P.K.; Dabrowski, Z.T. The Species Composition and Seasonal Dynamics of Thrips (Thysanoptera) Populations on Maize (Zea mays L.) in Southeastern Poland. J. Plant Prot. Res. 2011, 51, 210–216. [Google Scholar] [CrossRef]
- Habuštová, O.; Doležal, P.; Spitzer, L.; Svobodová, Z.; Hussein, H.; Sehnal, F. Impact of Cry1Ab toxin expression on the non-target insects dwelling on maize plants. J. Appl. Entomol. 2014, 138, 164–172. [Google Scholar] [CrossRef]
- Cagáň, L.; Praslička, J.; Huszár, J.; Šrobárová, A.; Roháčik, T.; Hudec, K.; Tancik, J.; Bokor, P.; Tóth, P.; Tóthová, M.; et al. Choroby a Škodcovia Poľných Plodín, 1st ed.; SAU: Nitra Skovakia, Slovakia, 2010; pp. 345–399. ISBN 978-80-552-0354-6. [Google Scholar]
- Zawirska, I.; Wałkowski, W. Fauna and Importance of Thrips (Thysanoptera) for Rye and Winter Wheat in Poland. Part I. Fauna of Thysanoptera on Rye and Winter Wheat in Poland. J. Plant Prot. Res. 2000, 40, 35–55. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.agro-article-caf2486c-4f47-490a-bed7-8076d34f837e (accessed on 3 March 2021).
- Zawirska, I. Fauna Thysanoptera na kukurydzy (Zea mays L.) w Polsce. Pr. Nauk. Inst. Ochr. Roślin 1969, 11, 81–88. [Google Scholar]
- Habuštová, O.; Sehnal, F. Results of a four-year study of the Bt corn impact on arthropod communities. Kosmos Probl. Nauk Biol. 2007, 56, 275–284. [Google Scholar]
- James, C. Global Status of Commercialized Biotech/GM Crops; Brief No. 43; ISAAA Brief—International Service for the Acquisition of Agri-Biotech Applications: Ithaca, NY, USA, 2011; 324p. [Google Scholar]
- Bourguet, D.; Chaufaux, J.; Micoud, A.; Delos, M.; Naibo, B.; Bombarde, F.; Marque, G.; Eychenne, N.; Pagliari, C. Ostrinia nubilalis parasitism and the field abundance of non-target insect in transgenic Bacillus thuringiensis corn (Zea mays). Environ. Biosaf. Res. 2002, 1, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Obrist, L.B.; Klein, H.; Dutton, A.; Bigler, F. Effects of Bt maize on Frankliniella tenuicornisand exposure of thrips predators to prey-mediated Bt toxin. Entomol. Exp. Appl. 2005, 115, 409–416. [Google Scholar] [CrossRef]
- Malchau, W. The preimaginal development of Frankliniella tenuicornis (Uzel, 1895) (Thysanoptera). Entomol. Nachr. Ber. 1990, 34, 129–134. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Langeluddecke, P.; Stauss, R.; van den Boom, T.; Weber, E.; Witzen-Berger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Svobodová, Z.; Skoková Habuštová, O.; Hutchison, W.D.; Hussein, H.M.; Sehnal, F. Risk Assessment of Genetically Engineered Maize Resistant to Diabrotica spp.: Influence on Above-Ground Arthropods in the Czech Republic. PLoS ONE 2015, 10, e0130656. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Li, Y.H.; Wu, K.M. Risk assessment and ecological effects of transgenic Bacillus thuringiensis crops on non-target organisms. J. Integr. Plant Biol. 2011, 53, 520–538. [Google Scholar] [CrossRef]
- Graham, S.H.; Stewart, S.D. Field Study Investigating Cry51Aa2.834_16 in Cotton for Control of Thrips (Thysanoptera: Thripidae) and Tarnished Plant Bugs (Hemiptera: Miridae). J. Econ. Entomol. 2018, 111, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Bereś, P.K.; Kucharczyk, H.; Kucharczyk, M. Thrips Abundance on Sweet Corn in Southeastern Poland and the Impact of Weather Conditions on Their Population Dynamics. Bull. Insectol. 2013, 66, 143–152. Available online: https://www.researchgate.net/publication/240004014_Thrips_abundance_on_sweet_corn_in_southeastern_Poland_and_the_impact_of_weather_conditions_on_their_population_dynamic (accessed on 2 February 2021).
- Reitz, S.R. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): The making of a pest. Florida Entomol. 2009, 92, 7–13. [Google Scholar] [CrossRef]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytical model for description of temperature dependent rate phenomenon in arthropods. Environ. Entomol. 1976, 24, 68–75. [Google Scholar]
- Sharpe, P.J.H.; Demichele, D.W. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef]
- Briere, J.F.; Pracros, P. Comparison of temperature- dependent growth models with the development of Lobesia botrana (Lepidoptera: Tortricidae). Environ. Entomol. 1998, 27, 94–101. [Google Scholar] [CrossRef]
- Patterson, D.T.; Westbrook, J.K.; Joyce, R.J.V.; Lingren, P.D.; Rogasik, J. Weeds, insects, and diseases. Clim. Change 1999, 43, 711–727. [Google Scholar] [CrossRef]
- Harrington, R.; Fleming, R.; Woiwod, I. Climate change impacts on insect management and conservation in temperate regions: Can they be predicted? Agric. For. Entomol. 2001, 3, 233–240. [Google Scholar] [CrossRef]
- Varikou, K.; Tsitsipis, J.A.; Alexandrakis, V.; Hoddle, M. Effect of temperature on the development and longevity of Pezothrips kellyanus (Bagnall) (Thysanoptera: Thripidae). Ann. Entomol. Soc. Am. 2009, 102, 835–841. [Google Scholar] [CrossRef]
- Dintenfass, L.P.; Bartell, D.P.; Scott, M.A. Predicting resurgence of western flower thrips (Thysanoptera: Thripidae) on onions after insecticide application in the Texas high plains. J. Econ. Entomol. 1987, 80, 502–506. [Google Scholar] [CrossRef]
- Varadharajan, S.; Veeraval, R. Populáciou dynamics of chilli thrips, Scirtothrips dorsalis Hood. Indian J. Ecol. 1995, 22, 27–30. [Google Scholar]
- Waiganjo, M.M.; Mueke, J.M.; Gitonga, L.M. Susceptible onion growth stages for selective and economic protection from onion thrips infestation. Acta Hort. 2008, 767, 193–200. [Google Scholar] [CrossRef]
- Lorini, I.; Junior, V.M. Population fluctuations of Thrips tabaci L. (Thysanoptera: Thripidae) on garlic crop. An. Soc. Entomol. Brasil. 1990, 19, 367–371. [Google Scholar] [CrossRef]
- Hamdy, M.K.; Salem, M. The effect of plantation dates of onion, temperature and relative humidity on the population density of the onion thrips, Thrips tabaci Lind. Ann. Agric. Sci. Univ. Ain. Shams 1994, V39, 417–424. [Google Scholar]
- Pierre, J.S.; Dedryvér, C.A. Un model de prevision des pullullations du puceron Sitobion avenae sur blé d’hiver. Acta Oecologica Oecologia Appl. 1984, V5, 152–172. [Google Scholar]
- Lewis, T. Flight and dispersal. In Thrips as Crop Pests; Lewis, T., Ed.; CABI: Oxon, UK, 1997; pp. 175–195. [Google Scholar]
- Moanaro, L.; Choudhary, J. Influence of weather parameters on population dynamics of thrips and mites on summer season cowpea in Eastern Plateau and Hill region of India. J. Agrometeorol. 2016, 18, 296–299. [Google Scholar] [CrossRef]
- Khan, S.M.; Ullah, Z. Population dynamics of insect pests of cotton in Dera Ismail Khan. Sarhad. J. Agric. 1994, 10, 285–290. [Google Scholar]
- Harris, H.M.; Drake, C.J.; Tate, H.D. Observation on the onion thrips (Thrips tabaci Lind). Iowa St. Coll. J. Sci. 1936, 10, 155–172. [Google Scholar]
- Smith, E.A.; Shields, E.J.; Nault, B.A. Impact of Abiotic Factors on Onion Thrips (Thysanoptera: Thripidae) Aerial Dispersal in an Onion Ecosystem. Environ. Entomol. 2016, 45, 1115–1122. [Google Scholar] [CrossRef]
- North, R.C.; Shelton, A.M. Ecology of Thysanoptera within cabbage fields. Environ. Entomol. 1986, 15, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Cluever, J.D.; Smith, H.A. A Photo-Based Key of Thrips (Thysanoptera) Associated with Horticultural Crops in Florida. Fla. Entomol. 2017, 100, 454–467. [Google Scholar] [CrossRef] [Green Version]
- Mound, L.A.; Collins, D.W.; Hastings, A. Thysanoptera Britannica et Hibernica—Thrips of the British Isles; Lucidcentral. Org, Identic Pty Ltd.: Queensland, Australia, 2018; Available online: https://keys.lucidcentral.org/keys/v3/british_thrips/ (accessed on 12 January 2021).
- Mound, L.A.; Tree, D.J.; Paris, D. OZ Thrips, Thysanoptera in Australia. 2022. Available online: http://www.ozthrips.org/ (accessed on 16 January 2022).
- Reed, J.T.; Allen, C.; Bagwell, R.; Cook, D.; Burris, E.; Freeman, B.; Leonard, R.; Lentz, G. A Key to the Thrips on Seedling Cotton in the Midsouthern United States. Bulletin 1156 was Published by the Office of Agricultural Communications, a Unit of the Division of Agriculture, Forestry, and Veterinary Medicine at Mississippi State University. Available online: https://www.mafes.msstate.edu/publications/bulletins/b1156.pdf (accessed on 2 February 2021).



| Species/Genus | 2013 (N) | 2014 (N) | 2015 (N) | 2013 (%) | 2014 (%) | 2015 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Bt | Bt | Non-Bt | Bt | Non-Bt | Bt | Non-Bt | Bt | Non-Bt | Bt | Non-Bt | Bt | |
| Limothrips denticornis | 32 | 26 | 4 | 0 | 6 | 1 | 1.80 | 1.46 | 0.93 | 0.00 | 0.49 | 0.08 |
| Limothrips cerealium | 70 | 80 | 85 | 104 | 254 | 271 | 3.94 | 4.50 | 19.77 | 24.19 | 20.85 | 22.25 |
| Haplothrips aculeatus | 399 | 441 | 49 | 33 | 50 | 25 | 22.44 | 24.80 | 11.40 | 7.67 | 4.11 | 2.05 |
| Frankliniella schultzei | 63 | 38 | 32 | 47 | 50 | 134 | 3.54 | 2.14 | 7.44 | 10.93 | 4.11 | 11.00 |
| Frankliniella occidentalis | 32 | 34 | 9 | 1 | 113 | 55 | 1.80 | 1.91 | 2.09 | 0.23 | 9.28 | 4.52 |
| Thrips tabaci | 1 | 2 | 3 | 3 | 10 | 11 | 0.06 | 0.11 | 0.70 | 0.70 | 0.82 | 0.90 |
| Aeolothrips fasciatus | 161 | 160 | 13 | 5 | 49 | 52 | 9.06 | 9.00 | 3.02 | 1.16 | 4.02 | 4.27 |
| Frankliniella tenuicornis | 88 | 84 | 23 | 13 | 68 | 67 | 4.95 | 4.72 | 5.35 | 3.02 | 5.58 | 5.50 |
| Chirothrips spp. | 36 | 31 | 5 | 1 | 0 | 2 | 2.02 | 1.74 | 1.16 | 0.23 | 0.00 | 0.16 |
| The total amount of thrips | 882 | 896 | 223 | 207 | 600 | 618 | 49.61 | 50.39 | 51.86 | 48.14 | 49.26 | 50.74 |
| 1778 | 430 | 1218 | 100 | 100 | 100 | |||||||
| Examined Parameter | Test Statistics F (DFn, DFd) | Bonferroni’s Multiple Comparisons Test (p) 1 |
|---|---|---|
| Occurrence of thrips Bt vs. Non-Bt | F (1, 46) = 0.0003072; p = 0.9861 | |
| Year of monitoring | F (2, 48) = 3.119; p = 0.053 | |
| Date of collection | F (25, 468) = 41.69; p < 0.0001 | Bt vs. Non-Bt: 1. 7. 2015 (BBCH 33); p = 0.0089 |
| Months of collections | F (4, 90) = 197.9; p < 0.0001 | Bt vs. Non-Bt: p > 0.9999 * |
| Yield of maize grain Bt vs. Non-Bt | F (1, 27) = 0.9746; p = 0.3323 | |
| Year of harvesting | F (2, 27) = 864.7; p < 0.0001 | 2013 vs. 2014: p < 0.0001 2013 vs. 2015: p = 0.0222 2014 vs. 2015: p < 0.0001 |
| Followed Parameters | T (+7) | p-Value | T (−7 + 7) | p-Value | R (+7) | p-Value | R (−7 + 7) | p-Value | R (−30 + 7) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Average No. of thrips on: Non-Bt 2013 | 0.355818 | 0.3473 | 0.35197 | 0.3529 | −0.42231 | 0.2575 | −0.46952 | 0.2022 | −0.28568 | 0.4562 |
| Bt 2013 | 0.364725 | 0.3345 | 0.37427 | 0.3210 | −0.40955 | 0.2737 | −0.46523 | 0.2070 | −0.28983 | 0.4493 |
| Non-Bt 2014 | 0.355418 | 0.3479 | −0.07175 | 0.8545 | −0.27904 | 0.4671 | −0.08482 | 0.8282 | −0.53828 | 0.1349 |
| Bt 2014 | 0.351807 | 0.3532 | −0.03245 | 0.9339 | −0.37886 | 0.3147 | −0.21572 | 0.5772 | −0.47690 | 0.1943 |
| Non-Bt 2015 | 0.172575 | 0.6828 | 0.11284 | 0.7902 | −0.03377 | 0.9367 | −0.27957 | 0.5025 | −0.67649 | 0.0952 |
| Bt 2015 | −0.054100 | 0.8988 | −0.33063 | 0.4238 | −0.21553 | 0.6082 | −0.18084 | 0.6682 | −0.46047 | 0.2984 |
| Non-Bt 2013–15 | 0.274293 | 0.1751 | −0.06663 | 0.7464 | −0.21733 | 0.2862 | −0.27033 | 0.1817 | −0.35888 | 0.0718 |
| Bt 2013–15 | 0.222794 | 0.2740 | −0.06597 | 0.7488 | −0.22819 | 0.2622 | −0.25954 | 0.2004 | −0.32175 | 0.1090 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagáň, Ľ.; Bokor, P.; Skoková Habuštová, O. Could the Presence of Thrips AFFECT the Yield Potential of Genetically Modified and Conventional Maize? Toxins 2022, 14, 502. https://doi.org/10.3390/toxins14070502
Cagáň Ľ, Bokor P, Skoková Habuštová O. Could the Presence of Thrips AFFECT the Yield Potential of Genetically Modified and Conventional Maize? Toxins. 2022; 14(7):502. https://doi.org/10.3390/toxins14070502
Chicago/Turabian StyleCagáň, Ľudovít, Peter Bokor, and Oxana Skoková Habuštová. 2022. "Could the Presence of Thrips AFFECT the Yield Potential of Genetically Modified and Conventional Maize?" Toxins 14, no. 7: 502. https://doi.org/10.3390/toxins14070502