Effects of Sesquiterpene Lactones on Primary Cilia Formation (Ciliogenesis)
Abstract
:1. Introduction
2. Results and Discussion
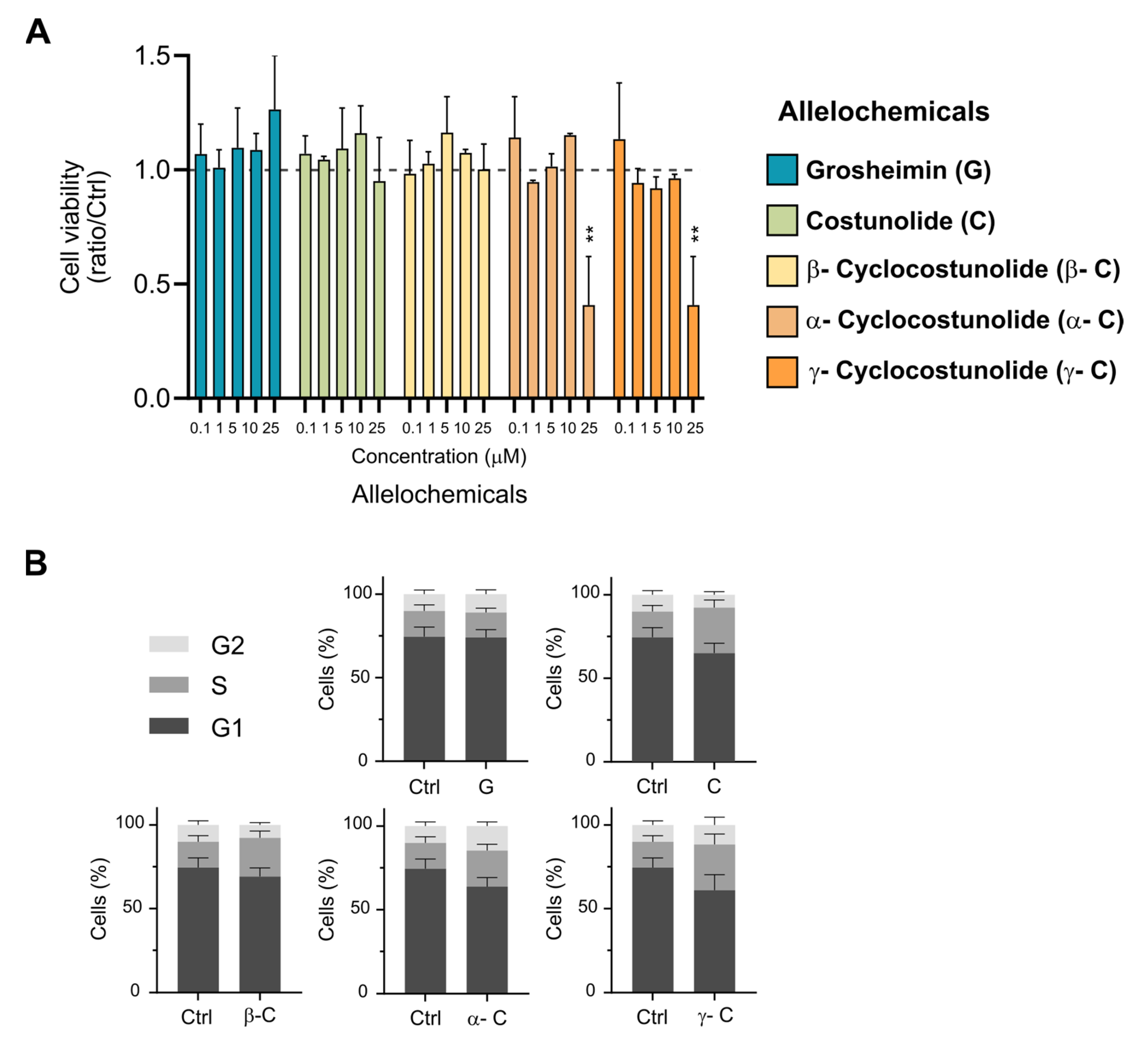
2.1. Effect of Sesquiterpene Lactones on Cell Viability and Cell Cycle Progression
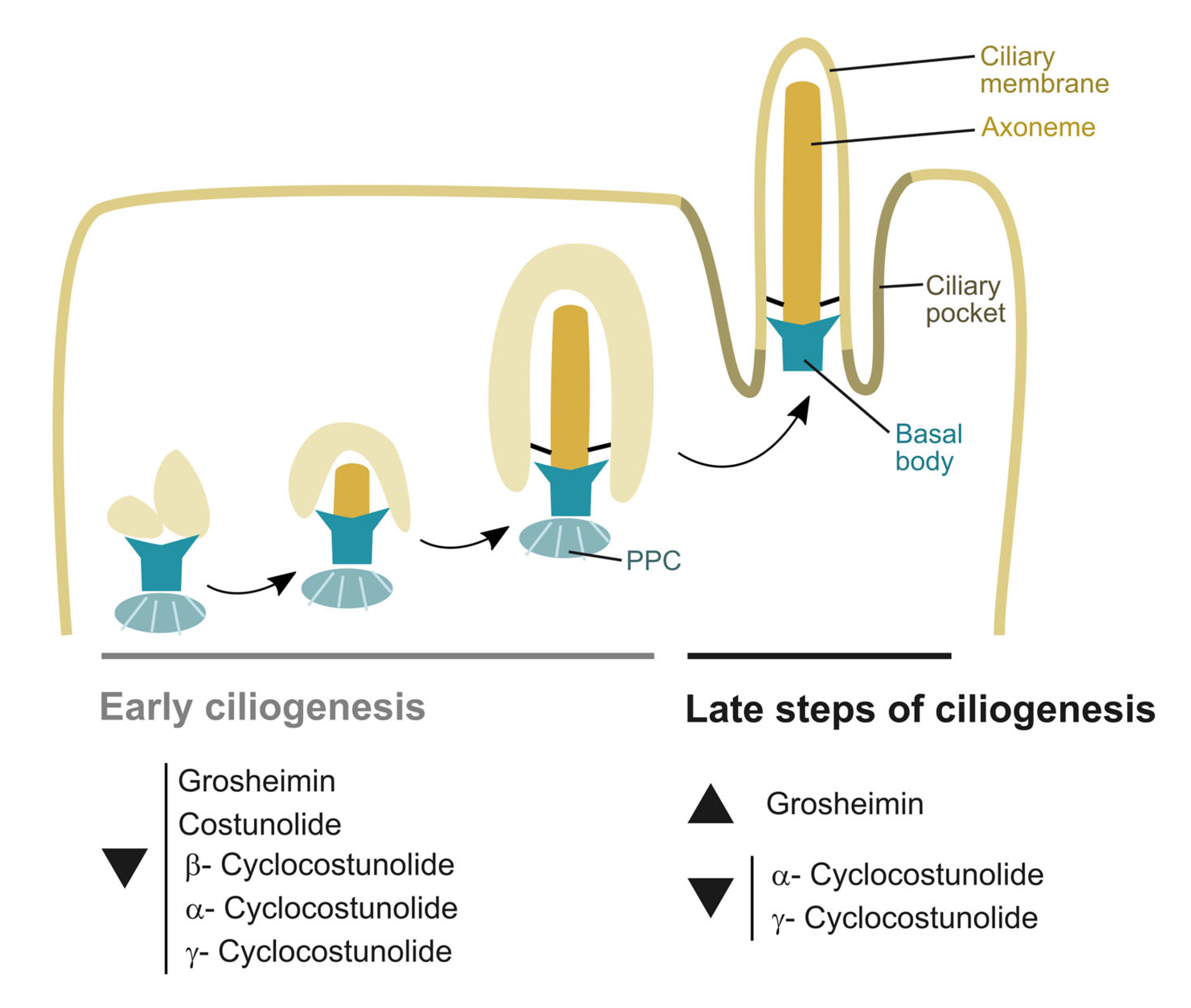
2.2. Effect of Sesquiterpene Lactones on Early Steps of Primary Cilia Formation
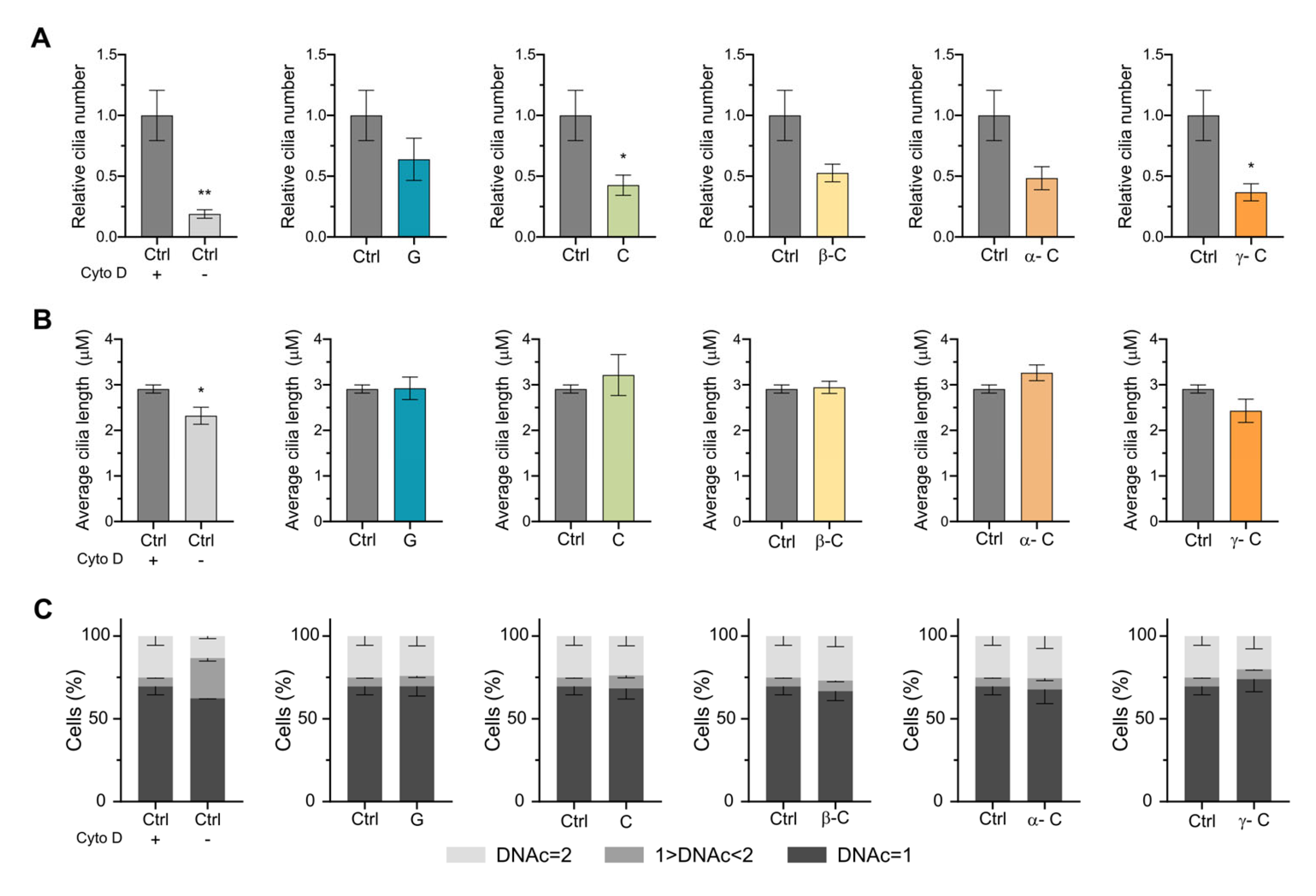
2.3. Effect of Sesquiterpene Lactones on Primary Cilia Formation Induced by Cytochalasin D
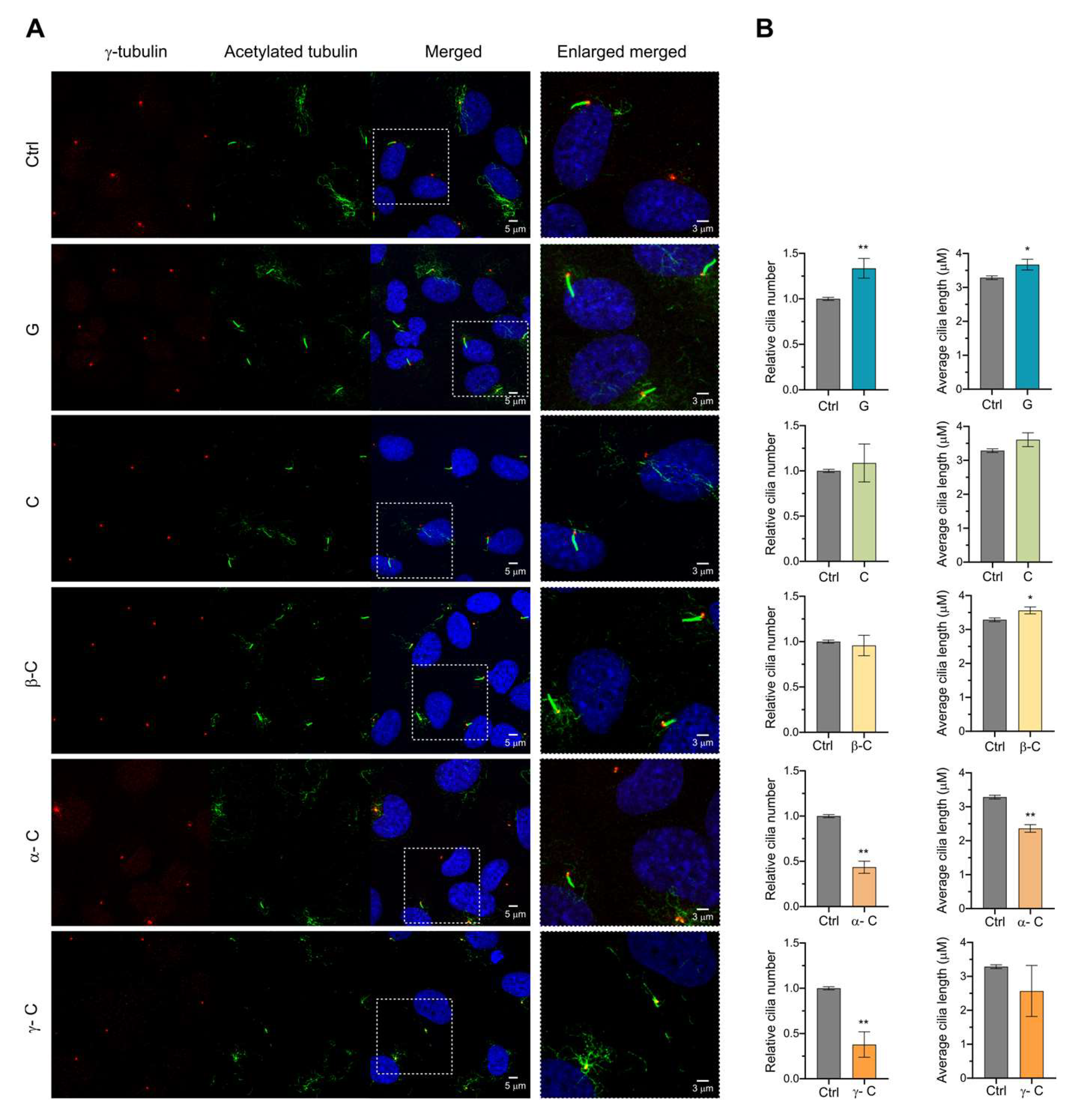
2.4. Effect of Sesquiterpene Lactones on Late Steps of Ciliogenesis
3. Conclusions
4. Materials and Methods
4.1. Plant Material and Extraction and Isolation of Test Compounds
4.2. Semi-Synthesis
4.3. Cell Culture Conditions and Biological Assays
4.4. Immunofluorescence (IF)
4.5. Microscopy
4.6. Cell Cycle Analysis and Flow Cytometry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, C.H.; Leroux, M.R. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat. Cell Biol. 2013, 15, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Mill, P.; Christensen, S.T.; Pedersen, L.B. Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat. Rev. Genet. 2023, 24, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.B.; Schroder, J.M.; Satir, P.; Christensen, S.T. The ciliary cytoskeleton. Compr. Physiol. 2012, 2, 779–803. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.A.; Korobeynikov, V.A.; Nikonova, A.S.; Zhang, P.; Makhov, P.; Deneka, A.Y.; Einarson, M.B.; Serebriiskii, I.G.; Liu, H.; Peterson, J.R.; et al. Unexpected Activities in Regulating Ciliation Contribute to off-target Effects of Targeted Drugs. Clin. Cancer Res. 2019, 25, 4179–4193. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Inaba, H.; Inagaki, M. Mechanisms of ciliogenesis suppression in dividing cells. Cell. Mol. Life Sci. 2017, 74, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Westlake, C.J. Recent advances in understanding assembly of the primary cilium membrane. Fac. Rev. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.C.; Anderson, K.V. The primary cilium: A signalling centre during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Molina, F.E.; Xi, Z.; Reales, E.; Wang, B.; Toomre, D. Exocyst complex mediates recycling of internal cilia. Curr. Biol. 2021, 31, 5580–5589.e5. [Google Scholar] [CrossRef]
- Hildebrandt, F.; Benzing, T.; Katsanis, N. Ciliopathies. N. Engl. J. Med. 2011, 364, 1533–1543. [Google Scholar] [CrossRef]
- Focsa, I.O.; Budisteanu, M.; Balgradean, M. Clinical and genetic heterogeneity of primary ciliopathies (Review). Int. J. Mol. Med. 2021, 48, 176. [Google Scholar] [CrossRef]
- Liu, H.; Kiseleva, A.A.; Golemis, E.A. Ciliary signalling in cancer. Nat. Rev. Cancer 2018, 18, 511–524. [Google Scholar] [CrossRef]
- Khan, N.A.; Garg, A.D.; Agostinis, P.; Swinnen, J.V. Drug-induced ciliogenesis in pancreatic cancer cells is facilitated by the secreted ATP-purinergic receptor signaling pathway. Oncotarget 2018, 9, 3507–3518. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.J.; Fritzler, M.J.; Rattner, J.B. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 2009, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Fernandez, A.; Varela, R.M.; Molinillo, J.M.; Torres, A.; Alves, P.L. Sesquiterpene lactones as allelochemicals. J. Nat. Prod. 2006, 69, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, I.A.; El-Baba, C.; Youssef, A.; Saliba, N.A.; Ghantous, A.; Darwiche, N. Lessons learned from the discovery and development of the sesquiterpene lactones in cancer therapy and prevention. Expert Opin. Drug Discov. 2022, 17, 1377–1405. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Maggi, F.; Nicoletti, M.; Baldan, V.; Dall Acqua, S. New Drugs from Old Natural Compounds: Scarcely Investigated Sesquiterpenes as New Possible Therapeutic Agents. Curr. Med. Chem. 2018, 25, 1241–1258. [Google Scholar] [CrossRef]
- Coricello, A.; Adams, J.D.; Lien, E.J.; Nguyen, C.; Perri, F.; Williams, T.J.; Aiello, F. A Walk in Nature: Sesquiterpene Lactones as Multi-Target Agents Involved in Inflammatory Pathways. Curr. Med. Chem. 2020, 27, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Golsteyn, R.M. Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells. Molecules 2017, 22, 459. [Google Scholar] [CrossRef]
- Mejias, F.J.R.; Duran, A.G.; Zorrilla, J.G.; Varela, R.M.; Molinillo, J.M.G.; Valdivia, M.M.; Macias, F.A. Acyl Derivatives of Eudesmanolides to Boost their Bioactivity: An Explanation of Behavior in the Cell Membrane Using a Molecular Dynamics Approach. ChemMedChem 2021, 16, 1297–1307. [Google Scholar] [CrossRef]
- Mejias, F.J.R.; Fernandez, I.P.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Calvino, J.J.; Trasobares, S.; Macias, F.A. Encapsulation of Cynara Cardunculus Guaiane-type Lactones in Fully Organic Nanotubes Enhances Their Phytotoxic Properties. J. Agric. Food Chem. 2022, 70, 3644–3653. [Google Scholar] [CrossRef]
- Cardenas, D.M.; Mejias, F.J.R.; Molinillo, J.M.G.; Macias, F.A. Synthesis of Vlasouliolides: A Pathway toward Guaiane-Eudesmane C(17)/C(15) Dimers by Photochemical and Michael Additions. J. Org. Chem. 2020, 85, 7322–7332. [Google Scholar] [CrossRef] [PubMed]
- Choodej, S.; Pudhom, K.; Mitsunaga, T. Inhibition of TNF-alpha-Induced Inflammation by Sesquiterpene Lactones from Saussurea lappa and Semi-Synthetic Analogues. Planta Med. 2018, 84, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, R.H.a.M.H. Saussurea lappa: A Comprehensive Review on its Pharmacological Activity and Phytochemistry. Curr. Tradit. Med. 2020, 6, 13–23. [Google Scholar] [CrossRef]
- Toyota, M.; Nagashima, F.; Asakawa, Y. Labdane type diterpenoids from the liverwort Frullania hamachiloba. Phytochemistry 1998, 27, 1789–1793. [Google Scholar] [CrossRef]
- Lu, Q.; Insinna, C.; Ott, C.; Stauffer, J.; Pintado, P.A.; Rahajeng, J.; Baxa, U.; Walia, V.; Cuenca, A.; Hwang, Y.S.; et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat. Cell Biol. 2015, 17, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Kukic, I.; Rivera-Molina, F.; Toomre, D. The IN/OUT assay: A new tool to study ciliogenesis. Cilia 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, O.V.; Golemis, E.A.; Pugacheva, E.N. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008, 68, 2058–2061. [Google Scholar] [CrossRef]
- Kiseleva, A.A.; Nikonova, A.S.; Golemis, E.A. Patterns of Ciliation and Ciliary Signaling in Cancer. Rev. Physiol. Biochem. Pharmacol. 2023, 185, 87–105. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Song, D.; Liu, Y.; Kong, W. Anticancer activity of Eremanthin against the human cervical cancer cells is due to G2/M phase cell cycle arrest, ROS-mediated necrosis-like cell death and inhibition of PI3K/AKT signalling pathway. J. BUON 2020, 25, 1547–1553. [Google Scholar]
- Zhang, Y.; Zhao, Y.; Ran, Y.; Guo, J.; Cui, H.; Liu, S. Alantolactone exhibits selective antitumor effects in HELA human cervical cancer cells by inhibiting cell migration and invasion, G2/M cell cycle arrest, mitochondrial mediated apoptosis and targeting Nf-kB signalling pathway. J. BUON 2019, 24, 2310–2315. [Google Scholar] [PubMed]
- Kim, J.; Lee, J.E.; Heynen-Genel, S.; Suyama, E.; Ono, K.; Lee, K.; Ideker, T.; Aza-Blanc, P.; Gleeson, J.G. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 2010, 464, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef]
- Smith, C.E.L.; Lake, A.V.R.; Johnson, C.A. Primary Cilia, Ciliogenesis and the Actin Cytoskeleton: A Little Less Resorption, a Little More Actin Please. Front. Cell Dev. Biol. 2020, 8, 622822. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Takasuka, T.; Ishibashi, S.; Ide, T. Cytochalasin D inhibits the progression from the Go to S phase at the mid-prereplicative stage in GC-7 cells stimulated with serum. Cell Struct. Funct. 1985, 10, 37–46. [Google Scholar] [CrossRef]
- Trendowski, M. Using cytochalasins to improve current chemotherapeutic approaches. Anticancer Agents Med. Chem. 2015, 15, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, P.; Marshall, W.F. Stages of ciliogenesis and regulation of ciliary length. Differentiation 2012, 83, S30–S42. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Rosenbaum, J.L. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 2008, 85, 23–61. [Google Scholar] [CrossRef]
- Luo, M.; Cao, M.; Kan, Y.; Li, G.; Snell, W.; Pan, J. The phosphorylation state of an aurora-like kinase marks the length of growing flagella in Chlamydomonas. Curr. Biol. 2011, 21, 586–591. [Google Scholar] [CrossRef]
- Hassounah, N.B.; Nagle, R.; Saboda, K.; Roe, D.J.; Dalkin, B.L.; McDermott, K.M. Primary cilia are lost in preinvasive and invasive prostate cancer. PLoS ONE 2013, 8, e68521. [Google Scholar] [CrossRef]
- Hassounah, N.B.; Nunez, M.; Fordyce, C.; Roe, D.; Nagle, R.; Bunch, T.; McDermott, K.M. Inhibition of Ciliogenesis Promotes Hedgehog Signaling, Tumorigenesis, and Metastasis in Breast Cancer. Mol. Cancer Res. 2017, 15, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.; Papon, L.; Cacheux, W.; Marques Sousa, P.; Lascano, V.; Tort, O.; Giordano, T.; Vacher, S.; Lemmers, B.; Mariani, P.; et al. Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 2014, 33, 2247–2260. [Google Scholar] [CrossRef]
- Sohara, E.; Luo, Y.; Zhang, J.; Manning, D.K.; Beier, D.R.; Zhou, J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J. Am. Soc. Nephrol. 2008, 19, 469–476. [Google Scholar] [CrossRef] [PubMed]
- DiBella, L.M.; Park, A.; Sun, Z. Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum. Mol. Genet. 2009, 18, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Conduit, S.E.; Ramaswamy, V.; Remke, M.; Watkins, D.N.; Wainwright, B.J.; Taylor, M.D.; Mitchell, C.A.; Dyson, J.M. A compartmentalized phosphoinositide signaling axis at cilia is regulated by INPP5E to maintain cilia and promote Sonic Hedgehog medulloblastoma. Oncogene 2017, 36, 5969–5984. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Facile synthesis of anhydrojudaicin and 11,13-dehydroanhydrojudaicin, two eudesmanolide-skeleton lactones with potential allelopathic activity. Phytochem. Lett. 2019, 31, 229–236. [Google Scholar] [CrossRef]
- Rao Vadaparthi, P.R.; Kumar, K.; Sarma, V.U.; Hussain, Q.A.; Babu, K.S. Estimation of Costunolide and Dehydrocostus Lactone in Saussurea lappa and its Polyherbal Formulations followed by their Stability Studies Using HPLC-DAD. Pharmacogn. Mag. 2015, 11, 180–190. [Google Scholar] [CrossRef]
- Yayli, N.; Baltaci, C.; Gök, Y.; Aydin, E.; Üçüncü, O. Sesquiterpene lactones from Centaurea helenioides Boiss. Turk. J. Chem. 2006, 30, 229–233. [Google Scholar]
- Cala, A.; Zorrilla, J.G.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Macias, F.A. Easy Access to Alkoxy, Amino, Carbamoyl, Hydroxy, and Thiol Derivatives of Sesquiterpene Lactones and Evaluation of Their Bioactivity on Parasitic Weeds. J. Agric. Food Chem. 2019, 67, 10764–10773. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo-Pineda, M.; Coto-Cid, J.M.; Romero, M.; Zorrilla, J.G.; Chinchilla, N.; Medina-Calzada, Z.; Varela, R.M.; Juárez-Soto, Á.; Macías, F.A.; Reales, E. Effects of Sesquiterpene Lactones on Primary Cilia Formation (Ciliogenesis). Toxins 2023, 15, 632. https://doi.org/10.3390/toxins15110632
Murillo-Pineda M, Coto-Cid JM, Romero M, Zorrilla JG, Chinchilla N, Medina-Calzada Z, Varela RM, Juárez-Soto Á, Macías FA, Reales E. Effects of Sesquiterpene Lactones on Primary Cilia Formation (Ciliogenesis). Toxins. 2023; 15(11):632. https://doi.org/10.3390/toxins15110632
Chicago/Turabian StyleMurillo-Pineda, Marina, Juan M. Coto-Cid, María Romero, Jesús G. Zorrilla, Nuria Chinchilla, Zahara Medina-Calzada, Rosa M. Varela, Álvaro Juárez-Soto, Francisco A. Macías, and Elena Reales. 2023. "Effects of Sesquiterpene Lactones on Primary Cilia Formation (Ciliogenesis)" Toxins 15, no. 11: 632. https://doi.org/10.3390/toxins15110632





