The Need for Next-Generation Antivenom for Snakebite Envenomation in India
Abstract
:1. Introduction
2. Variation in Snake Venom Composition and Antivenom Efficacy
3. Preclinical Studies of ‘Big Four’ Indian Snakes Using Indian Polyvalent Antivenoms
4. Lethal Envenoming by ‘Non-Big Four’ Snakes in India and the Antivenom Concern
| SI No. | Snake Species | Geographical Location of the Snake (s) Used for the Study | Antivenom (s) Used | Antivenom Neutralization Studies Performed | Observations/Inferences | Ref. |
|---|---|---|---|---|---|---|
| 1 | Naja naja | Northwestern (Rajasthan, Gujarat) | VINS | IAC | Antivenom efficacy varies according to the geographical location of the snake species. | [34] |
| 2 | Naja naja | Eastern India—(Burdwan District, West Bengal) Calcutta snake park, Kolkata | BSAV, PSAV | ELISA, WB, IAC | Both antivenoms showed poor immunological cross-reactivity to the low-molecular-mass proteins (<20 kDa) present in Naja naja venom. | [35] |
| 3 | Naja naja | Southern India—Tamil Nadu-Irula snake catcher’s society | BSAV, PSAV, VINS, and Virchow | ELISA, WB | The low molecular mass (<15 kDa) proteins showed poor immuno-recognition compared to the high- and mid-molecular-mass proteins. | [36] |
| 4 | Naja naja | Western India—Haffkine Institute, Mumbai | BSAV, PSAV, and Virchow | ELISA, WB, IAC, neutralization of enzyme activities and anti-coagulant activity | Poor recognition of proteins with low-molecular-mass (<20 kDa) toxins present in the cobra venom from Western parts of India. | [37] |
| 5 | Naja naja | Maharashtra (West India) | BSAV, PSAV, VINS, and Haffkine | ELISA, WB, LD50, ED50 | All antivenoms recognized the venom antigenic epitopes in the ‘big four’ snake species more efficiently than other snake species. | [48] |
| 6 | Naja naja | India (exact location not mentioned) | VINS, BSAV | LD50,ED50 | BSAV antivenom was very weak in recognizing venom from other krait and cobra species. However, the VPAV effectively neutralized venom from all Southeast Asian cobras, B. candidus, N. naja, and Ophiophagus hannah with varying potencies. | [49] |
| 7 | Naja naja | Western Ghats of India-Kerala | VINS, PSAV, Virchow | ELISA, WB | Antivenomics performed using VINS antivenom indicated that it detects and binds to low-molecular-mass proteins less effectively. | [50] |
| 8 | Naja naja | North—Punjab | BSAV, PSAV, Haffkine, and VINS | ELSA, WB, LD50, ED50 | This study showed that all the antivenoms failed to neutralize Naja naja venom from desert populations. This study reiterated the need for the development of a pan-India antivenom that is effective against all snake species. | [51] |
| South—(Tamil Nadu) | ||||||
| Southeast—(Andhra Pradesh) | ||||||
| East—(West Bengal) | ||||||
| Southwest—(Maharashtra) | ||||||
| Central—(Madhya Pradesh) | ||||||
| West—(Rajasthan) | ||||||
| 9 | Naja naja | Hindustan Park (Kolkotta, West Bengal) | Haffkines | LD50, Poteolytic and hemolytic inhibitory activities | This study suggests developing region-specific antivenoms for the effective management of snakebites. | [52] |
| Irula Snake Catchers (Chennai, Tamilnadu) | ||||||
| Haffkine Institute (Mumbai, Maharashtra) | ||||||
| 10 | Naja naja, Naja oxiana, and Naja kaouthia | Himachal Pradesh, West Bengal, Mizoram, Assam, Maharashtra, Tamil Nadu (Irula) and Arunachal Pradesh | VINS, PSAV | WB, IAC | Antivenomics indicated that low-molecular-mass proteins such as PLA2 and 3FTXs were recognized poorly by the antivenom. | [53] |
| 11 | Echis carinatus | Maharashtra (West India) | BSAV, PSAV, VINS, and Haffkine | ELISA, WB, LD50, ED50 | The detection and binding efficacies of antivenoms seems to vary among all of the snake venoms tested. | [48] |
| 12 | Echis carinatus | Tamil Nadu, Goa and Rajasthan | VINS | size-exclusion chromatography | Compared venom collected from Goa and Rajasthan; the E. carniatus venom collected from Tamil Nadu resulted in the formation of more venom–antivenom complexes, indicating binding efficacy. | [54] |
| 13 | Echis carinatus | Goa and Tamil Nadu | BSAV | IAC | Low-molecular-mass proteins, especially disintegrins, present in the venom showed poor binding to the antivenom tested. | [55] |
| 14 | Echis carinatus | Southern India—Tamil Nadu, Irula snake catcher’s society | BSAV, PSAV, Virchow | ELISA, WB, IAC, and pro-coagulant activity | The antivenoms poorly recognized the low-molecular-mass proteins (<20 kDa) present in E. carinatus venom. | [42] |
| 15 | Echis carinatus sochureki | Rajasthan (Northwest India) | BSAV, PSAV, VINS, and Haffkine | ELISA, WB, LD50, ED50 | The detection and binding efficacies of the antivenoms seems vary among all of the snake venoms tested. | [48] |
| 16 | Daboia russelii | Eastern India (Nadia and Burdwan District, West Bengal)—Calcutta Snake park | BSAV, PSAV, Virchow, and BE | ELISA, WB, IAC | All of the antivenoms failed to recognize low-molecular-mass proteins (<20 kDa). | [56] |
| 17 | Daboia russelii | Southern India-Tamil Nadu-Irula snake catcher’s society | BSAV, PSAV, Virchow, and BE | ELISA, WB, IAC, and neutralization of enzyme activities and pharmacological properties | Poor recognition of the low-molecular-mass protein(<20 kDa) of Naja naja venom from Western parts of India by all the antivenoms. | [57] |
| 18 | Daboia russelii | Southern India-Tamil Nadu-Irula snake catcher’s society | Haffkine, VINS, BE, and PSAV | ELISA, WB, LD50, ED50, and IAC | The immunological cross-reactivity was different towards all of the antivenoms. | [33] |
| 19 | Daboia russelii | Western India-Haffkine Institute, Mumbai | VINS and PSAV | ELISA, WB | Both the antivenoms exhibited poor cross-reactivity towards low-molecular-mass proteins (<18 kDa) in the crude venom. The study also demonstrated that monovalent antivenoms are better than polyvalent antivenoms. | [41] |
| 20 | Daboia russelii | North- Punjab | BSAV, PSAV, Haffkine, and VINS | ELISA, WB, LD50, ED50 | The antivenoms showed poor immunological cross-reactivity against all of the venoms used, indicating the need for pan-India effective antivenoms. | [58] |
| South-(Tamil Nadu) | ||||||
| Southeast-(Andhra Pradesh) | ||||||
| East-(West Bengal) | ||||||
| Southwest-(Maharashtra) | ||||||
| Central-(Madhya Pradesh) | ||||||
| 21 | Daboia russelii | Tamil Nadu region (South India) | VINS | ELISA, LD50, procoagulant activity and neutralization | Compared to high-molecular-mass venom proteins, the low-molecular-mass proteins were poorly recognized by the antivenom. | [59] |
| 22 | Bungarus caeruleus | Southern India-Tamil Nadu-Irula snake catcher’s society | BSAV, PSAV, and BE | ELISA, WB, IAC | Poor recognition of low-molecular-mass proteins (<15 kDa) such as three-finger toxins and phospholipase A2 by the antivenoms. | [40] |
| 23 | Bungarus caeruleus | South-eastern India, unspecified locales of India, supplied by Latoxan (France) | VINS, Neuro Polyvalent Antivenom (NBAV), and Bungarus candidus Monovalent Antivenom | ELISA, LD50, ED50 | All venoms showed better immuno-reactivity profiles towards VINS antivenom. Also, compared to venom from Pakistan and Sri Lanka, Indian venom was effectively neutralized by the antivenoms. | [60] |
| 24 | Bungarus sindanus | Bikaner, Rajasthan | Haffkine and PSAV | ELISA, WB, LD50, and ED50 | The antivenom effectively neutralized B. caeruleus venom, whereas B. sindanus and B. romulusi showed poor cross-reactivity profiles towards the antivenom. | [61] |
| Bungarus sindanus | Pune, Maharashtra | |||||
| Bungarus caeruleus | Pune, Maharashtra | |||||
| Bungarus romulusi | Bannerghatta, Karnataka | |||||
| 25 | Bungarus caeruleus | Punjab (North India) | BSAV, Haffkine, PSAV, and VINS | ELISA, WB, LD50, ED50 | All the antivenoms recognized the venom antigenic epitopes in the ‘big four’ snake species and showed varied immunological cross-reactivity towards venom from other species. | [48] |
| 26 | Bungarus sindanus | Rajasthan (Northwest India) | ||||
| 27 | Bungarus fasciatus | West Bengal (East India) | ||||
| 28 | Naja kaouthia | Arunachal Pradesh (Northeast India) | ||||
| 29 | Naja kaouthia | West Bengal (East India) | ||||
| 30 | Naja kaouthia | North East India (Assam—Guwahati and Jamurighat) | VINS | WB, IAC | The VINS polyvalent antivenom could not recognize the few three-finger toxins present in Naja kaouthia venom. | [62] |
| 31 | Naja kaouthia | East India (Kolkata, West Bengal, and Arunachal Pradesh) | BSAV, Haffkine, PSAV, VINS, and Thai monovalent N. kaouthia antivenom (QSMI) | LD50, ED50, ELISA | The study concluded that intraspecies venom variation affects antivenom efficacy. | [63] |
| 32 | Naja kaouthia | Eastern India-(Burdwan District, West Bengal)-Calcutta snake park, Kolkata | BSAV, PSAV | ELISA, WB, IAC | Both antivenoms showed poor immunological cross-reactivity profiles towards the low-molecular mass proteins (<20 kDa) present in N.kaouthia venom | [35] |
| 32 | Naja kaouthia | Assam | BSAV, PSAV, Virchow, VINS | ELISA, WB | The polyvalent antivenoms poorly recognized the low-molecular-mass proteins (<15 kDa) present in N.kaouthia venom from northeastern India. | [64] |
| 34 | Naja kaouthia | North-East India and Bangladesh | VINS, Haffkine, and BSAV | WB, ED50, LD50, IAC | Antivenoms showed better immunological cross-reactivity towards high-molecular-mass components. VINS antivenom poorly recognized low-molecular-mass proteins. | [32] |
| 35 | Trimeresurus malabaricus | Western Ghats of India-Kerala | VINS, PSAV, Virchow | ELISA, WB | Compared to Russell’s viper venom, all of the antivenoms showed poor immunological cross-reactivity towards Malabar pit viper venom proteins. | [65] |
5. General Strategies for Determining the Omes and Omics of Snake Venom
5.1. Snake Venom Proteomics
5.2. Genomics and Venom Gland Transcriptomics
5.3. Immunological Cross-Reactivity Studies and Antivenomics
6. Alternatives to Antivenom
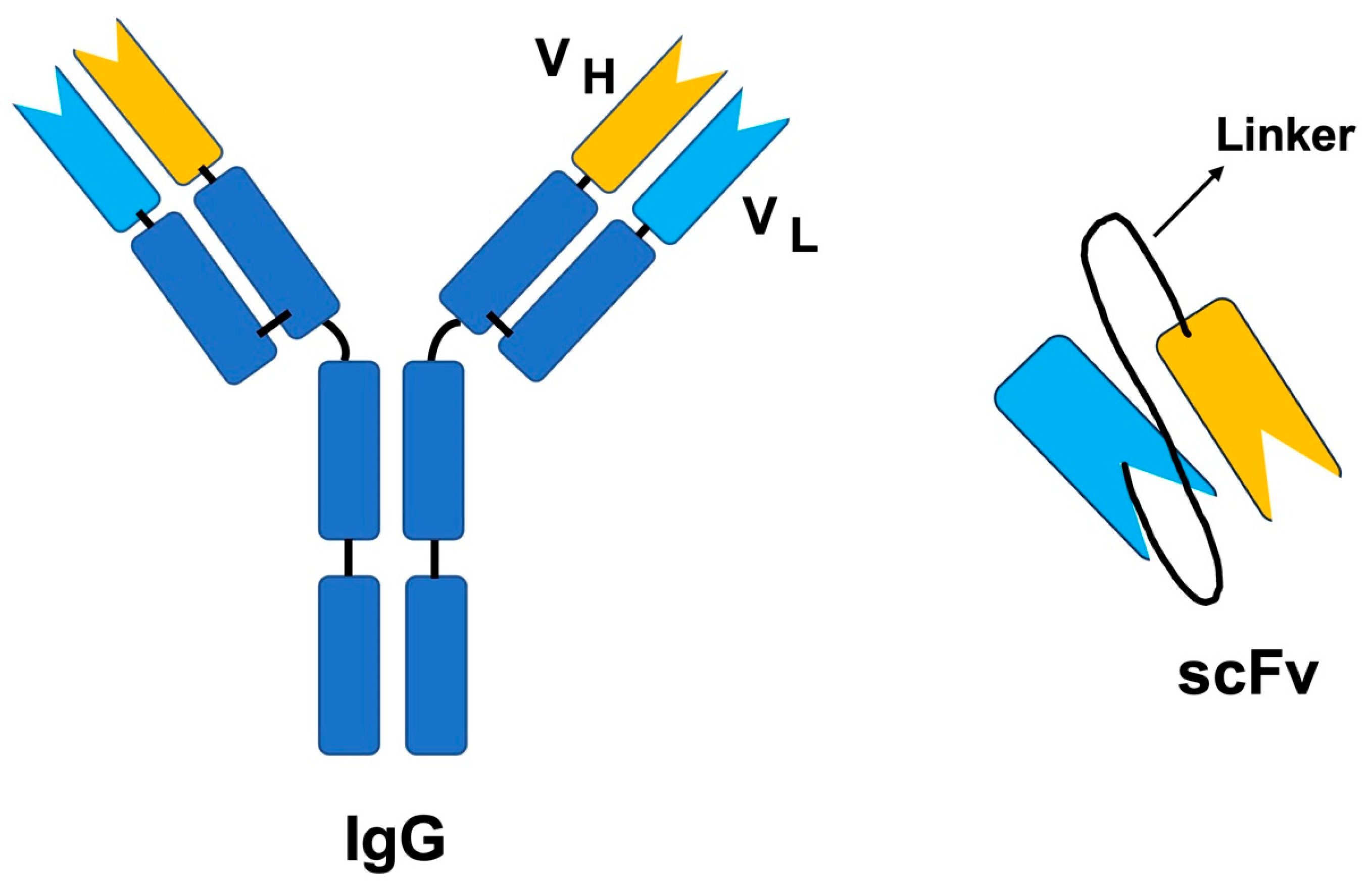
6.1. Aptamers
6.2. Camel Antivenoms
6.3. Phage Display
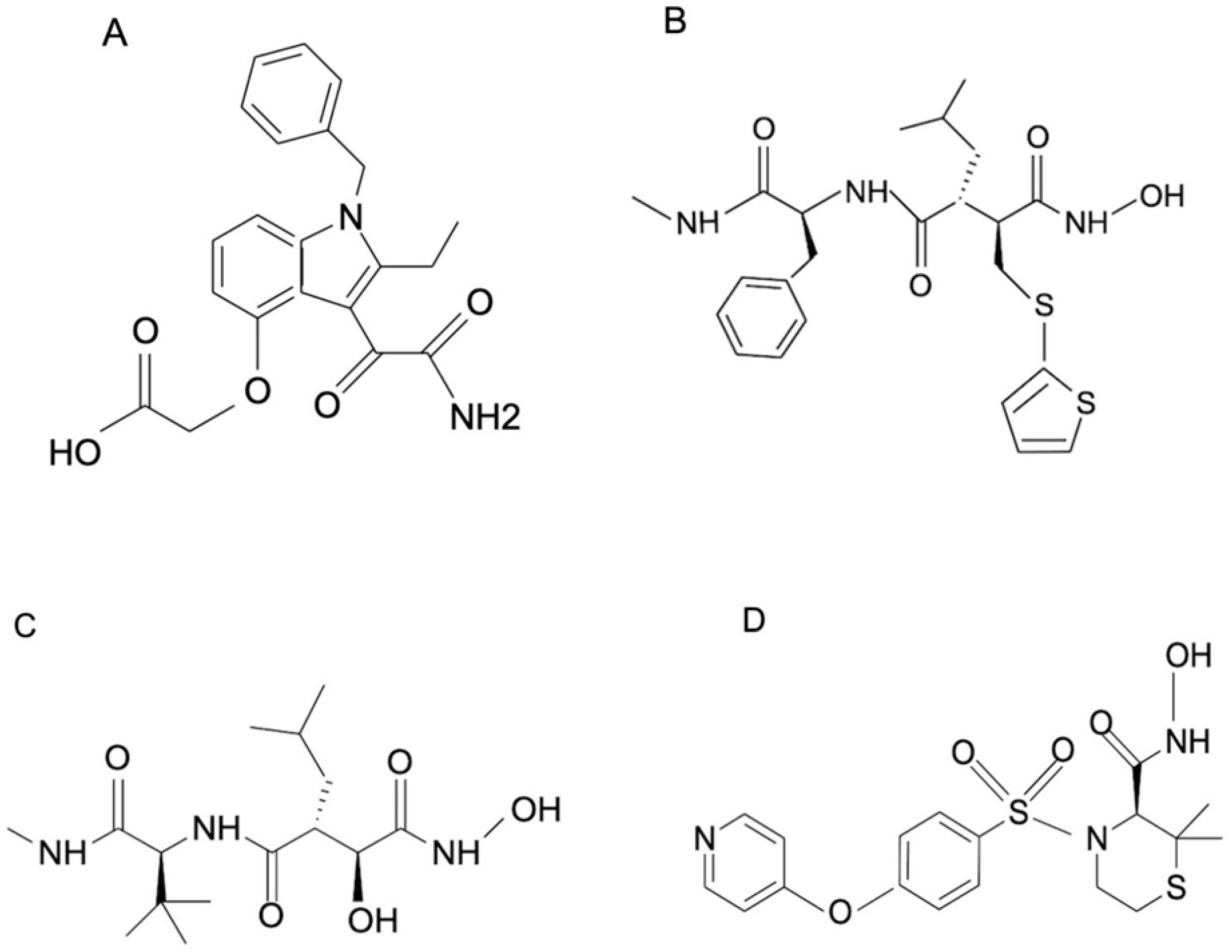
6.4. Small-Molecule Inhibitors
| SI No. | Small-Molecule Inhibitor | Snake Species | Protein | Study Design | Ref. |
|---|---|---|---|---|---|
| 1 | Varespladib | Bothrops asper, Calloselasma rhodostoma, Deinagkistrodon acutus, Daboia russelii, Echis carinatus, Echis ocellatus, and Oxyuranus scutellatus. | Phospholipase A2 | In vitro | [133] |
| 2 | Varespladib | Daboia siamensis | Phospholipase A2 | In vitro | [136] |
| 3 | Batimastat | Crotalus atrox | Group I (PI) metalloprotease | In vitro and in silico | [137] |
| 4 | Marimastat | Crotalus atrox | Group I (PI) metalloprotease | In vitro and in silico | [137] |
| 5 | Varespladib | Naja ashei, Naja katiensis, and Naja nubiae | Phospholipase A2 | In vitro | [138] |
| 6 | Prinomastat | Naja ashei, Naja katiensis, and Naja nubiae | Phospholipase A2 | In vitro | [138] |
6.5. Natural Products
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lancet. Snake-bite envenoming: A priority neglected tropical disease. Lancet 2017, 390, 2. [Google Scholar] [CrossRef] [PubMed]
- Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 18 May 2023).
- Roberts, N.L.S.; Johnson, E.K.; Zeng, S.M.; Hamilton, E.B.; Abdoli, A.; Alahdab, F.; Alipour, V.; Ancuceanu, R.; Andrei, C.L.; Anvari, D.; et al. Global mortality of snakebite envenoming between 1990 and 2019. Nat. Commun. 2022, 13, 6160. [Google Scholar]
- Chippaux, J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- World Health Organisation-Snakebite Information and Data Platform. Available online: https://snbdatainfo.who.int/ (accessed on 18 May 2023).
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, M.S.; Hemshekhar, M.; Sunitha, K.; Thushara, R.M.; Jnaneshwari, S.; Kemparaju, K.; Girish, K.S. Snake venom induced local toxicities: Plant secondary metabolites as an auxiliary therapy. Mini Rev. Med. Chem. 2013, 13, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef]
- Menezes, M.C.; Furtado, M.F.; Travaglia-Cardoso, S.R.; Camargo, A.C.; Serrano, S.M. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 2006, 47, 304–312. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wuster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Mackessy, S.P. Functional basis of a molecular adaptation: Prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon 2009, 53, 672–679. [Google Scholar] [CrossRef]
- Mackessy, S.P.; Leroy, J.; Mocino-Deloya, E.; Setser, K.; Bryson, R.W.; Saviola, A.J. Venom Ontogeny in the Mexican Lance-Headed Rattlesnake (Crotalus polystictus). Toxins 2018, 10, 271. [Google Scholar] [CrossRef]
- Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. Available online: https://apps.who.int/iris/bitstream/handle/10665/255657/9789241210133-eng.pdf#page=217 (accessed on 18 May 2023).
- Brown, N.I. Consequences of neglect: Analysis of the sub-Saharan African snake antivenom market and the global context. PLoS Negl. Trop. Dis. 2012, 6, e1670. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Gutierrez, J.M.; Calvete, J.J.; Wuster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef]
- Habib, A.G.; Brown, N.I. The snakebite problem and antivenom crisis from a health-economic perspective. Toxicon 2018, 150, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Leon, G.; Lomonte, B.; Angulo, Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug. Targets 2011, 10, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Bermudez-Mendez, E.; Fuglsang-Madsen, A.; Fons, S.; Lomonte, B.; Gutierrez, J.M.; Laustsen, A.H. Innovative Immunization Strategies for Antivenom Development. Toxins 2018, 10, 452. [Google Scholar] [CrossRef]
- Otero, R.; Fonnegra, R.; Jimenez, S.L.; Nunez, V.; Evans, N.; Alzate, S.P.; Garcia, M.E.; Saldarriaga, M.; Del Valle, G.; Osorio, R.G.; et al. Snakebites and ethnobotany in the northwest region of Colombia: Part I: Traditional use of plants. J. Ethnopharmacol. 2000, 71, 493–504. [Google Scholar] [CrossRef]
- Gimenes, S.N.C.; Sachett, J.A.G.; Colombini, M.; Freitas-de-Sousa, L.A.; Ibiapina, H.N.S.; Costa, A.G.; Santana, M.F.; Park, J.J.; Sherman, N.E.; Ferreira, L.C.L.; et al. Observation of Bothrops atrox Snake Envenoming Blister Formation from Five Patients: Pathophysiological Insights. Toxins 2021, 13, 800. [Google Scholar] [CrossRef]
- Puzari, U.; Fernandes, P.A.; Mukherjee, A.K. Pharmacological re-assessment of traditional medicinal plants-derived inhibitors as antidotes against snakebite envenoming: A critical review. J. Ethnopharmacol. 2022, 292, 115208. [Google Scholar] [CrossRef]
- Adriao, A.A.X.; Dos Santos, A.O.; de Lima, E.; Maciel, J.B.; Paz, W.H.P.; da Silva, F.M.A.; Pucca, M.B.; Moura-da-Silva, A.M.; Monteiro, W.M.; Sartim, M.A.; et al. Plant-Derived Toxin Inhibitors as Potential Candidates to Complement Antivenom Treatment in Snakebite Envenomations. Front. Immunol. 2022, 13, 842576. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Ainsworth, S.; Slagboom, J.; Alomran, N.; Pla, D.; Alhamdi, Y.; King, S.I.; Bolton, F.M.S.; Gutierrez, J.M.; Vonk, F.J.; Toh, C.H.; et al. The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Commun. Biol. 2018, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Sandesha, V.D.; Darshan, B.; Tejas, C.; Girish, K.S.; Kempaiah, K. A comparative cross-reactivity and paraspecific neutralization study on Hypnale hypnale, Echis carinatus, and Daboia russelii monovalent and therapeutic polyvalent anti-venoms. PLoS Negl. Trop. Dis. 2022, 16, e0010292. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.M.F.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Venom proteome of Bungarus sindanus (Sind krait) from Pakistan and in vivo cross-neutralization of toxicity using an Indian polyvalent antivenom. J. Proteom. 2019, 193, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Faisal, T.; Tan, K.Y.; Sim, S.M.; Quraishi, N.; Tan, N.H.; Tan, C.H. Proteomics, functional characterization and antivenom neutralization of the venom of Pakistani Russell’s viper (Daboia russelii) from the wild. J. Proteom. 2018, 183, 1–13. [Google Scholar] [CrossRef]
- Maduwage, K.; Silva, A.; O’Leary, M.A.; Hodgson, W.C.; Isbister, G.K. Efficacy of Indian polyvalent snake antivenoms against Sri Lankan snake venoms: Lethality studies or clinically focussed in vitro studies. Sci. Rep. 2016, 6, 26778. [Google Scholar] [CrossRef]
- Kalita, B.; Mackessy, S.P.; Mukherjee, A.K. Proteomic analysis reveals geographic variation in venom composition of Russell’s Viper in the Indian subcontinent: Implications for clinical manifestations post-envenomation and antivenom treatment. Expert. Rev. Proteom. 2018, 15, 837–849. [Google Scholar] [CrossRef]
- Deka, A.; Reza, M.A.; Faisal Hoque, K.M.; Deka, K.; Saha, S.; Doley, R. Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms. Toxicon 2019, 164, 31–43. [Google Scholar] [CrossRef]
- Pla, D.; Sanz, L.; Quesada-Bernat, S.; Villalta, M.; Baal, J.; Chowdhury, M.A.W.; Leon, G.; Gutierrez, J.M.; Kuch, U.; Calvete, J.J. Phylovenomics of Daboia russelii across the Indian subcontinent. Bioactivities and comparative in vivo neutralization and in vitro third-generation antivenomics of antivenoms against venoms from India, Bangladesh and Sri Lanka. J. Proteom. 2019, 207, 103443. [Google Scholar] [CrossRef]
- Sintiprungrat, K.; Watcharatanyatip, K.; Senevirathne, W.D.; Chaisuriya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Ratanabanangkoon, K. A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J. Proteom. 2016, 132, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Patra, A.; Kalita, B.; Mukherjee, A.K. Proteomics analysis to compare the venom composition between Naja naja and Naja kaouthia from the same geographical location of eastern India: Correlation with pathophysiology of envenomation and immunological cross-reactivity towards commercial polyantivenom. Expert Rev. Proteom. 2018, 15, 949–961. [Google Scholar]
- Chanda, A.; Mukherjee, A.K. Quantitative proteomics to reveal the composition of Southern India spectacled cobra (Naja naja) venom and its immunological cross-reactivity towards commercial antivenom. Int. J. Biol. Macromol. 2020, 160, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Kalita, B.; Patra, A.; Senevirathne, W.; Mukherjee, A.K. Proteomic analysis and antivenomics study of Western India Naja naja venom: Correlation between venom composition and clinical manifestations of cobra bite in this region. Expert Rev. Proteom. 2019, 16, 171–184. [Google Scholar] [CrossRef]
- Warrell, D.A.; Gutierrez, J.M.; Calvete, J.J.; Williams, D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013, 138, 38–59. [Google Scholar]
- Simpson, I.D.; Norris, R.L. Snakes of medical importance in India: Is the concept of the “Big 4” still relevant and useful? Wilderness Environ. Med. 2007, 18, 2–9. [Google Scholar] [CrossRef]
- Patra, A.; Chanda, A.; Mukherjee, A.K. Quantitative proteomic analysis of venom from Southern India common krait (Bungarus caeruleus) and identification of poorly immunogenic toxins by immune-profiling against commercial antivenom. Expert Rev. Proteom. 2019, 16, 457–469. [Google Scholar] [CrossRef]
- Kalita, B.; Patra, A.; Mukherjee, A.K. Unraveling the Proteome Composition and Immuno-profiling of Western India Russell’s Viper Venom for In-Depth Understanding of Its Pharmacological Properties, Clinical Manifestations, and Effective Antivenom Treatment. J. Proteome Res. 2017, 16, 583–598. [Google Scholar] [CrossRef]
- Patra, A.; Kalita, B.; Chanda, A.; Mukherjee, A.K. Proteomics and antivenomics of Echis carinatus carinatus venom: Correlation with pharmacological properties and pathophysiology of envenomation. Sci. Rep. 2017, 7, 17119. [Google Scholar] [CrossRef]
- Whitaker, R.; Martin, G. Diversity and Distribution of Medically Important Snakes of India. In Clinical Toxinology in Asia Pacific and Africa; Gopalakrishnakone, P., Faiz, A., Fernando, R., Gnanathasan, C., Habib, A., Yang, C.C., Eds.; Springer: Dordrecht, the Netherlands, 2015. [Google Scholar]
- Joseph, J.K.; Simpson, I.D.; Menon, N.C.; Jose, M.P.; Kulkarni, K.J.; Raghavendra, G.B.; Warrell, D.A. First authenticated cases of life-threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 85–90. [Google Scholar] [CrossRef]
- Kumar, K.S.; Narayanan, S.; Udayabhaskaran, V.; Thulaseedharan, N.K. Clinical and epidemiologic profile and predictors of outcome of poisonous snake bites—An analysis of 1500 cases from a tertiary care center in Malabar, North Kerala, India. Int. J. Gen. Med. 2018, 11, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Herath, N.; Wazil, A.; Kularatne, S.; Ratnatunga, N.; Weerakoon, K.; Badurdeen, S.; Rajakrishna, P.; Nanayakkara, N.; Dharmagunawardane, D. Thrombotic microangiopathy and acute kidney injury in hump-nosed viper (Hypnale species) envenoming: A descriptive study in Sri Lanka. Toxicon 2012, 60, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, M.; Yadav, P.; Mathur, R.; Midha, N.; Garg, M.K. Venom-Induced Consumption Coagulopathy Unresponsive to Antivenom After Echis carinatus sochureki Envenoming. Wilderness Environ. Med. 2021, 32, 221–225. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Tan, N.H.; Fung, S.Y.; Sim, S.M. Cross neutralisation of Southeast Asian cobra and krait venoms by Indian polyvalent antivenoms. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Vanuopadath, M.; Raveendran, D.; Nair, B.G.; Nair, S.S. Venomics and antivenomics of Indian spectacled cobra (Naja naja) from the Western Ghats. Acta Trop. 2022, 228, 106324. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Attarde, S.; Khochare, S.; Suranse, V.; Martin, G.; Casewell, N.R.; Whitaker, R.; Sunagar, K. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLoS Negl. Trop. Dis. 2021, 15, e0009150. [Google Scholar] [CrossRef]
- Shashidharamurthy, R.; Kemparaju, K. Region-specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern regional venom: A comparative study of the venoms from three different geographical distributions. Int. Immunopharmacol. 2007, 7, 61–69. [Google Scholar] [CrossRef]
- Deka, A.; Bhatia, S.; Santra, V.; Bharti, O.K.; Lalremsanga, H.T.; Martin, G.; Wuster, W.; Owens, J.B.; Graham, S.; Doley, R.; et al. Multilevel Comparison of Indian Naja Venoms and Their Cross-Reactivity with Indian Polyvalent Antivenoms. Toxins 2023, 15, 258. [Google Scholar] [CrossRef]
- Bhatia, S.; Blotra, A.; Vasudevan, K. Evaluating Antivenom Efficacy against Echis carinatus Venoms-Screening for In Vitro Alternatives. Toxins 2022, 14, 481. [Google Scholar] [CrossRef]
- Bhatia, S.; Blotra, A.; Vasudevan, K. Immunorecognition capacity of Indian polyvalent antivenom against venom toxins from two populations of Echis carinatus. Toxicon 2021, 201, 148–154. [Google Scholar] [CrossRef]
- Kalita, B.; Patra, A.; Das, A.; Mukherjee, A.K. Proteomic Analysis and Immuno-Profiling of Eastern India Russell’s Viper (Daboia russelii) Venom: Correlation between RVV Composition and Clinical Manifestations Post RV Bite. J. Proteome Res. 2018, 17, 2819–2833. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Singh, S.; Patra, A.; Mukherjee, A.K. Quantitative proteomic analysis and antivenom study revealing that neurotoxic phospholipase A2 enzymes, the major toxin class of Russell’s viper venom from southern India, shows the least immuno-recognition and neutralization by commercial polyvalent antivenom. Int. J. Biol. Macromol. 2018, 118, 375–385. [Google Scholar] [PubMed]
- Senji Laxme, R.R.; Khochare, S.; Attarde, S.; Suranse, V.; Iyer, A.; Casewell, N.R.; Whitaker, R.; Martin, G.; Sunagar, K. Biogeographic venom variation in Russell’s viper (Daboia russelii) and the preclinical inefficacy of antivenom therapy in snakebite hotspots. PLoS Negl. Trop. Dis. 2021, 15, e0009247. [Google Scholar] [CrossRef]
- Faisal, T.; Tan, K.Y.; Tan, N.H.; Sim, S.M.; Gnanathasan, C.A.; Tan, C.H. Proteomics, toxicity and antivenom neutralization of Sri Lankan and Indian Russell’s viper (Daboia russelii) venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200177. [Google Scholar] [CrossRef]
- Oh, A.M.F.; Tan, C.H.; Ariaranee, G.C.; Quraishi, N.; Tan, N.H. Venomics of Bungarus caeruleus (Indian krait): Comparable venom profiles, variable immunoreactivities among specimens from Sri Lanka, India and Pakistan. J. Proteom. 2017, 164, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Khochare, S.; Senji Laxme, R.R.; Attarde, S.; Dam, P.; Suranse, V.; Khaire, A.; Martin, G.; Captain, A. A Wolf in Another Wolf’s Clothing: Post-Genomic Regulation Dictates Venom Profiles of Medically-Important Cryptic Kraits in India. Toxins 2021, 13, 69. [Google Scholar] [CrossRef]
- Deka, A.; Gogoi, A.; Das, D.; Purkayastha, J.; Doley, R. Proteomics of Naja kaouthia venom from North East India and assessment of Indian polyvalent antivenom by third generation antivenomics. J. Proteom. 2019, 207, 103463. [Google Scholar] [CrossRef]
- Rashmi, U.; Khochare, S.; Attarde, S.; Laxme, R.R.S.; Suranse, V.; Martin, G.; Sunagar, K. Remarkable intrapopulation venom variability in the monocellate cobra (Naja kaouthia) unveils neglected aspects of India’s snakebite problem. J. Proteom. 2021, 242, 104256. [Google Scholar] [CrossRef]
- Kakati, H.; Patra, A.; Kalita, B.; Chanda, A.; Rapole, S.; Mukherjee, A.K. A comparison of two different analytical workflows to determine the venom proteome composition of Naja kaouthia from North-East India and immunological profiling of venom against commercial antivenoms. Int. J. Biol. Macromol. 2022, 208, 275–287. [Google Scholar] [CrossRef]
- Vanuopadath, M.; Shaji, S.K.; Raveendran, D.; Nair, B.G.; Nair, S.S. Delineating the venom toxin arsenal of Malabar pit viper (Trimeresurus malabaricus) from the Western Ghats of India and evaluating its immunological cross-reactivity and in vitro cytotoxicity. Int. J. Biol. Macromol. 2020, 148, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Mukherjee, A.K. Mass spectrometric analysis to unravel the venom proteome composition of Indian snakes: Opening new avenues in clinical research. Expert Rev. Proteom. 2020, 17, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef]
- Zaluzec, E.J.; Gage, D.A.; Watson, J.T. Matrix-assisted laser desorption ionization mass spectrometry: Applications in peptide and protein characterization. Protein Expr. Purif. 1995, 6, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Rey-Suarez, P.; Nunez, V.; Fernandez, J.; Lomonte, B. Integrative characterization of the venom of the coral snake Micrurus dumerilii (Elapidae) from Colombia: Proteome, toxicity, and cross-neutralization by antivenom. J. Proteom. 2016, 136, 262–273. [Google Scholar] [CrossRef]
- Degueldre, M.; Echterbille, J.; Smargiasso, N.; Damblon, C.; Gouin, C.; Mourier, G.; Gilles, N.; De Pauw, E.; Quinton, L. In-Depth Glyco-Peptidomics Approach Reveals Unexpected Diversity of Glycosylated Peptides and Atypical Post-Translational Modifications in Dendroaspis angusticeps Snake Venom. Int. J. Mol. Sci. 2017, 18, 2453. [Google Scholar] [CrossRef]
- Petras, D.; Heiss, P.; Sussmuth, R.D.; Calvete, J.J. Venom Proteomics of Indonesian King Cobra, Ophiophagus hannah: Integrating Top-Down and Bottom-Up Approaches. J. Proteome Res. 2015, 14, 2539–2556. [Google Scholar] [CrossRef]
- Rodrigues, C.R.; Teixeira-Ferreira, A.; Vargas, F.F.R.; Guerra-Duarte, C.; Costal-Oliveira, F.; Stransky, S.; Lopes-de-Souza, L.; Dutra, A.A.A.; Yarleque, A.; Bonilla, C.; et al. Proteomic profile, biological activities and antigenic analysis of the venom from Bothriopsis bilineata smaragdina (“loro machaco”), a pitviper snake from Peru. J. Proteom. 2018, 187, 171–181. [Google Scholar] [CrossRef]
- Wiezel, G.A.; Shibao, P.Y.T.; Cologna, C.T.; Morandi Filho, R.; Ueira-Vieira, C.; De Pauw, E.; Quinton, L.; Arantes, E.C. In-Depth Venome of the Brazilian Rattlesnake Crotalus durissus terrificus: An Integrative Approach Combining Its Venom Gland Transcriptome and Venom Proteome. J. Proteome Res. 2018, 17, 3941–3958. [Google Scholar] [CrossRef]
- Fox, J.W.; Ma, L.; Nelson, K.; Sherman, N.E.; Serrano, S.M. Comparison of indirect and direct approaches using ion-trap and Fourier transform ion cyclotron resonance mass spectrometry for exploring viperid venom proteomes. Toxicon 2006, 47, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Vanuopadath, M.; Sajeev, N.; Murali, A.R.; Sudish, N.; Kangosseri, N.; Sebastian, I.R.; Jain, N.D.; Pal, A.; Raveendran, D.; Nair, B.G.; et al. Mass spectrometry-assisted venom profiling of Hypnale hypnale found in the Western Ghats of India incorporating de novo sequencing approaches. Int. J. Biol. Macromol. 2018, 118, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; McCleary, R.J.R.; Kesherwani, M.; Kini, R.M.; Velmurugan, D. Comparison of proteomic profiles of the venoms of two of the ‘Big Four’ snakes of India, the Indian cobra (Naja naja) and the common krait (Bungarus caeruleus), and analyses of their toxins. Toxicon 2017, 135, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kerkkamp, H.M.; Kini, R.M.; Pospelov, A.S.; Vonk, F.J.; Henkel, C.V.; Richardson, M.K. Snake Genome Sequencing: Results and Future Prospects. Toxins 2016, 8, 360. [Google Scholar] [CrossRef]
- Castoe, T.A.; de Koning, A.P.; Hall, K.T.; Card, D.C.; Schield, D.R.; Fujita, M.K.; Ruggiero, R.P.; Degner, J.F.; Daza, J.M.; Gu, W.; et al. The Burmese python genome reveals the molecular basis for extreme adaptation in snakes. Proc. Natl. Acad. Sci. USA 2013, 110, 20645–20650. [Google Scholar] [CrossRef]
- Vonk, F.J.; Casewell, N.R.; Henkel, C.V.; Heimberg, A.M.; Jansen, H.J.; McCleary, R.J.; Kerkkamp, H.M.; Vos, R.A.; Guerreiro, I.; Calvete, J.J.; et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. USA 2013, 110, 20651–20656. [Google Scholar] [CrossRef]
- Dowell, N.L.; Giorgianni, M.W.; Kassner, V.A.; Selegue, J.E.; Sanchez, E.E.; Carroll, S.B. The Deep Origin and Recent Loss of Venom Toxin Genes in Rattlesnakes. Curr. Biol. 2016, 26, 2434–2445. [Google Scholar] [CrossRef]
- Suryamohan, K.; Krishnankutty, S.P.; Guillory, J.; Jevit, M.; Schroder, M.S.; Wu, M.; Kuriakose, B.; Mathew, O.K.; Perumal, R.C.; Koludarov, I.; et al. The Indian cobra reference genome and transcriptome enables comprehensive identification of venom toxins. Nat. Genet. 2020, 52, 106–117. [Google Scholar]
- Tattini, L.; D’Aurizio, R.; Magi, A. Detection of Genomic Structural Variants from Next-Generation Sequencing Data. Front. Bioeng. Biotechnol. 2015, 3, 92. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Z.J.; Li, Q.Y.; Lian, J.M.; Zhou, Y.; Lu, B.Z.; Jin, L.J.; Qiu, P.X.; Zhang, P.; Zhu, W.B.; et al. Evolutionary trajectories of snake genes and genomes revealed by comparative analyses of five-pacer viper. Nat. Commun. 2016, 7, 13107. [Google Scholar] [CrossRef]
- Shibata, H.; Chijiwa, T.; Oda-Ueda, N.; Nakamura, H.; Yamaguchi, K.; Hattori, S.; Matsubara, K.; Matsuda, Y.; Yamashita, A.; Isomoto, A.; et al. The habu genome reveals accelerated evolution of venom protein genes. Sci. Rep. 2018, 8, 11300. [Google Scholar] [PubMed]
- Pla, D.; Gutierrez, J.M.; Calvete, J.J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688–699. [Google Scholar] [PubMed]
- Silva, A.; Hodgson, W.C.; Isbister, G.K. Antivenom for Neuromuscular Paralysis Resulting From Snake Envenoming. Toxins 2017, 9, 143. [Google Scholar]
- Pla, D.; Rodriguez, Y.; Calvete, J.J. Third Generation Antivenomics: Pushing the Limits of the In Vitro Preclinical Assessment of Antivenoms. Toxins 2017, 9, 158. [Google Scholar] [CrossRef]
- Ascoet, S.; De Waard, M. Diagnostic and Therapeutic Value of Aptamers in Envenomation Cases. Int. J. Mol. Sci. 2020, 21, 3565. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.H.; Shamaileh, H.A.; Edwards, S.L.; Taran, E.; Veedu, R.N. Rapid one-step selection method for generating nucleic acid aptamers: Development of a DNA aptamer against alpha-bungarotoxin. PLoS ONE 2012, 7, e41702. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; Rusconi, C.P.; Sullenger, B.A. Aptamers: An emerging class of therapeutics. Annu. Rev. Med. 2005, 56, 555–583. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Alomran, N.; Chinnappan, R.; Alsolaiss, J.; Casewell, N.R.; Zourob, M. Exploring the Utility of ssDNA Aptamers Directed against Snake Venom Toxins as New Therapeutics for Snakebite Envenoming. Toxins 2022, 14, 469. [Google Scholar] [CrossRef]
- El-Aziz, T.M.A.; Ravelet, C.; Molgo, J.; Fiore, E.; Pale, S.; Amar, M.; Al-Khoury, S.; Dejeu, J.; Fadl, M.; Ronjat, M.; et al. Efficient functional neutralization of lethal peptide toxins in vivo by oligonucleotides. Sci. Rep. 2017, 7, 7202. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tsai, C.Y.; Hu, W.P.; Chang, L.S. DNA Aptamers against Taiwan Banded Krait alpha-Bungarotoxin Recognize Taiwan Cobra Cardiotoxins. Toxins 2016, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Anand, A.; Malhotra, A.; Khan, E.; Santra, V.; Kumar, A.; Sharma, T.K. Rational truncation of aptamer for cross-species application to detect krait envenomation. Sci. Rep. 2018, 8, 17795. [Google Scholar]
- Taiwe, G.S.; Montnach, J.; Nicolas, S.; De Waard, S.; Fiore, E.; Peyrin, E.; El-Aziz, T.M.A.; Amar, M.; Molgo, J.; Ronjat, M.; et al. Aptamer Efficacies for In Vitro and In Vivo Modulation of alphaC-Conotoxin PrXA Pharmacology. Molecules 2019, 24, 229. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Sola, M.; Jappe, E.C.; Oscoz, S.; Lauridsen, L.P.; Engmark, M. Biotechnological Trends in Spider and Scorpion Antivenom Development. Toxins 2016, 8, 226. [Google Scholar]
- Ye, F.; Zheng, Y.; Wang, X.; Tan, X.; Zhang, T.; Xin, W.; Wang, J.; Huang, Y.; Fan, Q. Recognition of Bungarus multicinctus venom by a DNA aptamer against beta-bungarotoxin. PLoS ONE 2014, 9, e105404. [Google Scholar]
- Anand, A.; Chatterjee, B.; Dhiman, A.; Goel, R.; Khan, E.; Malhotra, A.; Santra, V.; Salvi, N.; Khadilkar, M.V.; Bhatnagar, I.; et al. Complex target SELEX-based identification of DNA aptamers against Bungarus caeruleus venom for the detection of envenomation using a paper-based device. Biosens. Bioelectron. 2021, 193, 113523. [Google Scholar] [CrossRef]
- Devi, A.; Doley, R. Neutralization of Daboxin P activities by rationally designed aptamers. Toxicon 2021, 203, 93–103. [Google Scholar]
- Muyldermans, S. Single domain camel antibodies: Current status. J. Biotechnol. 2001, 74, 277–302. [Google Scholar]
- Richard, G.; Meyers, A.J.; McLean, M.D.; Arbabi-Ghahroudi, M.; MacKenzie, R.; Hall, J.C. In vivo neutralization of alpha-cobratoxin with high-affinity llama single-domain antibodies (VHHs) and a VHH-Fc antibody. PLoS ONE 2013, 8, e69495. [Google Scholar] [CrossRef]
- Harrison, R.A.; Hasson, S.S.; Harmsen, M.; Laing, G.D.; Conrath, K.; Theakston, R.D. Neutralisation of venom-induced haemorrhage by IgG from camels and llamas immunised with viper venom and also by endogenous, non-IgG components in camelid sera. Toxicon 2006, 47, 364–368. [Google Scholar] [CrossRef]
- Leon, G.; Lomonte, B.; Gutierrez, J.M. Anticomplementary activity of equine whole IgG antivenoms: Comparison of three fractionation protocols. Toxicon 2005, 45, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Gutierrez, J.M.; Rojas, G.; Nunez, V.; Diaz, A.; Miranda, E.; Uribe, A.F.; Silva, J.F.; Ospina, J.G.; Medina, Y.; et al. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: Correlation between safety and biochemical characteristics of antivenoms. Toxicon 1999, 37, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, D.G.; Theakston, R.D. Snake antivenoms. J. Toxicol. Clin. Toxicol. 2003, 41, 277–290; 317–327. [Google Scholar]
- Leon, G.; Monge, M.; Rojas, E.; Lomonte, B.; Gutierrez, J.M. Comparison between IgG and F(ab′)(2) polyvalent antivenoms: Neutralization of systemic effects induced by Bothrops asper venom in mice, extravasation to muscle tissue, and potential for induction of adverse reactions. Toxicon 2001, 39, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.A.; Owen, T.; Wagstaff, S.C.; Kinne, J.; Wernery, U.; Harrison, R.A. Analysis of camelid antibodies for antivenom development: Neutralisation of venom-induced pathology. Toxicon 2010, 56, 373–380. [Google Scholar] [CrossRef]
- Darvish, M.; Ebrahimi, S.A.; Shahbazzadeh, D.; Bagheri, K.P.; Behdani, M.; Shokrgozar, M.A. Camelid antivenom development and potential in vivo neutralization of Hottentotta saulcyi scorpion venom. Toxicon 2016, 113, 70–75. [Google Scholar] [PubMed]
- Meddeb-Mouelhi, F.; Bouhaouala-Zahar, B.; Benlasfar, Z.; Hammadi, M.; Mejri, T.; Moslah, M.; Karoui, H.; Khorchani, T.; El Ayeb, M. Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon 2003, 42, 785–791. [Google Scholar] [CrossRef]
- Darvish, M.; Behdani, M.; Shokrgozar, M.A.; Pooshang-Bagheri, K.; Shahbazzadeh, D. Development of protective agent against Hottentotta saulcyi venom using camelid single-domain antibody. Mol. Immunol. 2015, 68, 412–420. [Google Scholar] [CrossRef]
- Rodriguez-Valle, M.; McAlister, S.; Moolhuijzen, P.M.; Booth, M.; Agnew, K.; Ellenberger, C.; Knowles, A.G.; Vanhoff, K.; Bellgard, M.I.; Tabor, A.E. Immunomic Investigation of Holocyclotoxins to Produce the First Protective Anti-Venom Vaccine Against the Australian Paralysis Tick, Ixodes holocyclus. Front. Immunol. 2021, 12, 744795. [Google Scholar] [CrossRef]
- Cook, D.A.; Owen, T.; Wagstaff, S.C.; Kinne, J.; Wernery, U.; Harrison, R.A. Analysis of camelid IgG for antivenom development: Serological responses of venom-immunised camels to prepare either monospecific or polyspecific antivenoms for West Africa. Toxicon 2010, 56, 363–372. [Google Scholar] [CrossRef]
- Tanwar, P.D.; Ghorui, S.K.; Kochar, S.K.; Singh, R.; Patil, N.V. Production and preclinical assessment of camelid immunoglobulins against Echis sochureki venom from desert of Rajasthan, India. Toxicon 2017, 134, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khamehchian, S.; Zolfagharian, H.; Dounighi, N.M.; Tebianian, M.; Madani, R. Study on camel IgG purification: A new approach to prepare Naja Naja oxiana antivenom as passive immunization for therapy. Hum. Vaccin. Immunother. 2014, 10, 1633–1638. [Google Scholar] [CrossRef]
- Bazan, J.; Calkosinski, I.; Gamian, A. Phage display—A powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum. Vaccin. Immunother. 2012, 8, 1817–1828. [Google Scholar] [PubMed]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Roncolato, E.C.; Campos, L.B.; Pessenda, G.; Costa e Silva, L.; Furtado, G.P.; Barbosa, J.E. Phage display as a novel promising antivenom therapy: A review. Toxicon 2015, 93, 79–84. [Google Scholar] [PubMed]
- Lynagh, T.; Kiontke, S.; Meyhoff-Madsen, M.; Gless, B.H.; Johannesen, J.; Kattelmann, S.; Christiansen, A.; Dufva, M.; Laustsen, A.H.; Devkota, K.; et al. Peptide Inhibitors of the alpha-Cobratoxin-Nicotinic Acetylcholine Receptor Interaction. J. Med. Chem. 2020, 63, 13709–13718. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Leu, S.J.; Lee, Y.C.; Liu, C.I.; Lin, L.T.; Mwale, P.F.; Chiang, J.R.; Tsai, B.Y.; Chen, C.C.; Hung, C.S.; et al. Characterization of Chicken-Derived Single Chain Antibody Fragments against Venom of Naja Naja Atra. Toxins 2018, 10, 383. [Google Scholar] [CrossRef]
- Lee, C.H.; Liu, C.I.; Leu, S.J.; Lee, Y.C.; Chiang, J.R.; Chiang, L.C.; Mao, Y.C.; Tsai, B.Y.; Hung, C.S.; Chen, C.C.; et al. Chicken antibodies against venom proteins of Trimeresurus stejnegeri in Taiwan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200056. [Google Scholar] [CrossRef]
- Ahmadi, S.; Pucca, M.B.; Jurgensen, J.A.; Janke, R.; Ledsgaard, L.; Schoof, E.M.; Sorensen, C.V.; Caliskan, F.; Laustsen, A.H. An in vitro methodology for discovering broadly-neutralizing monoclonal antibodies. Sci. Rep. 2020, 10, 10765. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Wade, J.; Jenkins, T.P.; Boddum, K.; Oganesyan, I.; Harrison, J.A.; Villar, P.; Leah, R.A.; Zenobi, R.; Schoffelen, S.; et al. Discovery and optimization of a broadly-neutralizing human monoclonal antibody against long-chain alpha-neurotoxins from snakes. Nat. Commun. 2023, 14, 682. [Google Scholar]
- Silva, L.C.; Pucca, M.B.; Pessenda, G.; Campos, L.B.; Martinez, E.Z.; Cerni, F.A.; Barbosa, J.E. Discovery of human scFvs that cross-neutralize the toxic effects of B. jararacussu and C. d. terrificus venoms. Acta Trop. 2018, 177, 66–73. [Google Scholar]
- Liu, C.C.; Wu, C.J.; Chou, T.Y.; Liaw, G.W.; Hsiao, Y.C.; Chu, L.J.; Lee, C.H.; Wang, P.J.; Hsieh, C.H.; Chen, C.K.; et al. Development of a Monoclonal scFv against Cytotoxin to Neutralize Cytolytic Activity Induced by Naja atra Venom on Myoblast C2C12 Cells. Toxins 2022, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [PubMed]
- Alangode, A.; Reick, M. Sodium oleate, arachidonate, and linoleate enhance fibrinogenolysis by Russell’s viper venom proteinases and inhibit FXIIIa; a role for phospholipase A2 in venom induced consumption coagulopathy. Toxicon 2020, 186, 83–93. [Google Scholar]
- Rosenson, R.S.; Hislop, C.; Elliott, M.; Stasiv, Y.; Goulder, M.; Waters, D. Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J. Am. Coll. Cardiol. 2010, 56, 1079–1088. [Google Scholar] [CrossRef]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Menzies, S.K.; Clare, R.H.; Xie, C.; Westhorpe, A.; Hall, S.R.; Edge, R.J.; Alsolaiss, J.; Crittenden, E.; Marriott, A.E.; Harrison, R.A.; et al. In vitro and in vivo preclinical venom inhibition assays identify metalloproteinase inhibiting drugs as potential future treatments for snakebite envenoming by Dispholidus typus. Toxicon X 2022, 14, 100118. [Google Scholar]
- Isbister, G.K. Procoagulant snake toxins: Laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin. Thromb. Hemost. 2009, 35, 93–103. [Google Scholar]
- Xie, C.; Albulescu, L.O.; Still, K.B.M.; Slagboom, J.; Zhao, Y.; Jiang, Z.; Somsen, G.W.; Vonk, F.J.; Casewell, N.R.; Kool, J. Varespladib Inhibits the Phospholipase A2 and Coagulopathic Activities of Venom Components from Hemotoxic Snakes. Biomedicines 2020, 8, 165. [Google Scholar] [CrossRef]
- Escalante, T.; Franceschi, A.; Rucavado, A.; Gutierrez, J.M. Effectiveness of batimastat, a synthetic inhibitor of matrix metalloproteinases, in neutralizing local tissue damage induced by BaP1, a hemorrhagic metalloproteinase from the venom of the snake bothrops asper. Biochem. Pharmacol. 2000, 60, 269–274. [Google Scholar]
- Gutierrez, J.M.; Leon, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.; Liang, Q.; Isbister, G.K.; Hodgson, W.C. In Vitro Efficacy of Antivenom and Varespladib in Neutralising Chinese Russell’s Viper (Daboia siamensis) Venom Toxicity. Toxins 2023, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Layfield, H.J.; Williams, H.F.; Ravishankar, D.; Mehmi, A.; Sonavane, M.; Salim, A.; Vaiyapuri, R.; Lakshminarayanan, K.; Vallance, T.M.; Bicknell, A.B.; et al. Repurposing Cancer Drugs Batimastat and Marimastat to Inhibit the Activity of a Group I Metalloprotease from the Venom of the Western Diamondback Rattlesnake, Crotalus atrox. Toxins 2020, 12, 309. [Google Scholar] [CrossRef]
- Chowdhury, A.; Lewin, M.R.; Zdenek, C.N.; Carter, R.; Fry, B.G. The Relative Efficacy of Chemically Diverse Small-Molecule Enzyme-Inhibitors Against Anticoagulant Activities of African Spitting Cobra (Naja Species) Venoms. Front. Immunol. 2021, 12, 752442. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Lewin, M.R.; Williams, D.J.; Lomonte, B. Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins 2020, 12, 131. [Google Scholar] [CrossRef]
- Tan, C.H.; Lingam, T.M.C.; Tan, K.Y. Varespladib (LY315920) rescued mice from fatal neurotoxicity caused by venoms of five major Asiatic kraits (Bungarus spp.) in an experimental envenoming and rescue model. Acta Trop. 2022, 227, 106289. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.W.; Gerardo, C.J.; Samuel, S.P.; Kumar, S.; Kotehal, S.D.; Mukherjee, P.P.; Shirazi, F.M.; Akpunonu, P.D.; Bammigatti, C.; Bhalla, A.; et al. The BRAVO Clinical Study Protocol: Oral Varespladib for Inhibition of Secretory Phospholipase A2 in the Treatment of Snakebite Envenoming. Toxins 2022, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Shalinsky, D.R.; Brekken, J.; Zou, H.; Kolis, S.; Wood, A.; Webber, S.; Appelt, K. Antitumor efficacy of AG3340 associated with maintenance of minimum effective plasma concentrations and not total daily dose, exposure or peak plasma concentrations. Investig. New. Drugs 1998, 16, 303–313. [Google Scholar] [CrossRef]
- Shalinsky, D.R.; Brekken, J.; Zou, H.; Bloom, L.A.; McDermott, C.D.; Zook, S.; Varki, N.M.; Appelt, K. Marked antiangiogenic and antitumor efficacy of AG3340 in chemoresistant human non-small cell lung cancer tumors: Single agent and combination chemotherapy studies. Clin. Cancer Res. 1999, 5, 1905–1917. [Google Scholar]
- Gomez-Betancur, I.; Gogineni, V.; Salazar-Ospina, A.; Leon, F. Perspective on the Therapeutics of Anti-Snake Venom. Molecules 2019, 24, 3276. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, B.L.; Shivalingaiah, S. The anti-inflammatory activity of standard aqueous stem bark extract of Mangifera indica L. as evident in inhibition of Group IA sPLA2. An. Acad. Bras. Cienc. 2016, 88, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Alangode, A.; Rajan, K.; Nair, B.G. Snake antivenom: Challenges and alternate approaches. Biochem. Pharmacol. 2020, 181, 114135. [Google Scholar] [CrossRef]
- Alam, M.I.; Gomes, A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J. Ethnopharmacol. 2003, 86, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lohse, B.; Lomonte, B.; Engmark, M.; Gutierrez, J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon 2015, 104, 43–45. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wagstaff, S.C.; Wuster, W.; Cook, D.A.; Bolton, F.M.; King, S.I.; Pla, D.; Sanz, L.; Calvete, J.J.; Harrison, R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bera, I.; Chakraborty, S.; Ghoshal, N.; Bhattacharyya, D. Aristolochic acid and its derivatives as inhibitors of snake venom L-amino acid oxidase. Toxicon 2017, 138, 1–17. [Google Scholar] [CrossRef]
- Vishwanath, B.S.; Gowda, T.V. Interaction of aristolochic acid with Vipera russelli phospholipase A2: Its effect on enzymatic and pathological activities. Toxicon 1987, 25, 929–937. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Doley, R.; Saikia, D. Isolation of a snake venom phospholipase A2 (PLA2) inhibitor (AIPLAI) from leaves of Azadirachta indica (Neem): Mechanism of PLA2 inhibition by AIPLAI in vitro condition. Toxicon 2008, 51, 1548–1553. [Google Scholar] [CrossRef]
- Venkatachalapathi, A.; Sangeeth, T.; Ali, M.A.; Tamilselvi, S.S.; Paulsamy, S.; Al-Hemaidc, F.M.A. Ethnomedicinal assessment of Irula tribes of Walayar valley of Southern Western Ghats, India. Saudi J. Biol. Sci. 2018, 25, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Mohanakumari, H.P.; Nagaraju, S.; Vishwanath, B.S.; Kemparaju, K. Hyaluronidase and protease activities from Indian snake venoms: Neutralization by Mimosa pudica root extract. Fitoterapia 2004, 75, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, K.T.; Nagaraju, S.; Nandini, S.U.; Kemparaju, K. Neutralization of local and systemic toxicity of Daboia russelii venom by Morus alba plant leaf extract. Phytother. Res. 2009, 23, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Gopi, K.; Anbarasu, K.; Renu, K.; Jayanthi, S.; Vishwanath, B.S.; Jayaraman, G. Quercetin-3-O-rhamnoside from Euphorbia hirta protects against snake Venom induced toxicity. Biochim. Biophys. Acta 2016, 1860, 1528–1540. [Google Scholar] [CrossRef]
- Alam, M.I.; Auddy, B.; Gomes, A. Isolation, purification and partial characterization of viper venom inhibiting factor from the root extract of the Indian medicinal plant sarsaparilla (Hemidesmus indicus R. Br.). Toxicon 1994, 32, 1551–1557. [Google Scholar] [CrossRef]
- Alam, M.I.; Gomes, A. Viper venom-induced inflammation and inhibition of free radical formation by pure compound (2-hydroxy-4-methoxy benzoic acid) isolated and purified from anantamul (Hemidesmus indicus R. BR) root extract. Toxicon 1998, 36, 207–215. [Google Scholar] [CrossRef]
- Alam, M.I.; Gomes, A. An experimental study on evaluation of chemical antagonists induced snake venom neutralization. Indian J. Med. Res. 1998, 107, 142–146. [Google Scholar]
- Machiah, D.K.; Gowda, T.V. Purification of a post-synaptic neurotoxic phospholipase A2 from Naja naja venom and its inhibition by a glycoprotein from Withania somnifera. Biochimie 2006, 88, 701–710. [Google Scholar] [CrossRef]
- Deepa, M.; Veerabasappa Gowda, T. Purification and characterization of a glycoprotein inhibitor of toxic phospholipase from Withania somnifera. Arch. Biochem. Biophys. 2002, 408, 42–50. [Google Scholar] [CrossRef]
- Lizano, S.; Domont, G.; Perales, J. Natural phospholipase A2 myotoxin inhibitor proteins from snakes, mammals and plants. Toxicon 2003, 42, 963–977. [Google Scholar] [CrossRef]
- Chandra, V.; Jasti, J.; Kaur, P.; Srinivasan, A.; Betzel, C.; Singh, T.P. Structural basis of phospholipase A2 inhibition for the synthesis of prostaglandins by the plant alkaloid aristolochic acid from a 1.7 A crystal structure. Biochemistry 2002, 41, 10914–10919. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R., II; Francisco, A.F.; Moreira-Dill, L.S.; Quintero, A.; Guimaraes, C.L.S.; Fernandes, C.A.H.; Takeda, A.A.S.; Zanchi, F.B.; Caldeira, C.A.S.; Pereira, P.S.; et al. Isolation and structural characterization of bioactive compound from Aristolochia sprucei aqueous extract with anti-myotoxic activity. Toxicon X 2020, 7, 100049. [Google Scholar] [CrossRef] [PubMed]


| SI NO. | Taxonomic Family | Species Name | Common Name | Geographical Distribution in India | Antivenom Available and Licensed in India | Nature of Available Antivenom (s) | Antivenom Manufacturing Countries |
|---|---|---|---|---|---|---|---|
| Category-1 | |||||||
| 1 | Elapidae | Bungarus caeruleus | Indian krait | Throughout | Yes | Polyvalent antivenom | India, Pakistan |
| 2 | Naja naja | Indian cobra | Throughout | Yes | Polyvalent antivenom | India, Pakistan | |
| 3 | Naja kaouthia | Monocellate cobra | Northeast | No | Monovalent and Polyvalent | Myanmar, Thailand, Vietnam | |
| 4 | Viperidae | Daboia russelii | Russell’s viper | Throughout | Yes | Polyvalent antivenom | India, Pakistan |
| 5 | Echis carinatus | Saw-scaled viper | Throughout | Yes | Polyvalent antivenom | India, Pakistan, Iran, Uzbekistan, Spain | |
| 6 | Hypnale hypnale | Hump-nosed pit viper | Southwest | No | NA | - | |
| Category-2 | |||||||
| 7 | Elapidae | Bungarus bungaroides | Northeastern hill krait | Northeast | No | NA | - |
| 8 | Bungarus fasciatus | Banded krait | Northeast | No | Polyvalent and monovalent antivenoms | Thailand, Indonesia | |
| 9 | Bungarus lividus | Lesser black krait | Northeast | No | NA | - | |
| 10 | Bungarus niger | Greater black krait | Northeast | No | NA | - | |
| 11 | Bungarus sindanus | Sind krait | Northwest | No | Polyvalent antivenom | Pakistan | |
| 12 | Bungarus walli | Wall’s krait | Northeast and Southwest | No | NA | - | |
| 13 | Naja oxiana | Central Asian cobra | North and Northwest | No | Polyvalent | Iran, Pakistan, Uzbekistan, Egypt | |
| 14 | Naja sagittifera | Andaman cobra | Andaman Islands | No | NA | - | |
| 15 | Ophiophagus hannah | King cobra | South, Northeast, Andaman Islands | No | Polyvalent and monovalent | Thailand | |
| 16 | Viperidae | Gloydius himalayanus | Himalayan pit viper | North | No | NA | - |
| 17 | Protobothrops jerdonii | Jerdon’s pit viper | Northeast | No | NA | - | |
| 18 | Protobothrops kaulbacki | Kaulback’s lance-headed pit viper | Northeast | No | NA | - | |
| 19 | Protobothrops mucrosquamatus | Brown-spotted pit viper | Northeast | No | Monovalent antivenom | China | |
| 20 | Trimeresurus gramineus | Common bamboo pit viper | South and East | No | NA | - | |
| 21 | Craspedocephalus malabaricus | Malabarian pit viper | Southwest | No | NA | - | |
| 22 | Macrovipera lebetina | Levantine viper | Northeast | No | |||
| 23 | Protobothrops himalayanus | NA | North | No | NA | - | |
| 24 | Trimeresurus andersonii | Andaman Pitviper | Andaman Islands | No | NA | - | |
| 25 | Trimeresurus erythrurus | Bamboo pitviper | East | No | Monovalent | Thailand | |
| 26 | Trimeresurus gumprechti | Gumprecht’s green pit viper | Northeast | No | NA | - | |
| 27 | Craspedocephalus macrolepis | large-scaled pit viper | South | No | NA | - | |
| 28 | Trimeresurus salazar | Salazar’s pit viper | Northeast | No | NA | - | |
| 29 | Trimeresurus septentrionalis | Nepal pitviper | North | No | NA | - | |
| 30 | Craspedocephalus strigatus | horseshoe pit viper | South | No | NA | - | |
| 31 | Trimeresurus yunnanensis | Yunnan bamboo pitviper | North | No | NA | - | |
| SI No. | Name | Antivenom Manufacturer | Listed in WHO Database | Nature of Antivenom | Stated Efficacy |
|---|---|---|---|---|---|
| 1 | Polyvalent Snake Antivenin | Biological E Limited (Telangana, Hyderabad) | Yes | Polyvalent (raised against big four Indian snakes) | After reconstitution, each mL of Polyvalent snake venom antiserum neutralizes not less than: Indian Cobra venom—0.60 mg Common Krait venom—0.45 mg Russell’s viper venom—0.60 mg Saw scaled viper venom—0.45 mg (The stated efficacies of each of these antivenoms are indicated based on the LD50 and ED50 values obtained after performing In vivo studies) |
| 2 | Snake Venom Antiserum I.P. | VINS Bioproducts Ltd. (Telangana, Hyderabad) | Yes | ||
| 3 | Polyvalent Snake Antivenom | Bharat Serums & Vaccines (Mumbai, Maharashtra) | Yes | ||
| 4 | Snake antivenin I.P. | Haffkine Biopharmaceutical Corporation Ltd. (Mumbai, Maharashtra) | Yes | ||
| 5 | Virchow (V-ASV) | Virchow biotech private limited (Telangana, Hyderabad) | No | ||
| 6 | Snake Venom Antiserum I.P. | Premium Serums & Vaccines Pvt. Ltd. (Pune, Maharashtra) | No | ||
| 7 | Snake Venom Antiserum I.P. | Mediclone Biotech (Chennai, Tamil Nadu) | No | ||
| 8 | Polyvalent Anti Snake Venom Serum I.P. | King Institute of Preventative Medicine and Research (Chennai, Tamil Nadu) | Yes |
| SI No. | Species | Protein | Protein Family | Nature of Aptamer | Methodology Used for Selecting Aptamer | Efficacy/Efficiency | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Daboia russelii | Daboxin P | Phospholipase A2 | Nucleic acid aptamer | Entropy fragment-based approach and seed and grow method | Showed PLA2 inhibitory and anticoagulant activities. | [101] |
| 2 | Bungarus multicinctus | β-bungarotoxin (β-BuTx) | Neurotoxin (three-finger toxin) | DNA aptamer | plate-SELEX | The designed aptamer βB-1 was specific to β-BuTx and could differentiate B. multicinctus venom among the other snake venoms tested. | [99] |
| 3 | Bungarus caeruleus | α-Toxin | Neurotoxin (three-finger toxin) | Truncated aptamer | Truncated aptamer | A truncated DNA aptamer, α-Tox-T2, generated to fight against the α-Toxin of Bungarus multicinctus was also able to detect Bungarus caeruleus venom. | [96] |
| 4 | Bungarus multicinctus | α-bungarotoxin | Neurotoxin (three-finger toxin) | DNA aptamer | Single-step selection on a glass coverslip using designed aptamers | Simple one-step selection could be applied for the rapid production of DNA and RNA aptamers. | [90] |
| 5 | N. atra | Cardiotoxins | Neurotoxin (three-finger toxin) | DNA aptamer | Neogene Biomedicals Corporation synthesized the labeled single-stranded DNA samples | The aptamers designed to fight against Bungarus multicinctus α-bungarotoxin inhibited cytotoxicity and membrane damage induced by Naja atra cardiotoxins. | [95] |
| 6 | Bungarus caeruleus | β-Bungarotoxin | Neurotoxin (three-finger toxin) | DNA aptamer | SELEX | The designed aptamer could discriminate B. caeruleus venom from Russell’s, Cobra, and Saw-scaled viper’s venom and was specific to β-Bungarotoxin. | [100] |
| 7 | C. rhodostoma and B. atrox | Ancrod and batroxobin | Snake venom serine protease | ssDNA aptamers | SELEX | The toxin-specific aptamers were found to exhibit in vitro cross-reactivity against the different isoforms present in various snake species. | [93] |
| SI No. | Snake Species | Neutralizing Toxin/Protein (Antigen) | Phage Bound Molecule | Study Design | Reference |
|---|---|---|---|---|---|
| 1 | Naja kaouthia | α−cobratoxin | 8-mer peptide | In vitro | [120] |
| 2 | Naja naja atra | Naja naja atra proteins (NNA proteins) | Single-chain variable fragment (scFv) | In vitro and in vivo | [121] |
| 3 | Trimeresurus stejnegeri | Whole venom | scFv | In vitro and in vivo | [122] |
| 4 | Naja. nigricollis, Naja. mossambica, and Naja. melanoleuca | Whole venom | scFv | In vitro | [123] |
| 5 | Naja kaouthia | Whole venom | Monoclonal antibody | In vitro and in vivo | [124] |
| 6 | Bothrops jararacussu and Crotalus durissus terrificus | Whole venom | scFv | In vitro and in vivo | [125] |
| SI No. | Species | Plant Extract/Compound | Protein Family | Nature of Plant Product | Neutralization Studies | Reference |
|---|---|---|---|---|---|---|
| 1 | Russell’s viper | Aristolochic acid | Phospholipase A2 L-amino acid Oxidase | Nitrophenanthrene carboxylic acids | In vitro cell based assays and in vivo mouse model | [101] |
| 2 | Daboia russelli, Naja naja, Naja kaouthia | Leaf extract of Azadirachta indica | Phospholipase A2 | Non- terpenoids | In vitro assays and in vivo mouse model | [99] |
| 3 | Daboia russelli, Naja naja, Echis carinatus | Root extract of Mimosa pudica | Hyaluronidase and Protease | Not determined | In vitro assays | [96] |
| 4 | Daboia russelli | Leaf extract of Morus alba | Snake venom metalloproteinase and hyaluronidase | Not determined | In vitro assays and in vivo mouse model | [90] |
| 5 | Naja naja | Quercetin-3-O-α-rhamnoside | Phospholipase A2, hyaluronidase and hemolytic activity | Flavonoid glycoside | In vitro assays and in vivo mouse model | [95] |
| 6 | Naja kaouthia, Ophiophagus hannah, Daboia russelii, and Echis carinatus | 2-hydroxy-4-methoxy benzoic acid | Phospholipase A2, snake venom metalloproteinase | Salicylates derivative | In vitro assays and in vivo mouse model | [100] |
| 7 | Naja naja | Withania sominifera Glycoprotein | Phospholipase A2 | Glycoprotein | In vitro assays and in vivo mouse model | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanuopadath, M.; Rajan, K.; Alangode, A.; Nair, S.S.; Nair, B.G. The Need for Next-Generation Antivenom for Snakebite Envenomation in India. Toxins 2023, 15, 510. https://doi.org/10.3390/toxins15080510
Vanuopadath M, Rajan K, Alangode A, Nair SS, Nair BG. The Need for Next-Generation Antivenom for Snakebite Envenomation in India. Toxins. 2023; 15(8):510. https://doi.org/10.3390/toxins15080510
Chicago/Turabian StyleVanuopadath, Muralidharan, Karthika Rajan, Aswathy Alangode, Sudarslal Sadasivan Nair, and Bipin Gopalakrishnan Nair. 2023. "The Need for Next-Generation Antivenom for Snakebite Envenomation in India" Toxins 15, no. 8: 510. https://doi.org/10.3390/toxins15080510





