Analytical Size Exclusion Chromatography Coupled with Mass Spectrometry in Parallel with High-Throughput Venomics and Bioassaying for Venom Profiling
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEC Optimisation
2.2. Analytical SEC Implemented to Venom Research
2.2.1. Post-Column HT-Venomics after SEC-Fractionation
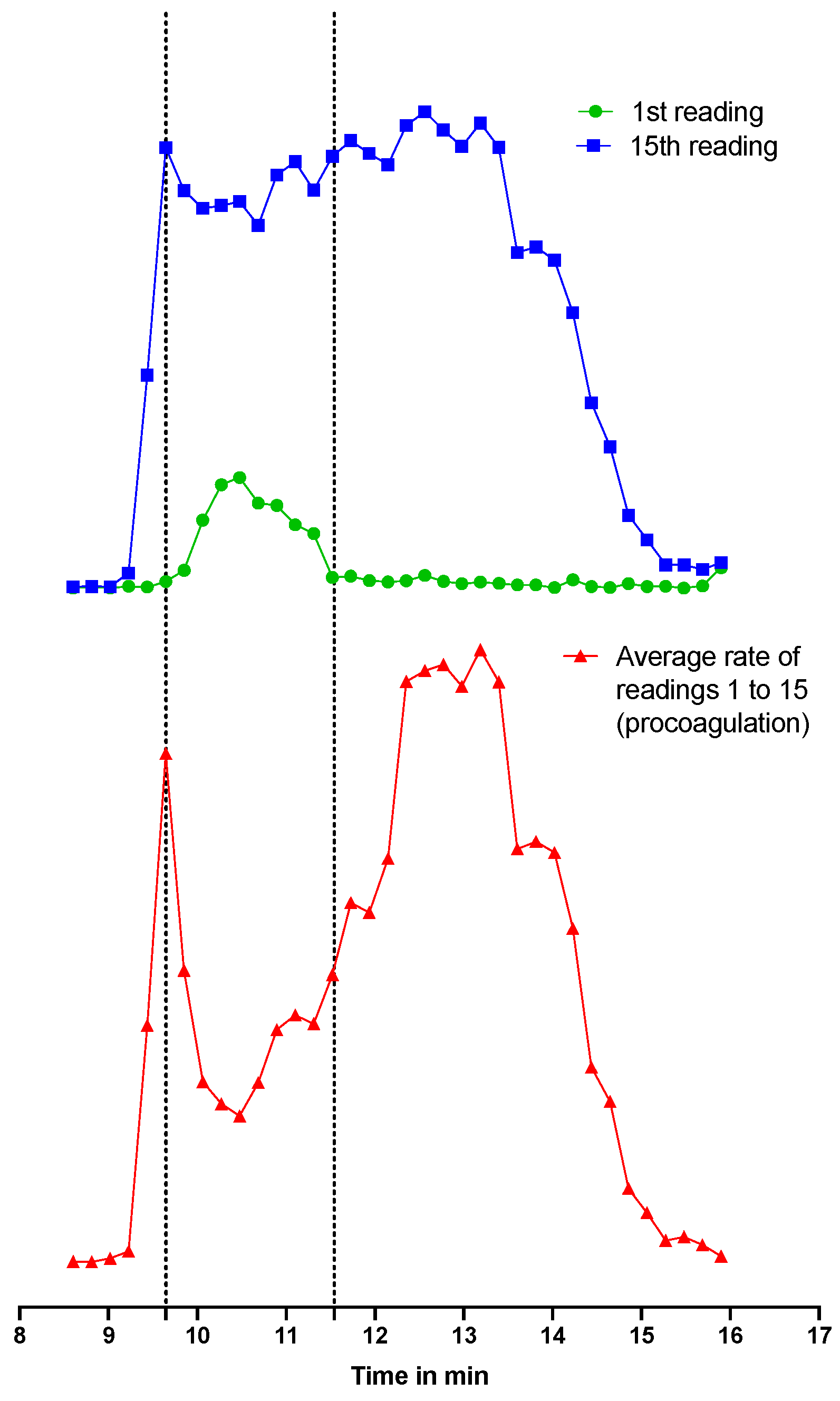
2.2.2. Post-Column Bioassays after SEC-Fractionation
2.2.3. Integration and Correlation of Post-SEC Bioassay and HT-Venomics Data
2.2.4. Application and Results of On-Line SEC-MS Analysis
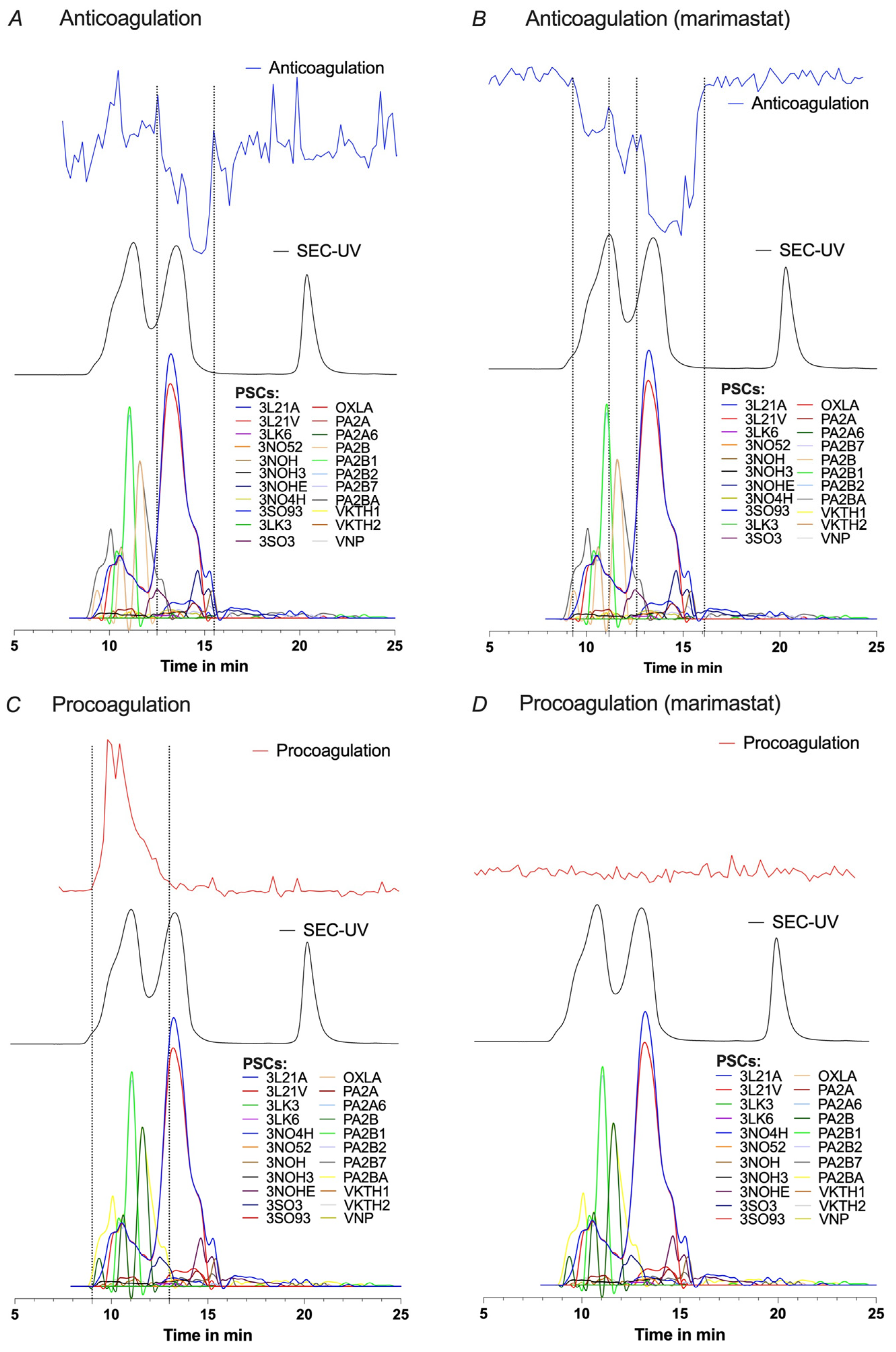
Bungarus Multicinctus
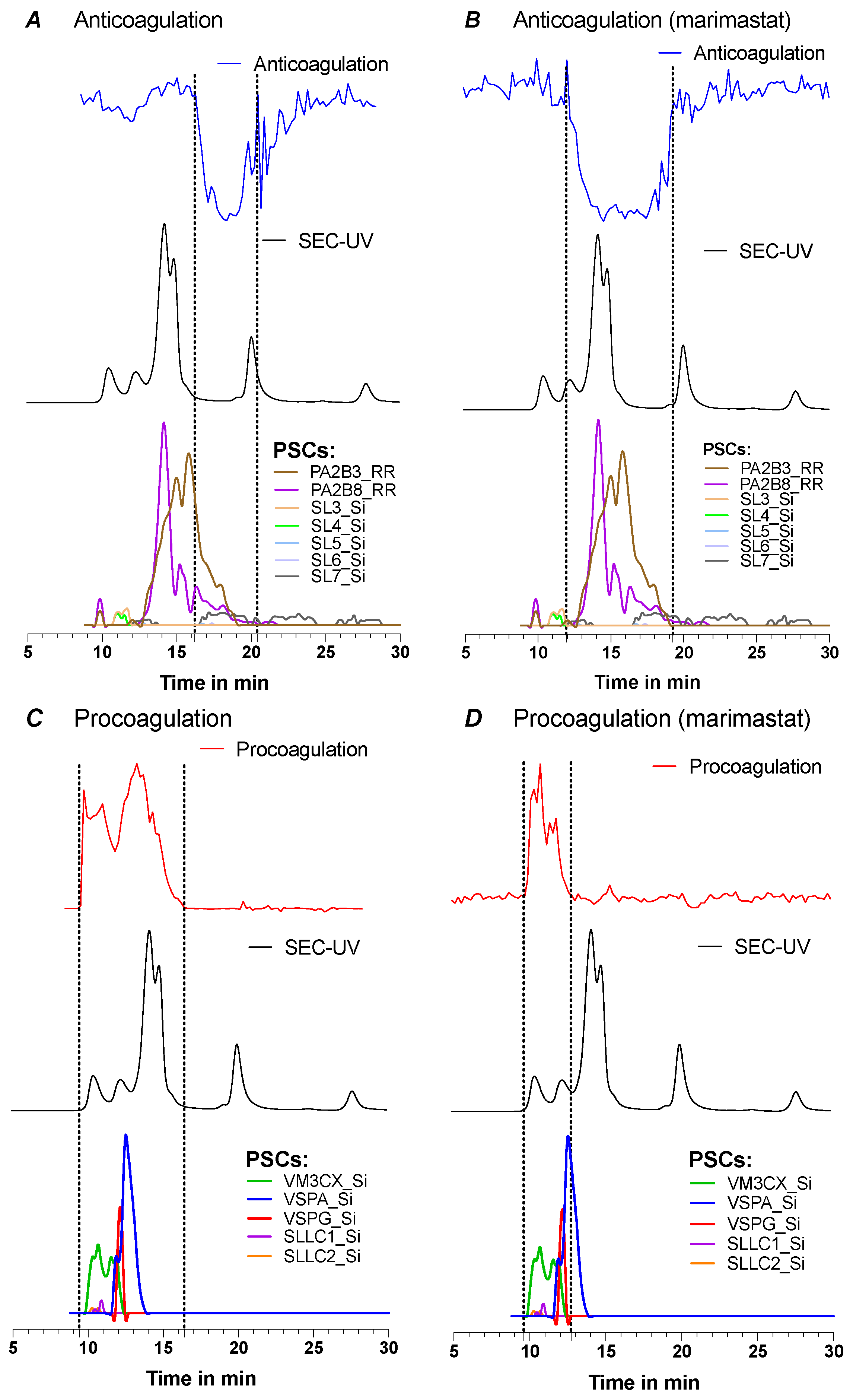
Daboia Russelii
2.2.5. Comparison of All Acquired Post-Column Data Sets
3. Conclusions
4. Methods
4.1. Reagents
4.1.1. Chemicals and Solutions
4.1.2. Venoms
4.2. SEC-MS Measurements
4.3. Nanofractionation
4.4. HT-Venomics
4.5. Plasma Coagulation Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, Venomics, Antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Winkel, K.D.; Wickramaratna, J.C.; Hodgson, W.C.; Wüster, W. Effectiveness of Snake Antivenom: Species and Regional Venom Variation and Its Clinical Impact. J. Toxicol. Toxin Rev. 2003, 22, 23–34. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.M.F.; Tan, K.Y.; Tan, N.H.; Tan, C.H. Proteomics and Neutralization of Bungarus multicinctus (Many-Banded Krait) Venom: Intra-Specific Comparisons between Specimens from China and Taiwan. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 247, 109063. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the Drought: New Strategies for Improving the Flow of Affordable, Effective Antivenoms in Asia and Africa. J. Proteomics 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement Ahead: Structure, Function and Mechanism of Snake Venom Phospholipase A2 Enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Snake Venom Three-Finger Toxins and Their Potential in Drug Development Targeting Cardiovascular Diseases. Biochem. Pharmacol. 2020, 181, 114105. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S.; Swenson, S. Snake Venom Metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef]
- Serrano, S.M.T. The Long Road of Research on Snake Venom Serine Proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic Toxins from Snake Venom: Structural Characterization and Mechanism of Catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake Venomics. Strategy and Applications. J. Mass Spectrom. 2007, 42, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- McNay, J.L.; Fernandez, E.J. How Does a Protein Unfold on a Reversed-Phase Liquid Chromatography Surface? J. Chromatogr. A 1999, 849, 135–148. [Google Scholar] [CrossRef]
- Gekko, K.; Ohmae, E.; Kameyama, K.; Takagi, T. Acetonitrile-Protein Interactions: Amino Acid Solubility and Preferential Solvation. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998, 1387, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kony, D.B.; Hünenberger, P.H.; van Gunsteren, W.F. Molecular Dynamics Simulations of the Native and Partially Folded States of Ubiquitin: Influence of Methanol Cosolvent, PH, and Temperature on the Protein Structure and Dynamics. Protein Sci. 2007, 16, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.T.; Pitts, M.; Tongyoo, P.; Rojnuckarin, P.; Wilkinson, M.C. Snake Venom Metalloproteinases and Their Peptide Inhibitors from Myanmar Russell’s Viper Venom. Toxins 2017, 9, 15. [Google Scholar] [CrossRef]
- Stubblefield, E.; Mueller, G.C. Effects of Sodium Chloride Concentration on Growth, Biochemical Composition, and Metabolism of HeLa Cells. Cancer Res. 1960, 20, 1646–1655. [Google Scholar]
- Sterling, H.J.; Batchelor, J.D.; Wemmer, D.E.; Williams, E.R. Effects of Buffer Loading for Electrospray Ionization Mass Spectrometry of a Noncovalent Protein Complex That Requires High Concentrations of Essential Salts. J. Am. Soc. Mass Spectrom. 2010, 21, 1045–1049. [Google Scholar] [CrossRef]
- Slagboom, J.; Mladić, M.; Xie, C.; Kazandjian, T.D.; Vonk, F.; Somsen, G.W.; Casewell, N.R.; Kool, J. High Throughput Screening and Identification of Coagulopathic Snake Venom Proteins and Peptides Using Nanofractionation and Proteomics Approaches. PLoS Negl. Trop. Dis. 2020, 14, e0007802. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.M.A.E.; Bourgoin-Voillard, S.; Combemale, S.; Beroud, R.; Fadl, M.; Seve, M.; De Waard, M. Fractionation and Proteomic Analysis of the Walterinnesia Aegyptia Snake Venom Using OFFGEL and MALDI-TOF-MS Techniques. Electrophoresis 2015, 36, 2594–2605. [Google Scholar] [CrossRef]
- Slagboom, J.; Derks, R.J.E.; Sadighi, R.; Somsen, G.W.; Ulens, C.; Casewell, N.R.; Kool, J. High-Throughput Venomics. J. Proteome Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Still, K.B.M.; Nandlal, R.S.S.; Slagboom, J.; Somsen, G.W.; Casewell, N.R.; Kool, J. Multipurpose HTS Coagulation Analysis: Assay Development and Assessment of Coagulopathic Snake Venoms. Toxins 2017, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Faisal, T.; Tan, K.Y.; Sim, S.M.; Quraishi, N.; Tan, N.H.; Tan, C.H. Proteomics, Functional Characterization and Antivenom Neutralization of the Venom of Pakistani Russell’s Viper (Daboia russelii) from the Wild. J. Proteomics 2018, 183, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Lee, W.; Xu, X.; Zhang, Y.; Zhao, R.; Zhang, Y.; Wang, W. Venom Gland Transcriptomes of Two Elapid Snakes (Bungarus multicinctus and Naja Atra) and Evolution of Toxin Genes. BMC Genom. 2011, 12, 1. [Google Scholar] [CrossRef]
- Goyon, A.; Fekete, S.; Beck, A.; Veuthey, J.L.; Guillarme, D. Unraveling the Mysteries of Modern Size Exclusion Chromatography—The Way to Achieve Confident Characterization of Therapeutic Proteins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1092, 368–378. [Google Scholar] [CrossRef]
- Goyon, A.; Beck, A.; Colas, O.; Sandra, K.; Guillarme, D.; Fekete, S. Evaluation of Size Exclusion Chromatography Columns Packed with Sub-3 Μm Particles for the Analysis of Biopharmaceutical Proteins. J. Chromatogr. A 2017, 1498, 80–89. [Google Scholar] [CrossRef]
- Castells, R.C.; Castells, C.B.; Castillo, M.A. Influence of Differences between Sample and Mobile Phase Viscosities on the Shape of Chromatographic Elution Profiles. J. Chromatogr. A 1997, 775, 73–79. [Google Scholar] [CrossRef]
- Sang, Y.; Roest, M.; De Laat, B.; De Groot, P.G.; Huskens, D. Interplay between Platelets and Coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Gantsova, E.A.; Andreeva, T.V.; Starkov, V.G.; Ziganshin, R.H.; Anh, H.N.; Thao, N.T.T.; Khoa, N.C.; Tsetlin, V.I. Venoms of Kraits Bungarus multicinctus and Bungarus fasciatus Contain Anticoagulant Proteins. Dokl. Biochem. Biophys. 2015, 460, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Frank, N.; Turchioe, B.J. Anticoagulant Activity of Krait, Coral Snake, and Cobra Neurotoxic Venoms with Diverse Proteomes Are Inhibited by Carbon Monoxide. Blood Coagul. Fibrinolysis 2019, 30, 379–384. [Google Scholar] [CrossRef]
- Wang, N.; Wu, X.; Zhang, S.; Shen, W.; Li, N. Local Tissue Necrosis and Thrombocytopenia Following Bungarus multicinctus Envenomation in a Child. Arch. Argent. Pediatr. 2021, 119, E80–E83. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, H.; Yu, H.; Gao, W.; Lai, R.; Liu, J.; Liang, X. A Novel Serine Protease Inhibitor from Bungarus fasciatus Venom. Peptides 2008, 29, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Fung, S.Y.; Ponnudurai, G. Enzymatic and Immunological Properties of Bungarus flaviceps (Red-Headed Krait) Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 147–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.L.; Bon, C. An Activator of Blood Coagulation Factor X from the Venom of Bungarus fasciatus. Toxicon 1995, 33, 1277–1288. [Google Scholar] [CrossRef]
- Ziganshin, R.H.; Kovalchuk, S.I.; Arapidi, G.P.; Starkov, V.G.; Hoang, A.N.; Thi Nguyen, T.T.; Nguyen, K.C.; Shoibonov, B.B.; Tsetlin, V.I.; Utkin, Y.N. Quantitative Proteomic Analysis of Vietnamese Krait Venoms: Neurotoxins Are the Major Components in Bungarus multicinctus and Phospholipases A2 in Bungarus fasciatus. Toxicon 2015, 107, 197–209. [Google Scholar] [CrossRef]
- Shan, L.-L.; Gao, J.-F.; Zhang, Y.-X.; Shen, S.-S.; He, Y.; Wang, J.; Ma, X.-M.; Ji, X. Proteomic Characterization and Comparison of Venoms from Two Elapid Snakes (Bungarus multicinctus and Naja Atra) from China. J. Proteomics 2016, 138, 83–94. [Google Scholar] [CrossRef]
- Xie, C.; Albulescu, L.-O.; Bittenbinder, M.A.; Somsen, G.W.; Vonk, F.J.; Casewell, N.R.; Kool, J. Neutralizing Effects of Small Molecule Inhibitors and Metal Chelators on Coagulopathic Viperinae Snake Venom Toxins. Biomedicines 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Slagboom, J.; Albulescu, L.O.; Somsen, G.W.; Vonk, F.J.; Casewell, N.R.; Kool, J. Neutralising Effects of Small Molecule Toxin Inhibitors on Nanofractionated Coagulopathic Crotalinae Snake Venoms. Acta Pharm. Sin. B 2020, 10, 1835–1845. [Google Scholar] [CrossRef]
- Faisal, T.; Tan, K.Y.; Tan, N.H.; Sim, S.M.; Gnanathasan, C.A.; Tan, C.H. Proteomics, Toxicity and Antivenom Neutralization of Sri Lankan and Indian Russell’s Viper (Daboia russelii) Venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, 1–15. [Google Scholar] [CrossRef]
- Mukherjee, A.K. A Major Phospholipase A2 from Daboia russelii Russelii Venom Shows Potent Anticoagulant Action via Thrombin Inhibition and Binding with Plasma Phospholipids. Biochimie 2014, 99, 153–161. [Google Scholar] [CrossRef]
- Saikia, D.; Majumdar, S.; Mukherjee, A.K. Mechanism of in Vivo Anticoagulant and Haemolytic Activity by a Neutral Phospholipase A2 Purified from Daboia russelii Russelii Venom: Correlation with Clinical Manifestations in Russell’s Viper Envenomed Patients. Toxicon 2013, 76, 291–300. [Google Scholar] [CrossRef]
- Sharma, M.; Iyer, J.K.; Shih, N.; Majumder, M.; Mattaparthi, V.S.K.; Mukhopadhyay, R.; Doley, R. Daboxin p, a Major Phospholipase A2 Enzyme from the Indian Daboia russelii Russelii Venom Targets Factor x and Factor Xa for Its Anticoagulant Activity. PLoS ONE 2016, 11, e0153770. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Dutta, S.; Mackessy, S.P. A New C-Type Lectin (RVsnaclec) Purified from Venom of Daboia russelii Russelii Shows Anticoagulant Activity via Inhibition of FXa and Concentration-Dependent Differential Response to Platelets in a Ca2+-Independent Manner. Thromb. Res. 2014, 134, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Mackessy, S.P.; Dutta, S. Characterization of a Kunitz-Type Protease Inhibitor Peptide (Rusvikunin) Purified from Daboia russelii Russelii Venom. Int. J. Biol. Macromol. 2014, 67, 154–162. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, J.R.; Lu, H.J.; Zhao, L.; Chen, J.; Zhang, H.F.; Wei, X.S.; Zhang, L.Y.; Wu, X.B.; Lee, W.H. Immunoreactivity and Neutralization Study of Chinese Bungarus multicinctus Antivenin and Lab-Prepared Anti-Bungarotoxin Antisera towards Purified Bungarotoxins and Snake Venoms. PLoS Negl. Trop. Dis. 2020, 14, e0008873. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zheng, Y.; Wang, X.; Tan, X.; Zhang, T.; Xin, W.; Wang, J.; Huang, Y.; Fan, Q.; Wang, J. Recognition of Bungarus multicinctus Venom by a DNA Aptamer against β-Bungarotoxin. PLoS ONE 2014, 9, 2–9. [Google Scholar] [CrossRef]
- Lardeux, H.; Duivelshof, B.L.; Colas, O.; Beck, A.; McCalley, D.V.; Guillarme, D.; D’Atri, V. Alternative Mobile Phase Additives for the Characterization of Protein Biopharmaceuticals in Liquid Chromatography—Mass Spectrometry. Anal. Chim. Acta 2021, 1156, 338347. [Google Scholar] [CrossRef]
- Gowda, D.C.; Jackson, C.M.; Hensley, P.; Davidson, E.A. Factor X-Activating Glycoprotein of Russell’s Viper Venom. Polypeptide Composition and Characterization of the Carbohydrate Moieties. J. Biol. Chem. 1994, 269, 10644–10650. [Google Scholar] [CrossRef]
- Chen, H.S.; Chen, J.M.; Lin, C.W.; Khoo, K.H.; Tsai, I.H. New Insights into the Functions and N-Glycan Structures of Factor X Activator from Russell’s Viper Venom. FEBS J. 2008, 275, 3944–3958. [Google Scholar] [CrossRef]
- Takeda, S. Structural Aspects of the Factor X Activator RVV-X from Russell’s Viper Venom. In Toxins and Hemostasis: From Bench to Bedside; Kini, R.M., Clemetson, K.J., Markland, F.S., McLane, M.A., Morita, T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 465–484. ISBN 978-90-481-9295-3. [Google Scholar]
- Mladic, M.; de Waal, T.; Burggraaff, L.; Slagboom, J.; Somsen, G.W.; Niessen, W.M.A.; Manjunatha Kini, R.; Kool, J. Rapid Screening and Identification of ACE Inhibitors in Snake Venoms Using At-Line Nanofractionation LC-MS. Anal. Bioanal. Chem. 2017, 409, 5987–5997. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Yang, X.; Li, H.; Ambrogelly, A.; Pollard, D.J. A High Throughput Ultra Performance Size Exclusion Chromatography Assay for the Analysis of Aggregates and Fragments of Monoclonal Antibodies. Pharm. Bioprocess. 2014, 2, 141–156. [Google Scholar] [CrossRef]
- Mattos, C.; Ringe, D. Proteins in Organic Solvents. Curr. Opin. Struct. Biol. 2001, 11, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Maroun, R.C. Snake Venom Serine Proteinases: Sequence Homology vs. Substrate Specificity, a Paradox to Be Solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 Biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar] [CrossRef]
- Fry, B.G.; Wüster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular Evolution and Phylogeny of Elapid Snake Venom Three-Finger Toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef]




| Mascot Code | Name | HT-Venomics Retrieved Mass | MS Accurate Mass |
|---|---|---|---|
| Bungarus multicinctus | |||
| 3L21A_BUNMU | α-Bungarotoxin | 7978.60 | 7978.38 |
| 3L21V_BUNMU | α-Bungarotoxin Isoform V31 | 8003.63 | 8005.39 |
| 3LKB_BUNMU | κ-bungarotoxin | 7260.22 | 7259.92 |
| 3NO5I_BUNMU | γ-bungarotoxin | 7519.33 | 7517.11 |
| 3NOH_BUNMU | Toxin BMLCL | 9012.78 | 9012.52 |
| 3NOHE_BUNMU | Muscarinic toxin BM14 | 9068.89 | 9066.61 |
| PA2A_BUNMU | Acidic PLA2 | 12,808.56 | 12,808.20 |
| PA2B2_BUNMU | Basic PLA2 ß-bungarotoxin A2 chain | 13,656.20 | 13,652.88 |
| PA2B_BUNMU
+ VKTH3_BUNMU | Basic PLA2 ß-bungarotoxin A-AL2 chain + Kunitz-type serine protease inhibitor homolog beta-bungarotoxin B3 chain | 13,575.11 + 7205.28 = total 20,780.39 | 20,776.95 |
| PA2B7_BUNMU + VKTH2_BUNMU | Basic PLA2 ß-bungarotoxin A7 chain + Kunitz-type serine protease inhibitor homolog beta-bungarotoxin B2 chain | 13,445.08 + 7186.37 = total 20,631.45 | 20,634.90 |
| Daboia russelii | |||
| PA2B_DABRR | Basic PLA2 RVV-VD | 13,602.94 | 13,603.94 |
| PA2B5_DABRR | Basic PLA2 VRV-PL-V | 13,563.13 | 13,568.91 |
| PA2B8_DABRR | Basic PLA2 VRV-PL-VIIIa | 13,587.20 | 13,585.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzioglu, S.; Bittenbinder, M.A.; Slagboom, J.; van de Velde, B.; Casewell, N.R.; Kool, J. Analytical Size Exclusion Chromatography Coupled with Mass Spectrometry in Parallel with High-Throughput Venomics and Bioassaying for Venom Profiling. Toxins 2023, 15, 552. https://doi.org/10.3390/toxins15090552
Terzioglu S, Bittenbinder MA, Slagboom J, van de Velde B, Casewell NR, Kool J. Analytical Size Exclusion Chromatography Coupled with Mass Spectrometry in Parallel with High-Throughput Venomics and Bioassaying for Venom Profiling. Toxins. 2023; 15(9):552. https://doi.org/10.3390/toxins15090552
Chicago/Turabian StyleTerzioglu, Sedef, Mátyás A. Bittenbinder, Julien Slagboom, Bas van de Velde, Nicholas R. Casewell, and Jeroen Kool. 2023. "Analytical Size Exclusion Chromatography Coupled with Mass Spectrometry in Parallel with High-Throughput Venomics and Bioassaying for Venom Profiling" Toxins 15, no. 9: 552. https://doi.org/10.3390/toxins15090552






