Comprehensive Analysis of Bufadienolide and Protein Profiles of Gland Secretions from Medicinal Bufo Species
Abstract
:1. Introduction
2. Results
2.1. Qualitative Analysis of Bufadienolides by UPLC-Q-TOF/MS
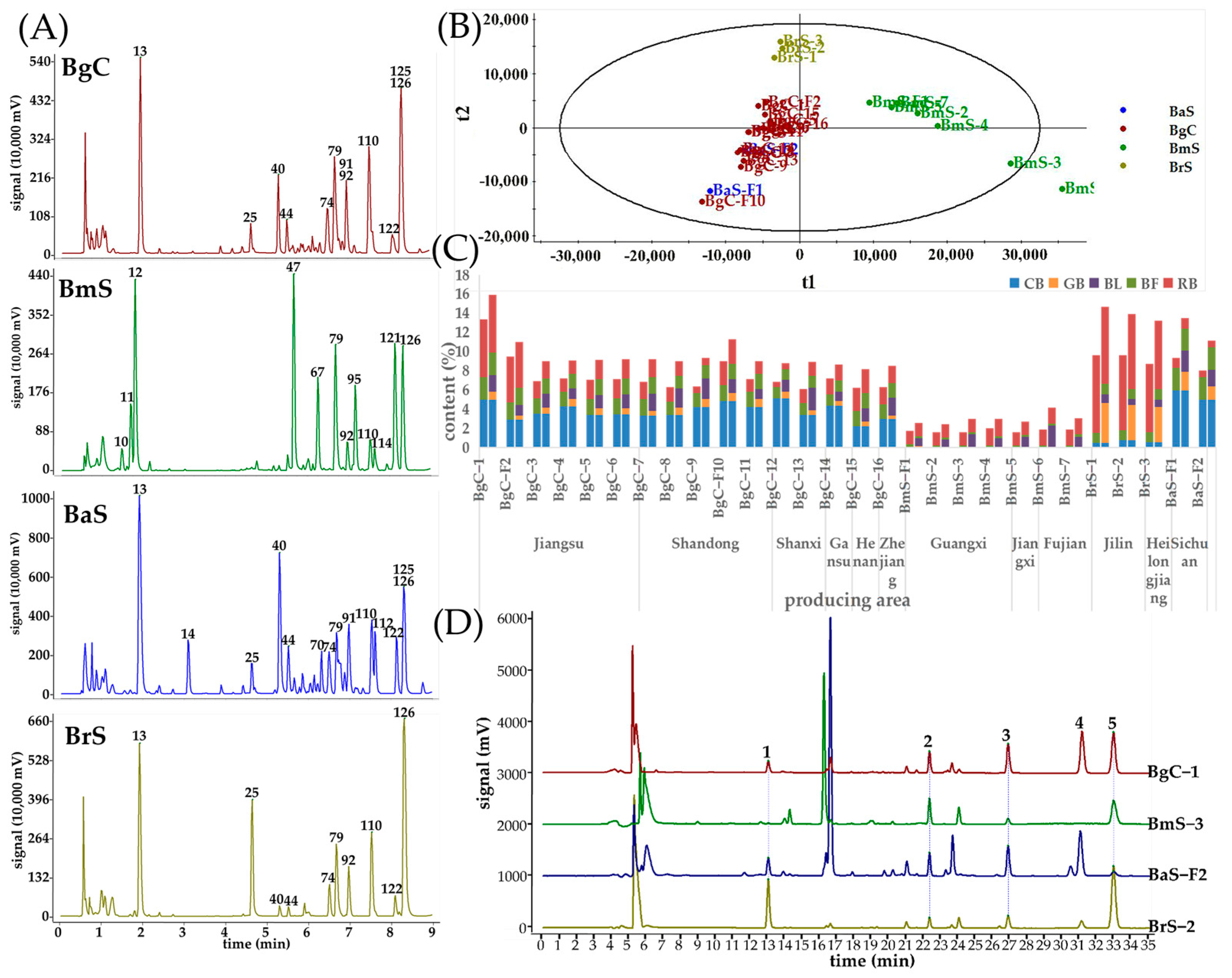
2.2. The Comparison between TV Components from Different Species
2.3. Quantitative Analysis of Five Marker Bufadienolides
2.4. The Comparison of Bufadienolides from Different Regions in the Same Species
2.5. Total Protein Content Determination
2.6. SDS-PAGE Analysis
2.7. Protein Identification and Bioinformatic Analysis
2.8. The Comparison of TV Proteins from Different Species
2.9. The Comparison of TV Proteins from Different Regions in the Same Species
3. Discussion
3.1. Micromolecules in TV
3.2. Macromolecules in TV
3.3. Integrated Analysis Supported Taxonomic Relationship of Toads
3.4. Future Research
4. Conclusions
5. Materials and Methods
5.1. Materials
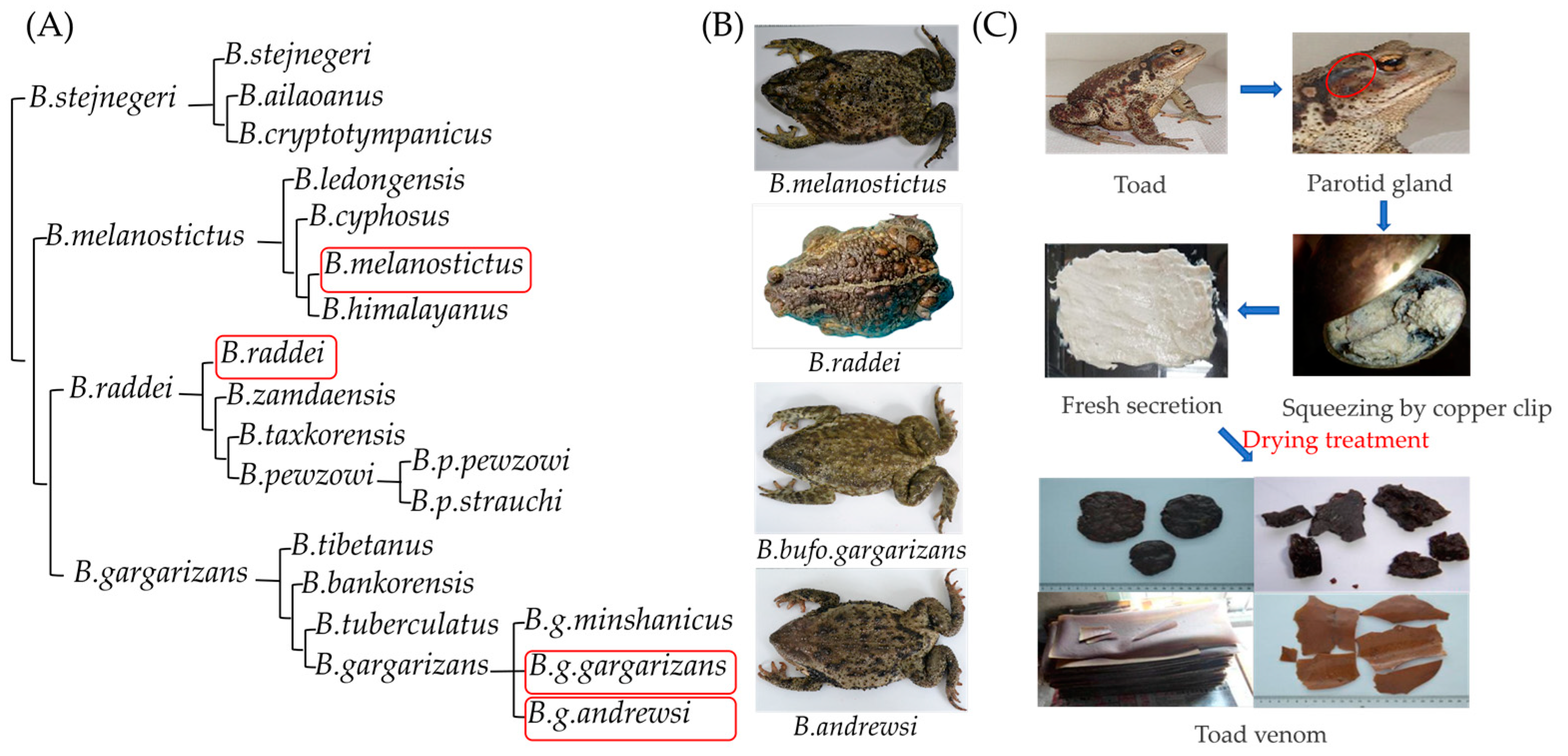
5.2. TV Samples
5.3. UPLC-Q-TOF/MS Analysis
5.4. Quantitative Analysis of Bufadienolides
5.5. Determination of Total Proteins by Bradford Method
5.6. SDS-PAGE Analysis
5.7. In-Solution Digestion of Proteins
5.8. Nano LC-MS/MS Analysis and Protein Identification
5.9. Statistical Analysis and Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia (Part I), 11th ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 401–402.
- Wei, W.L.; Hou, J.J.; Wang, X.; Yu, Y.; Li, H.J.; Li, Z.W.; Feng, Z.J.; Qu, H.; Wu, W.Y.; Guo, D.A. Venenum bufonis: An overview of its traditional use, natural product chemistry, pharmacology, pharmacokinetics and toxicology. J. Ethnopharmacol. 2019, 237, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Asrorov, A.M.; Kayumov, M.; Mukhamedov, N.; Yashinov, A.; Mirakhmetova, Z.; Huang, Y.; Yili, A.; Aisa, H.A.; Tashmukhamedov, M.; Salikhov, S.; et al. Toad venom bufadienolides and bufotoxins: An updated review. Drug Dev. Res. 2023, 84, 815–838. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhou, J.; Ma, H.Y.; Zhu, Z.H.; Qian, D.W.; Duan, J.A.; Wu, Q.N. Identification of proteins in toad venom by NanoLC-LTQ-Orbitrap Velos Pro. Chin. Pharm. J. 2017, 52, 675–680. [Google Scholar]
- Yang, M.Y.; Huan, W.W.; Zhang, G.B.; Li, J.; Xia, F.Y.; Durrani, R.; Zhao, W.; Lu, J.D.; Peng, X.M.; Gao, F. Identification of protein quality markers in toad venom from Bufo gargarizans. Molecules 2023, 28, 3628. [Google Scholar] [CrossRef] [PubMed]
- Mariano, D.O.C.; Messias, M.D.G.; Spencer, P.J.; Pimenta, D.C. Protein identification from the parotoid macrogland secretion of Duttaphrynus melanostictus. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2019, 25, e20190029. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.G.; Xv, R.X.; Ma, H.Y.; Zhou, J.; Xi, X.P.; Wu, Q.A.; Duan, J.A.; Zhou, M.; Chen, T.B. Identification of <10 KD peptides in the water extraction of Venenum Bufonis from Bufo gargarizans using Nano LC-MS/MS and de novo sequencing. J. Pharm. Biomed. Anal. 2018, 157, 156–164. [Google Scholar] [PubMed]
- Wang, J.J.; Guo, H.B.; Xu, D.H.; Yu, C.L.; Xv, R.X.; Wu, Q.A.; Di, L.Q.; Cheng, H.B.; Duan, J.A.; Zhou, J.; et al. Cell affinity screening combined with nanoLC-MS/MS based peptidomics for identifying cancer cell binding peptides from Bufo bufo gargarizans. J. Pharm. Biomed. Anal. 2021, 206, 114354. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Zehl, M.; Leitner, A.; Wu, X.Y.; Wang, Z.M.; Kopp, B. Comparison of toad venoms from different Bufo species by HPLC and LC-DAD-MS/MS. J. Ethnopharmacol. 2010, 131, 368–376. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Z.; Liu, Y.; Wang, D.; Liu, N.; Yoshikawa, M. Quality evaluation of traditional Chinese drug toad venom from different origins through a simultaneous determination of bufogenins and indole alkaloids by HPLC. Chem. Pharm. Bull. 2005, 53, 1582–1586. [Google Scholar] [CrossRef]
- Wang, P.F.; Fang, Y.G.; Li, Z.Y.; Chen, L.M.; Wang, Z.M.; Zou, Z.M.; Gao, H.M. Study on quality control method of toad venom based on characteristic chromatogram and QAMS. Chin. J. Chin. Mater. Med. 2018, 43, 2863–2871. [Google Scholar]
- Fang, Y.G.; Wang, P.F.; Zhu, H.D.; Chen, L.M.; Wang, Z.M.; Gao, H.M.; Fu, X.T.; Nie, J. Study on quality standard of Bufonis Venenum and its processed slice, toad venom powder. Chin. J. Chin. Mater. Med. 2020, 45, 1726–1733. [Google Scholar]
- Kowalski, K.; Marciniak, P.; Rosinski, G.; Rychlik, L. Toxic activity and protein identification from the parotoid gland secretion of the common toad Bufo bufo. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 205, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liao, Y.Q.; Hong, Q.; Li, H.; Cao, Y.; Chen, W.; Liu, J.Y.; Tu, P.F.; Li, J.; Song, Y.L. Widely quasi-quantitative analysis of both metabolites and tryptic peptides in animal-originated medicinal materials: Bufonis Venenum as a case. J. Pharm. Biomed. Anal. 2023, 223, 115143. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Tian, W.S.; Jiang, J.P.; Wu, G.F.; Li, J.; Wang, Y.S. Fauna Sinica: Amphibia; Science Press: Beijing, China, 2009; Volume 2, pp. 489–490. [Google Scholar]
- Wang, Y.M.; Li, Z.Y.; Wang, J.J.; Wu, X.Y.; Gao, H.M.; Wang, Z.M. Bufadienolides and polyhydroxycholestane derivatives from Bufo bufo gargarizans. J. Asian Nat. Prod. Res. 2015, 17, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Han, L.Y.; Luo, M.Y.; Bian, B.L.; Guan, M.; Yang, H.; Han, C.; Li, N.; Li, T.; Li, S.L.; et al. Multi-component identification and target cell-based screening of potential bioactive compounds in toad venom by UPLC coupled with high-resolution LTQ-Orbitrap MS and high-sensitivity Qtrap MS. Anal. Bioanal. Chem. 2018, 410, 4419–4435. [Google Scholar] [CrossRef]
- Cao, Y.T.; Wu, J.H.; Pan, H.Y.; Wang, L.H. Chemical profile and multicomponent quantitative analysis for the quality evaluation of toad venom from different origins. Molecules 2019, 24, 3595. [Google Scholar] [CrossRef] [PubMed]
- Zulfiker, A.H.M.; Sohrabi, M.; Qi, J.; Matthews, B.; Wei, M.Q.; Grice, I.D. Multi-constituent identification in Australian cane toad skin extracts using high-performance liquid chromatography high-resolution tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 129, 260–272. [Google Scholar] [CrossRef]
- Su, R.; Zhang, S.; Zhang, X.Y.; Wang, S.N.; Zhang, W.Y. Neglected skin-associated microbial communities: A unique immune defense strategy of Bufo raddei under environmental heavy metal pollution. Environ. Sci. Pollut. Res. Int. 2023, 30, 22330–22342. [Google Scholar] [CrossRef]
- Sousa-Filho, L.M.; Freitas, C.D.; Lobo, M.D.; Monteiro-Moreira, A.C.; Silva, R.O.; Santana, L.A.; Ribeiro, R.A.; Souza, M.H.; Ferreira, G.P.; Pereira, A.C.; et al. Biochemical profile, biological activities, and toxic effects of proteins in the Rhinella schneideri parotoid gland secretion. J. Exp. Zool. A. Ecol. Genet. Physiol. 2016, 325, 511–523. [Google Scholar] [CrossRef]
- Li, F.J.; Hu, J.H.; Ren, X.; Zhou, C.M.; Liu, Q.; Zhang, Y.Q. Toad venom: A comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch. Pharm. 2021, 354, e2100060. [Google Scholar] [CrossRef]
- He, R.R.; Ma, H.Y.; Zhou, J.; Zhu, Z.H.; Lv, X.; Li, Q.; Wang, H.B.; Yan, Y.Q.; Luo, N.C.; Di, L.Q.; et al. High resolution mass profile of bufadienolides and peptides combing with anti-tumor cell screening and multivariate analysis for the quality evaluation of Bufonis Venenum. Molecules 2019, 24, 1943. [Google Scholar] [CrossRef]
- Sun, B.; Li, M.L.; Ding, Y.H.; Zhang, Y.; Xia, B.; Guo, S.Y.; Wang, S.L.; Bian, B.L.; Si, N.; Zhao, H.Y. Systematic comparative study of two kinds of Bufonis Venenum derived from different Bufo bufo gargarizans subspecies based on metabolomics and antitumor activity. Chin. J. Chin. Mater. Med. 2023, 48, 1280–1288. [Google Scholar]
- Zhao, Y.; Jin, Y.; Wei, S.S.; Lee, W.H.; Zhang, Y. Purification and characterization of an irreversible serine protease inhibitor from skin secretions of Bufo andrewsi. Toxicon 2005, 46, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jin, Y.; Lee, W.H.; Zhang, Y. Purification of a lysozyme from skin secretions of Bufo andrewsi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 142, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, X.J.; Li, Z.; Zhou, A.C.; Tiffany-Castiglioni, E.; Xie, L.J.; Qian, Y.C. Identification of anti-tumor components from toad venom. Oncol. Lett. 2017, 14, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.N.; Litvinchuk, S.N.; Maslova, I.; Dahn, H.; Messenger, K.R.; Andersen, D.; Jowers, M.J.; Kojima, Y.; Skorinov, D.V.; Yasumiba, K.; et al. From Gondwana to the Yellow Sea, evolutionary diversifications of true toads Bufo sp. in the Eastern Palearctic and a revisit of species boundaries for Asian lineages. eLife 2022, 11, e70494. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.N.; Yang, W.Z.; Fu, J.Z. Population genetic structure and species status of Asiatic Toads (Bufo gargarizans) in Western China. Zoolog. Sci. 2015, 32, 427–434. [Google Scholar] [CrossRef]
- Frost, D.R.; Grant, T.; Faivovich, J.; Bain, R.H.; Haas, A.; Haddad, C.F.B.; De SÁ, R.O.; Channing, A.; Wilkinson, M.; Donnellan, S.C.; et al. The Amphibian Tree of Life. Bull. Am. Mus. Nat. Hist. 2006, 297, 219–220. [Google Scholar] [CrossRef]
- Edwards, R.J.; Tuipulotu, D.E.; Amos, T.G.; O’Meally, D.; Richardson, M.F.; Russell, T.L.; Vallinoto, M.; Carneiro, M.; Ferrand, N.; Wilkins, M.R.; et al. Draft genome assembly of the invasive cane toad, Rhinella marina. Gigascience 2018, 7, giy095. [Google Scholar] [CrossRef]

| No. | Name | RT (min) | [M+H]+ Detected | [M+H]+ Expected | Error (ppm) | Formula | Bufo Species 1 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| BgC | BmS | BrS | BaS | |||||||
| 1 | succinyl arginine | 0.60 | 275.1359 | 275.1355 | 1.5 | C10H18N4O5 | ++++ | − | ++++ | ++++ |
| 2 | adipyl arginine | 0.79 | 303.1674 | 303.1668 | 2.0 | C12H22N4O5 | +++ | − | +++ | ++++ |
| 3 | serotonin | 0.89 | 177.1028 | 177.1028 | 0.0 | C10H12N2O | + | − | − | + |
| 4 | N-methyl serotonin | 0.97 | 191.1185 | 191.1184 | 0.5 | C11H14N2O | ++ | ++ | ++ | ++ |
| 5 | N,N-dimethyl serotonin | 1.03 | 205.1345 | 205.1341 | 1.9 | C12H16N2O | ++ | + | +++ | ++ |
| 6 | N,N,N-trimethyl serotonin | 1.04 | 219.1497 | 219.1497 | 0.0 | C13H18N2O | +++ | +++ | +++ | ++++ |
| 7 | pimeloyl arginine | 1.12 | 317.1830 | 317.1825 | 1.6 | C13H24N4O5 | +++ | + | +++ | ++++ |
| 8 | dehydrobufotenine | 1.30 | 203.1188 | 203.1184 | 2.0 | C12H14N2O | +++ | ++ | +++ | +++ |
| 9 | bufothionine | 1.40 | 283.0751 | 283.0753 | −0.7 | C12H14N2O4S | ++ | ++ | ++ | ++ |
| 10 | suberoyl-L-histidine | 1.53 | 312.1562 | 312.1559 | 1.0 | C14H21N3O5 | + | +++ | + | + |
| 11 | suberoyl-L-1-methylhistidine | 1.73 | 326.1717 | 326.1716 | 0.3 | C15H23N3O5 | ++ | ++++ | ++ | +++ |
| 12 | unknown | 1.82 | 245.1866 | 245.1865 | 0.4 | C12H24N2O3 | + | ++++ | ++ | − |
| 13 | suberoyl arginine | 1.95 | 331.1984 | 331.1981 | 0.9 | C14H26N4O5 | ++++ | ++ | ++++ | ++++ |
| 14 | sebacyl arginine isomer | 2.42 | 359.2296 | 359.2294 | 0.6 | C16H30N4O5 | +++ | ++ | +++ | +++ |
| 15 | unknown | 2.85 | 416.2432 2 | 416.2437 | −1.2 | C24H30O5 | + | − | + | + |
| 16 | azelayl arginine | 3.12 | 345.2142 | 345.2138 | 1.2 | C15H28N4O5 | + | − | + | ++++ |
| 17 | 11α-hydroxyltelocinobufagin | 3.95 | 419.2418 | 419.2434 | −3.8 | C24H34O6 | ++ | ++ | + | ++ |
| 18 | 19-hydroxyltelocinobufagin | 4.22 | 419.2423 | 419.2434 | −2.6 | C24H34O6 | ++ | + | ++ | ++ |
| 19 | 5,12β-dihydroxycinobufagin | 4.23 | 475.2322 | 475.2332 | −2.1 | C26H34O8 | + | ++ | + | + |
| 20 | ψ-bufarenogin | 4.44 | 417.2277 | 417.2277 | 0.0 | C24H32O6 | +++ | ++ | ++ | +++ |
| 21 | gamabufotalin 3-O-succinoyl arginine ester or its isomer | 4.48 | 656.3658 | 659.3656 | 0.3 | C34H50N4O9 | + | − | ++ | + |
| 22 | 16-acetoxybufarenogin | 4.53 | 475.2328 | 475.2332 | −0.8 | C26H34O8 | + | ++ | + | + |
| 23 | 16β-Hydroxyl-pseudobufarenogin | 4.56 | 433.2215 | 433.2226 | −2.6 | C24H32O7 | − | − | + | ++ |
| 24 | 3-oxo-12β-hydroxyl desacetylcinobufagin | 4.56 | 415.2111 | 415.2121 | −2.4 | C24H30O6 | − | − | + | ++ |
| 25 | gamabufotalin | 4.65 | 403.2485 | 403.2484 | 0.2 | C24H34O5 | +++ | ++ | ++++ | ++++ |
| 26 | bufarenogin | 4.72 | 417.2272 | 417.2277 | 0.0 | C24H32O6 | +++ | ++ | ++ | +++ |
| 27 | 11α,19-dihydroxylmarinobufagin | 4.75 | 433.2221 | 433.2226 | −1.2 | C24H32O7 | − | − | + | ++ |
| 28 | gamabufotalin 3-O-succinoyl arginine ester or its isomer | 4.76 | 659.3663 | 659.3656 | 1.1 | C34H50N4O9 | + | − | ++ | + |
| 29 | 16-O-acetylarenobufagin | 4.77 | 475.2334 | 475.2332 | 0.4 | C26H34O8 | ++ | +++ | ++ | ++ |
| 30 | 1β-hydroxylbufalin | 4.78 | 403.2476 | 403.2484 | −2.0 | C24H34O5 | ++ | ++ | − | +++ |
| 31 | gamabufotalin 3-O-succinoyl arginine ester or its isomer | 5.08 | 659.3654 | 659.3656 | −0.3 | C34H50N4O9 | + | − | ++ | + |
| 32 | arenobufagin/hellebrigenin 3-O-succinoyl arginine | 5.15 | 673.3824 | 673.3813 | 1.6 | C35H52N4O9 | − | − | − | + |
| 33 | arenobufagin 3-O-adipoyl arginine ester | 5.19 | 701.3748 | 701.3762 | −2.0 | C36H52N4O10 | + | − | − | +++ |
| 34 | 19-oxo-desacetylcinobufagin | 5.19 | 415.2129 | 415.2121 | 1.9 | C24H30O6 | ++ | − | − | ++ |
| 35 | hellebrigenol | 5.19 | 419.2427 | 419.2434 | −1.7 | C24H34O6 | ++ | ++ | + | ++ |
| 36 | hellebrigenol 3-O-suberoyl arginine ester or its isomer | 5.21 | 731.4226 | 731.4231 | −0.7 | C38H58N4O10 | + | − | + | +++ |
| 37 | 1β-Hydroxylarenobufagin | 5.23 | 433.2211 | 433.2226 | −3.5 | C24H32O7 | − | − | + | + |
| 38 | gamabufotalin isomer | 5.28 | 403.2479 | 403.2484 | −1.2 | C24H34O5 | ++ | ++ | + | +++ |
| 39 | 5-hydroxy bufotalin | 5.28 | 461.2531 | 461.2539 | −1.7 | C26H36O7 | ++ | ++ | + | ++ |
| 40 | arenobufagin | 5.31 | 417.2281 | 417.2277 | 1.0 | C24H32O6 | ++++ | +++ | +++ | ++++ |
| 41 | cinobufaginol isomer | 5.42 | 459.2371 | 459.2383 | −2.6 | C26H34O7 | ++ | ++ | + | ++ |
| 42 | hellebrigenin 3-O-adipoyl arginine ester | 5.48 | 701.3774 | 701.3762 | 1.7 | C37H56N4O9 | + | − | + | +++ |
| 43 | arenobufagin/hellebrigenin 3-O-pimeloyl arginine ester | 5.51 | 715.3908 | 715.3918 | −1.4 | C37H54N4O10 | − | − | − | ++ |
| 44 | hellebrigenin | 5.52 | 417.2276 | 417.2277 | −0.2 | C24H32O6 | ++++ | +++ | +++ | ++++ |
| 45 | gamabufotalin 3-O-adipoyl arginine ester or its isomer | 5.58 | 687.3976 | 687.3969 | 1.0 | C36H54N4O9 | − | + | − | − |
| 46 | hellebrigenol 3-O-suberoyl arginine ester or its isomer | 5.66 | 731.4214 | 731.4231 | −2.3 | C38H58N4O10 | + | − | + | ++ |
| 47 | 19-hydroxybufalin | 5.68 | 403.2494 | 403.2484 | 2.5 | C24H34O5 | +++ | ++++ | ++ | +++ |
| 48 | gamabufotalin 3-O-adipoyl arginine ester or its isomer | 5.76 | 687.3953 | 687.3969 | −1.9 | C36H54N4O9 | + | − | + | ++ |
| 49 | 5β-Hydroxyl-14α-artebufogenin | 5.78 | 401.2325 | 401.2328 | −0.7 | C24H32O5 | ++ | ++ | + | +++ |
| 50 | cinobufaginol | 5.80 | 459.2391 | 459.2383 | 1.7 | C26H34O7 | +++ | − | ++ | +++ |
| 51 | monohydroxylbufotalin | 5.81 | 461.2528 | 461.2539 | −2.4 | C26H36O7 | ++ | + | ++ | +++ |
| 52 | hellebrigenin/arenobufagin 3-O-suberoyl arginine ester or its isomer | 5.88 | 729.4062 | 729.4075 | −1.8 | C38H56N4O10 | ++ | − | + | ++++ |
| 53 | unknown | 5.91 | 615.4006 | 615.4009 | −0.5 | C35H54N2O7 | − | ++ | − | − |
| 54 | gamabufotalin 3-O-suberoyl arginine ester or its isomer | 5.92 | 715.4272 | 715.4282 | −1.4 | C38H58N4O9 | ++ | − | +++ | +++ |
| 55 | bufotalinin | 5.92 | 415.2113 | 415.2121 | −1.9 | C24H30O6 | ++ | ++ | +++ | ++ |
| 56 | desacetylbufotalin | 6.00 | 403.2481 | 403.2484 | −0.7 | C24H34O5 | +++ | +++ | ++ | +++ |
| 57 | resibufaginol | 6.03 | 401.2324 | 401.2328 | −1.0 | C24H32O5 | ++ | +++ | +++ | ++ |
| 58 | desacetylcinobufaginol | 6.03 | 417.2255 | 417.2277 | −5.3 | C24H32O6 | − | − | − | + |
| 59 | 19-oxo-cinobufotalin | 6.05 | 473.2178 | 473.2175 | 0.6 | C26H32O8 | +++ | − | ++ | +++ |
| 60 | bufotalinin 3-O-suberoyl arginine ester | 6.11 | 727.3909 | 727.3918 | −1.2 | C38H54N4O10 | + | − | + | ++ |
| 61 | gamabufotalin 3-O-pimeloyl arginine ester or its isomer | 6.12 | 701.4124 | 701.4126 | −0.3 | C37H56N4O9 | ++ | − | + | +++ |
| 62 | 1β-hydroxylcinobufagin | 6.12 | 459.2368 | 459.2383 | −3.3 | C26H34O7 | − | − | − | − |
| 63 | argentinogenin | 6.15 | 415.2115 | 415.2121 | −1.4 | C24H30O6 | ++ | + | + | ++ |
| 64 | 19-oxo-cinobufotalin 3-O-suberoyl arginine ester | 6.15 | 785.3965 | 785.3973 | −1.0 | C40H56N4O12 | + | − | − | +++ |
| 65 | bufalin 3-O-succinoyl arginine ester or its isomer | 6.16 | 643.3726 | 643.3707 | 3.0 | C34H50N4O8 | +++ | + | ++ | ++++ |
| 66 | 19-oxo-bufalin | 6.23 | 401.2323 | 401.2328 | −1.2 | C24H32O5 | +++ | +++ | ++ | +++ |
| 67 | unknown | 6.26 | 629.4162 | 629.4139 | 3.7 | C32H52N8O5 | − | ++++ | − | − |
| 68 | 19-oxo-cinobufotalin-3-suberate methylhistidine | 6.26 | 710.4012 | 710.4017 | −0.7 | C34H50N3O9 | − | +++ | − | − |
| 69 | gamabufotalin 3-O-suberoyl arginine ester or its isomer | 6.30 | 715.4261 | 715.4282 | −2.9 | C38H58N4O9 | + | − | + | ++ |
| 70 | hellebrigenin/arenobufagin 3-O-suberoyl arginine ester or its isomer | 6.34 | 729.4077 | 729.4075 | 0.3 | C38H56N4O10 | +++ | − | + | ++++ |
| 71 | cinobufaginol 3-O-suberoyl arginine ester or its isomer | 6.38 | 771.4172 | 771.4180 | −1.0 | C40H58N4O11 | ++ | − | + | +++ |
| 72 | bufalin 3-O-glutaryl arginine ester or its isomer | 6.45 | 657.3846 | 657.3863 | −2.6 | C35H52N4O8 | ++ | − | + | +++ |
| 73 | telocinobufagin 3-O-suberoyl arginine ester | 6.50 | 715.4296 | 715.4282 | 2.0 | C38H58N4O9 | ++ | − | ++ | ++++ |
| 74 | telocinobufagin | 6.52 | 403.2480 | 403.2484 | −1.0 | C24H34O5 | ++++ | ++ | +++ | ++++ |
| 75 | 12β-hydroxylcinobufagin | 6.57 | 459.2376 | 459.2383 | −1.5 | C26H34O7 | ++ | − | + | +++ |
| 76 | bufotalin 3-O-suberoyl-L-histidine or its isomer | 6.62 | 738.3953 | 738.3966 | −1.8 | C40H55N3O10 | − | + | − | − |
| 77 | resibufogenin 3-O-succinyl arginine ester or its isomer | 6.66 | 641.3535 | 641.3550 | −2.3 | C34H48N4O8 | ++ | − | ++ | + |
| 78 | desacetylcinobufagin | 6.69 | 401.2325 | 401.2328 | −0.7 | C24H32O5 | +++ | + | ++ | +++ |
| 79 | bufotalin | 6.69 | 445.2589 | 445.2590 | −0.2 | C26H36O6 | ++++ | ++++ | ++++ | ++++ |
| 80 | unknown | 6.74 | 627.3989 | 627.4009 | −3.2 | C36H54N2O7 | − | +++ | − | + |
| 81 | cinobufaginol 3-O-suberoyl arginine ester or its isomer | 6.74 | 771.4191 | 771.4180 | 1.4 | C40H58N4O11 | ++ | − | + | ++++ |
| 82 | desacetylcinobufagin 3-O-suberoyl arginine ester or its isomer | 6.74 | 713.4122 | 713.4126 | −0.6 | C38H56N4O9 | + | − | +++ | +++ |
| 83 | cinobufagin 3-O-succinoyl arginine ester or its isomer | 6.76 | 699.3596 | 699.3605 | −1.3 | C36H50N4O10 | +++ | − | + | +++ |
| 84 | bufalin 3-O-adipoyl arginine ester | 6.81 | 671.4021 | 671.4020 | 0.1 | C36H54N4O8 | ++ | − | + | ++++ |
| 85 | resibufagin | 6.82 | 399.2173 | 399.2171 | 0.5 | C24H30O5 | +++ | + | +++ | +++ |
| 86 | 19-oxo-cinobufagin | 6.89 | 457.2230 | 457.2226 | 0.9 | C26H32O7 | +++ | + | ++ | ++++ |
| 87 | resibufogenin 3-O-glutaryl arginine ester or its isomer | 6.92 | 655.3706 | 655.3707 | −0.2 | C35H50N4O8 | − | − | ++ | − |
| 88 | telocinobufagin 3-O-suberoyl arginine ester isomer | 6.95 | 715.4286 | 715.4282 | 1.4 | C38H58N4O9 | ++ | − | + | +++ |
| 89 | 3-oxo-Δ4-resibufogenin | 6.98 | 381.2062 | 381.2066 | −1.0 | C24H28O4 | ++ | − | + | ++ |
| 90 | 3-keto-cinobufagin | 6.98 | 441.2273 | 441.2277 | −0.9 | C26H32O6 | ++ | − | + | ++ |
| 91 | cinobufotalin | 7.00 | 459.2381 | 459.2383 | −0.4 | C26H34O7 | ++++ | − | +++ | ++++ |
| 92 | marinobufagin | 7.00 | 401.2323 | 401.2328 | −1.2 | C24H32O5 | +++ | +++ | ++++ | ++ |
| 93 | bufotalin 3-O-suberoyl-L-histidine or its isomer | 7.00 | 738.3961 | 738.3966 | −0.7 | C40H55N3O10 | − | ++ | − | − |
| 94 | bufalin 3-O-suberoyl arginine ester or its isomer | 7.01 | 699.4313 | 699.4333 | −2.9 | C38H58N4O8 | − | − | − | + |
| 95 | unknown | 7.19 | 671.4271 | 627.4245 | 3.9 | C34H54N8O6 | − | ++++ | − | − |
| 96 | resibufagin 3-O-suberoyl arginine ester or its isomer | 7.07 | 711.3968 | 711.3969 | −0.1 | C38H54N4O9 | + | − | + | ++ |
| 97 | bufotalin 3-O-suberoyl-L-3-methyl histidine | 7.12 | 752.4113 | 752.4122 | −1.3 | C41H57N3O10 | − | ++ | − | + |
| 98 | 19-hydroxybufalin 3-O-suberoyl-L-histidine or its isomer | 7.14 | 680.3892 | 680.3911 | −2.8 | C38H53N3O8 | + | + | − | − |
| 99 | 19-oxo-cinobufagin 3-O-suberoyl arginine ester or its isomer | 7.14 | 769.4028 | 769.4024 | 0.5 | C40H56N4O11 | ++ | − | + | +++ |
| 100 | bufotalin 3-O-suberoyl arginine ester or its isomer | 7.17 | 757.4387 | 757.4388 | −0.1 | C40H60N4O10 | ++ | − | ++ | +++ |
| 101 | 19-hydroxybufalin 3-O-suberoyl-L-3-methyl histidine or its isomer | 7.17 | 694.4036 | 694.4067 | −4.5 | C39H55N3O8 | − | + | − | − |
| 102 | bufalin 3-O-pimeloyl arginine ester or its isomer | 7.19 | 685.4171 | 685.4176 | −0.7 | C37H56N4O8 | +++ | − | ++ | +++ |
| 103 | marinobufagin 3-O-suberoyl arginine ester or its isomer | 7.20 | 713.4130 | 713.4126 | 0.6 | C38H56N4O9 | + | − | + | ++ |
| 104 | resibufogenin 3-O-adipoyl arginine ester or its isomer | 7.27 | 669.3846 | 669.3863 | −2.5 | C36H52N4O8 | + | + | + | ++ |
| 105 | cinobufagin 3-O-adipoyl arginine ester or its isomer | 7.30 | 727.3907 | 727.3918 | −1.5 | C38H54N4O10 | ++ | − | + | +++ |
| 106 | marinobufagin 3-O-suberoyl arginine ester or its isomer | 7.38 | 713.4116 | 713.4126 | −1.4 | C38H56N4O9 | − | − | + | + |
| 107 | cinobufotalin 3-O-suberoyl arginine ester | 7.40 | 771.4157 | 771.4180 | −3.0 | C40H58N4O11 | + | − | − | ++ |
| 108 | 19-hydroxybufalin 3-O-suberoyl-L-histidine or its isomer | 7.45 | 680.3909 | 680.3911 | −0.3 | C38H53N3O8 | − | + | − | − |
| 109 | resibufogenin 3-O-suberoyl arginine ester or its isomer | 7.48 | 697.4169 | 697.4176 | −1.0 | C38H56N4O8 | + | − | + | ++ |
| 110 | bufalin | 7.55 | 387.2531 | 387.2535 | −1.0 | C24H34O4 | ++++ | +++ | ++++ | ++++ |
| 111 | 19-hydroxybufalin 3-O-suberoyl-L-3-methyl histidine or its isomer | 7.61 | 694.4064 | 694.4067 | −0.4 | C39H55N3O8 | − | ++ | − | ++ |
| 112 | bufalin 3-O-suberoyl arginine ester or its isomer | 7.62 | 699.4321 | 699.4333 | −1.7 | C38H58N4O8 | +++ | + | +++ | ++++ |
| 113 | resibufogenin 3-O-pimeloyl arginine ester or its isomer | 7.67 | 683.4014 | 683.4020 | −0.9 | C37H54N4O8 | + | − | ++ | − |
| 114 | unknown | 7.64 | 613.4211 | 613.4217 | −1.0 | C36H56N2O6 | − | +++ | + | − |
| 115 | unknown | 7.69 | 597.3895 | 597.3804 | −1.5 | C35H52N2O6 | − | +++ | − | − |
| 116 | cinobufagin 3-O-pimeloyl arginine ester or its isomer | 7.74 | 741.4074 | 741.4075 | −0.1 | C39H56N4O10 | ++ | − | + | +++ |
| 117 | hellebrigenin 3-O-hemisuberate | 7.85 | 573.3052 | 573.3064 | −2.1 | C32H44O9 | + | − | + | ++ |
| 118 | resibufogenin 3-O-suberoyl-L-1-methyl histidine or its isomer | 8.09 | 692.3909 | 692.3911 | −0.3 | C39H53N3O8 | ||||
| 119 | bufalin 3-O-sebacyl arginine ester or its isomer | 7.87 | 727.4630 | 727.4646 | −2.2 | C40H62N4O8 | ++ | − | + | ++ |
| 120 | resibufogenin 3-O-suberoyl arginine ester or its isomer | 8.10 | 697.4158 | 697.4176 | −2.8 | C38H56N4O8 | ++ | − | +++ | +++ |
| 121 | unknown | 8.12 | 611.4075 | 611.4073 | 0.3 | C37H50N6O2 | − | ++++ | ++ | − |
| 122 | cinobufagin 3-O-suberoyl arginine ester | 8.14 | 755.4223 | 755.4231 | −1.1 | C40H58N4O10 | +++ | − | ++ | ++++ |
| 123 | gamabufotalin 3-O-hemisuberate | 8.16 | 559.3248 | 559.3271 | −4.1 | C32H46O8 | + | − | ++ | ++ |
| 124 | 22,23-Epoxyresibufogenin | 8.32 | 401.2332 | 401.2328 | 1.0 | C24H32O5 | ++ | − | + | + |
| 125 | cinobufagin | 8.33 | 443.2433 | 443.2434 | −0.2 | C26H34O6 | ++++ | + | +++ | ++++ |
| 126 | resibufogenin | 8.33 | 385.2377 | 385.2379 | −0.5 | C24H32O4 | ++++ | ++++ | ++++ | +++ |
| No. | Source | Content 1 (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CB | GB | BL | BF | RB | BF+CB+RB | CB+GB+BL+BF+RB | Total Proteins | ||
| BgC-1 | Nantong, Jiangsu | 4.97 | 0.89 | 1.69 | 2.39 | 5.99 | 13.35 | 15.93 | 17.0 |
| BgC-F2 | Haimen, Jiangsu | 2.94 | 0.38 | 1.15 | 1.75 | 4.80 | 9.49 | 11.02 | 11.5 |
| BgC-3 | Xuzhou, Jiangsu | 3.52 | 0.62 | 1.45 | 1.61 | 1.80 | 6.93 | 9.00 | 11.8 |
| BgC-4 | Rugao, Jiangsu | 4.28 | 0.82 | 1.05 | 1.57 | 1.36 | 7.21 | 9.08 | 11.6 |
| BgC-5 | Shanxian, Shandong | 4.27 | 0.81 | 1.04 | 1.57 | 1.31 | 7.15 | 9.00 | 12.4 |
| BgC-6 | Baoying, Jiangsu | 3.43 | 0.69 | 1.35 | 1.65 | 2.01 | 7.09 | 9.13 | 14.2 |
| BgC-7 | Linyi, Shandong | 3.35 | 0.50 | 1.79 | 1.72 | 1.82 | 6.89 | 9.18 | 13.0 |
| BgC-8 | Taicang, Jiangsu | 3.48 | 0.60 | 1.49 | 1.64 | 2.01 | 7.13 | 9.22 | 21.7 |
| BgC-9 | Xianyang, Shanxi | 5.13 | 0.73 | 1.18 | 1.17 | 0.59 | 6.89 | 8.80 | 23.0 |
| BgC-10 | Longnan, Gansu | 4.38 | 0.47 | 1.01 | 1.16 | 1.65 | 7.19 | 8.67 | 24.4 |
| BgC-11 | Linyi, Shandong | 3.44 | 0.73 | 1.99 | 1.36 | 1.51 | 6.31 | 9.03 | 21.7 |
| BgC-12 | Dezhou, Shandong | 4.25 | 0.80 | 2.15 | 1.42 | 0.74 | 6.41 | 9.36 | 23.5 |
| BgC-13 | Linfen, Shanxi | 3.40 | 0.46 | 2.39 | 1.22 | 1.45 | 6.07 | 8.92 | 12.8 |
| BgC-14 | Zhoukou, Henan | 2.23 | 0.51 | 1.38 | 1.58 | 2.45 | 6.26 | 8.15 | 6.9 |
| BgC-15 | Ningbo, Zhejiang | 2.96 | 0.36 | 1.86 | 1.54 | 1.83 | 6.33 | 8.55 | 8.0 |
| BgC-F16 | Linyi, Shandong | 4.85 | 0.86 | 1.41 | 1.64 | 2.50 | 8.99 | 11.26 | 15.6 |
| BmS-F1 | Guilin, Guangxi | 0.12 | 0.04 | 0.80 | 0.25 | 1.36 | 1.73 | 2.57 | 9.9 |
| BmS-2 | Pingnan, Guangxi | - | 0.04 | 0.79 | 0.22 | 1.40 | 1.62 | 2.45 | 20.4 |
| BmS-3 | Guilin, Guangxi | - | 0.02 | 1.37 | 0.20 | 1.43 | 1.63 | 3.02 | 16.7 |
| BmS-4 | Guilin, Guangxi | - | 0.05 | 0.91 | 0.21 | 1.81 | 2.02 | 2.98 | 11.9 |
| BmS-5 | Ganzhou, Jiangxi | - | 0.02 | 1.09 | 0.20 | 1.41 | 1.61 | 2.72 | 8.3 |
| BmS-6 | Zhangzhou, Fujian | - | 0.02 | 2.26 | 0.22 | 1.64 | 1.86 | 4.14 | 11.8 |
| BmS-7 | Wuyishan, Fujian | - | 0.03 | 1.12 | 0.23 | 1.69 | 1.92 | 3.07 | 4.8 |
| BrS-1 | Yanji, Jilin | 0.47 | 4.19 | 0.89 | 1.10 | 8.03 | 9.60 | 14.68 | 13.7 |
| BrS-2 | Wuchang, Heilongjiang | 0.55 | 3.70 | 0.78 | 1.06 | 7.12 | 8.73 | 13.21 | 12.9 |
| BrS-3 | Huadian, Jilin | 0.80 | 3.61 | 0.68 | 1.00 | 7.81 | 9.61 | 13.90 | 10.1 |
| BaS-F1 | Chengdu, Sichuan | 5.97 | 1.92 | 2.20 | 2.33 | 1.08 | 9.38 | 13.50 | 19.1 |
| BaS-F2 | Yibin, Sichuan | 4.98 | 1.40 | 1.71 | 2.38 | 0.68 | 8.04 | 11.15 | 20.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Chen, L.; Wang, P.; Liu, Y.; Wang, Y.; Wang, Z.; Ma, Y.; Gao, H. Comprehensive Analysis of Bufadienolide and Protein Profiles of Gland Secretions from Medicinal Bufo Species. Toxins 2024, 16, 159. https://doi.org/10.3390/toxins16030159
Fang Y, Chen L, Wang P, Liu Y, Wang Y, Wang Z, Ma Y, Gao H. Comprehensive Analysis of Bufadienolide and Protein Profiles of Gland Secretions from Medicinal Bufo Species. Toxins. 2024; 16(3):159. https://doi.org/10.3390/toxins16030159
Chicago/Turabian StyleFang, Yunge, Liangmian Chen, Pengfei Wang, Yating Liu, Yuxiu Wang, Zhimin Wang, Yue Ma, and Huimin Gao. 2024. "Comprehensive Analysis of Bufadienolide and Protein Profiles of Gland Secretions from Medicinal Bufo Species" Toxins 16, no. 3: 159. https://doi.org/10.3390/toxins16030159