Isolation and Functional Characterization of Erythrofibrase: An Alfa-Fibrinogenase Enzyme from Trimeresurus erythrurus Venom of North-East India
Abstract
:1. Introduction
2. Results
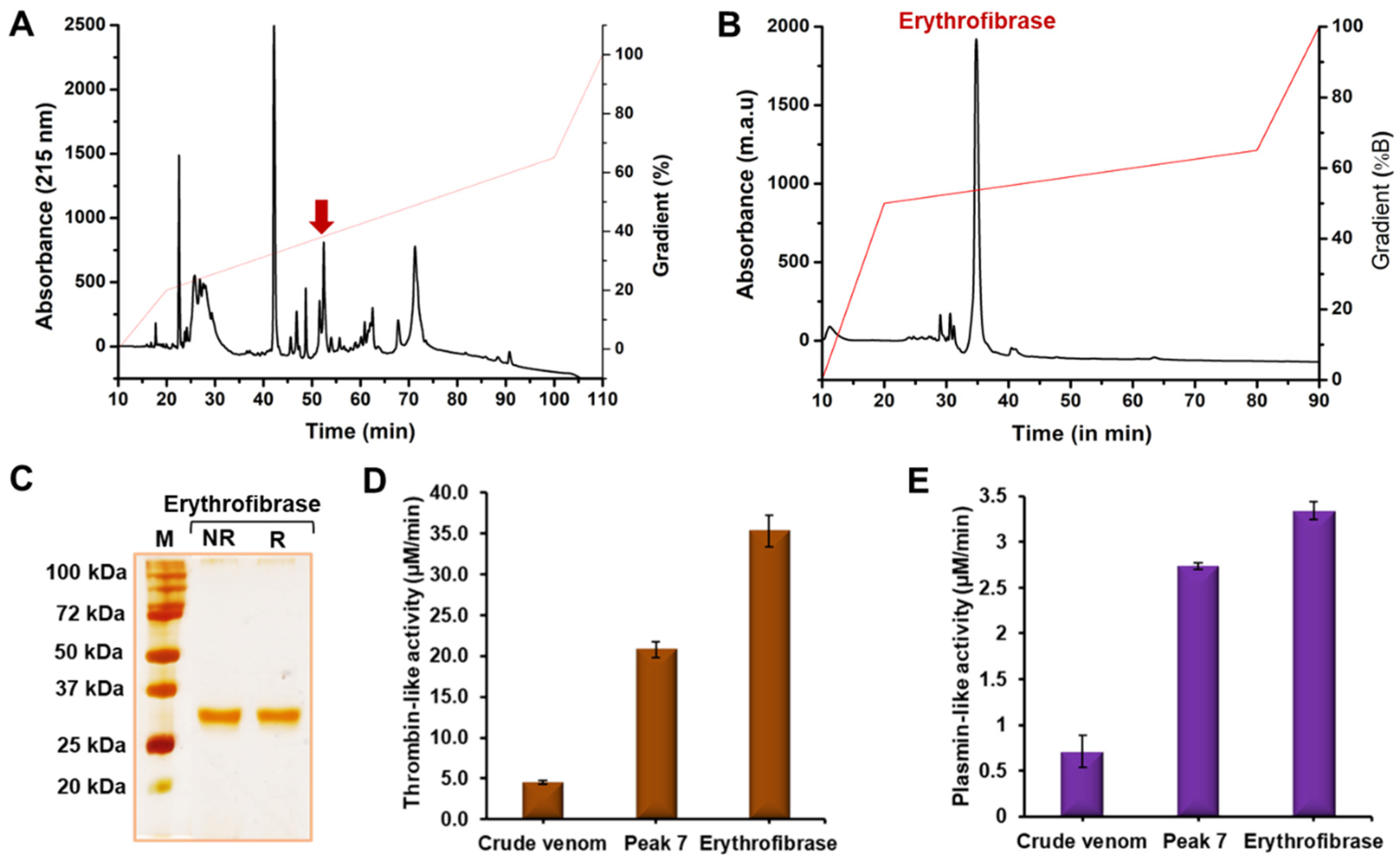
2.1. Purification of Erythrofibrase from Trimeresurus erythrurus Venom
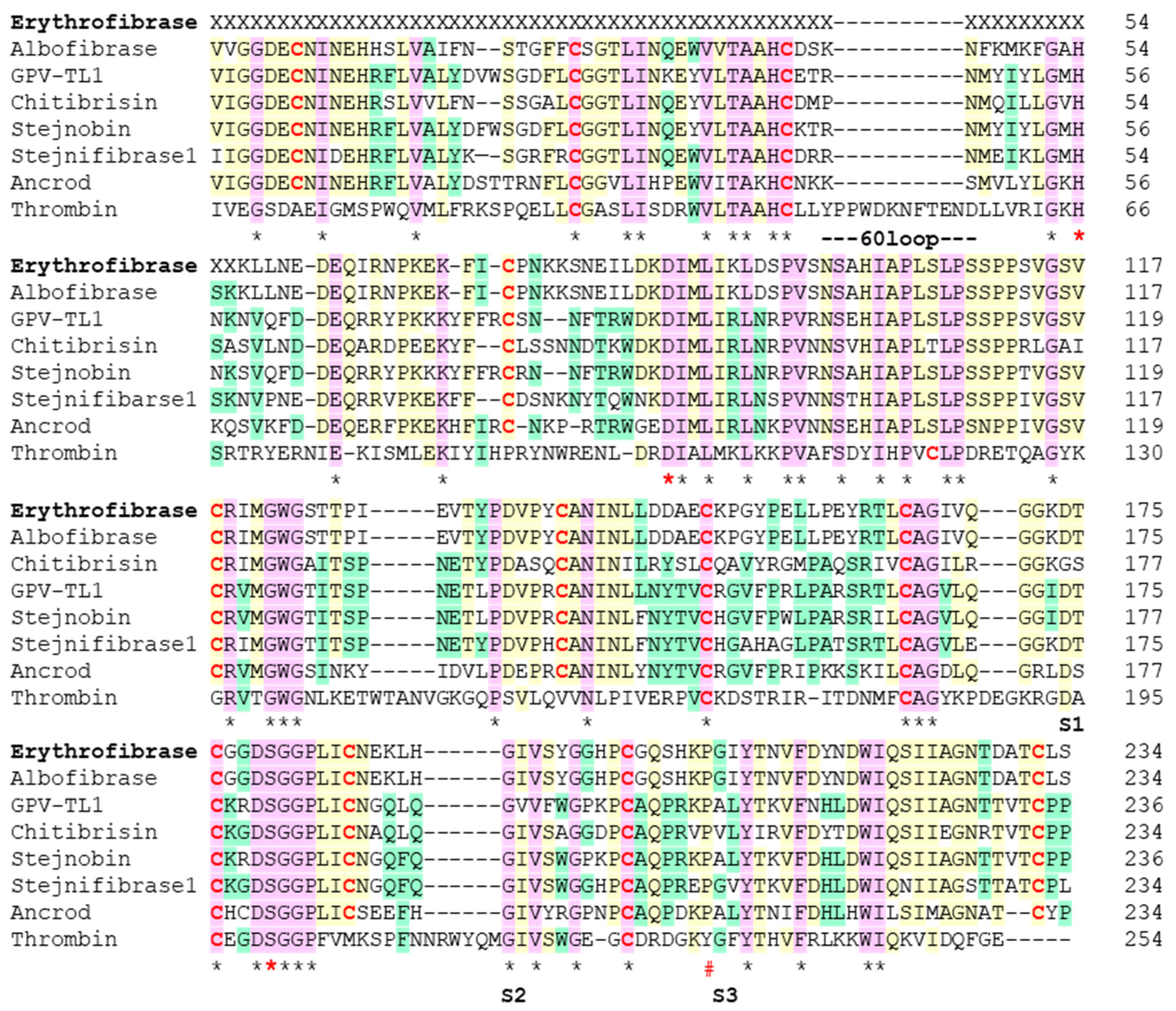
2.2. Sequence Alignment of Erythrofibrase
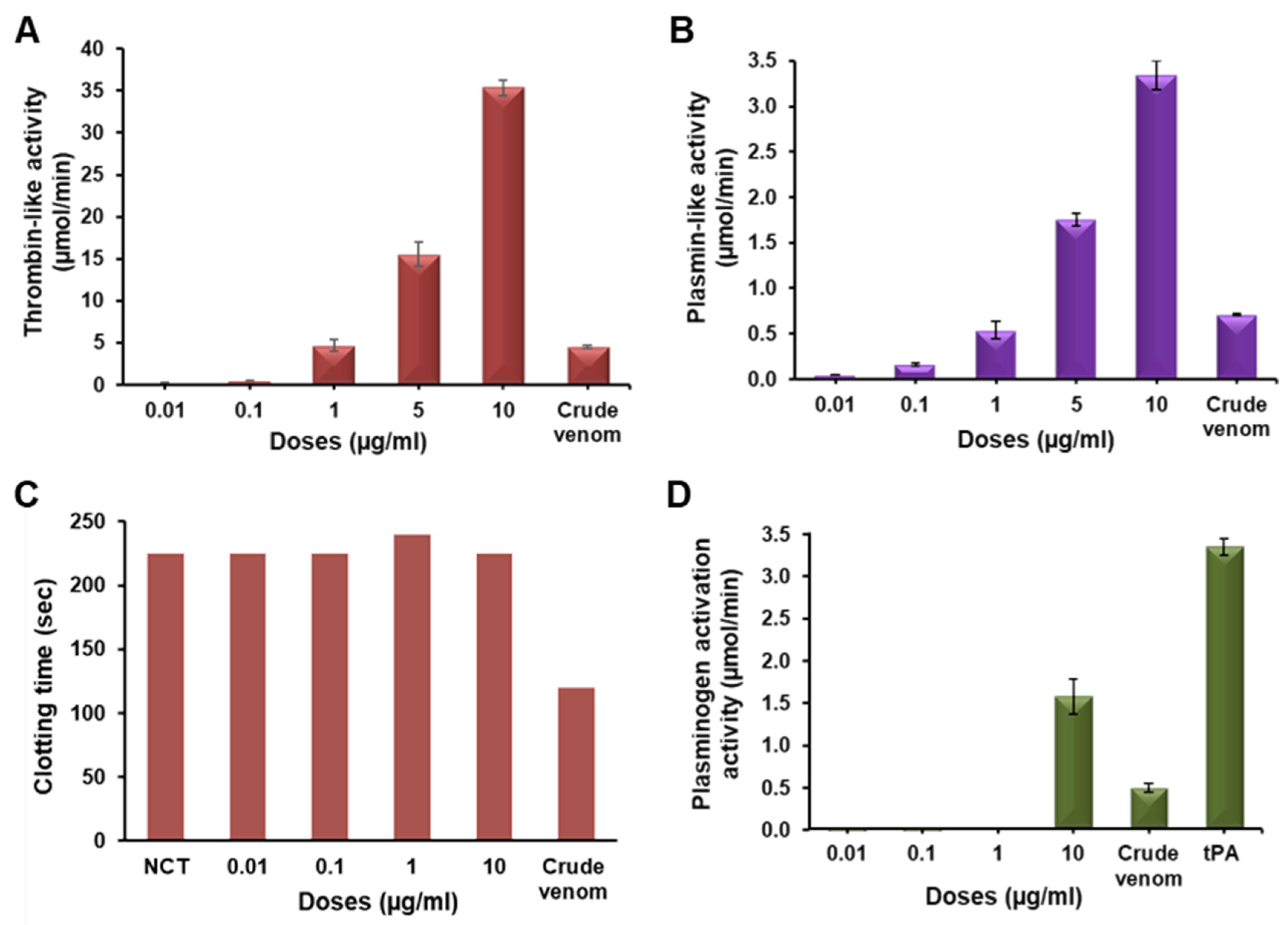
2.3. Functional Characterization of Erythrofibrase
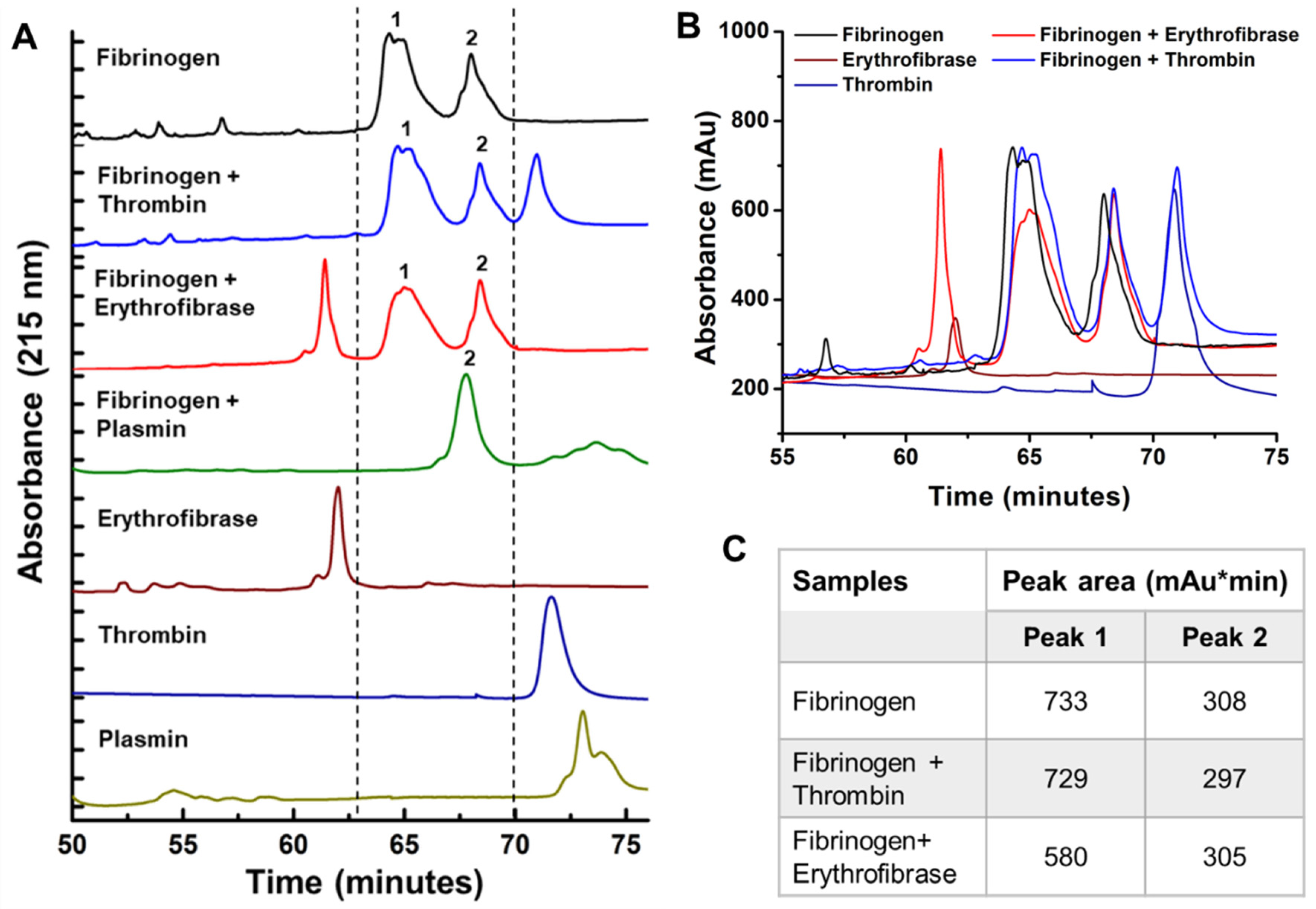
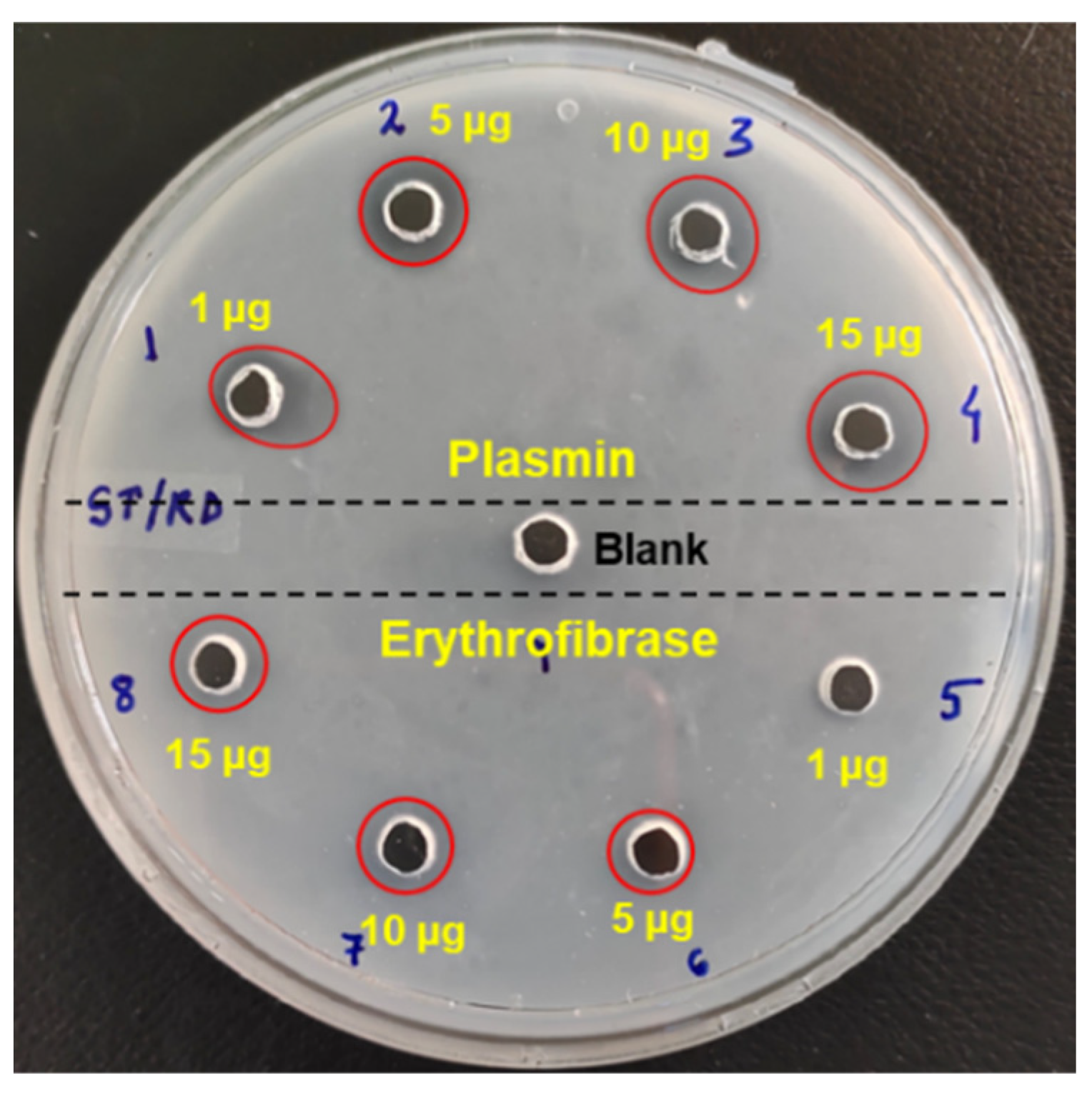
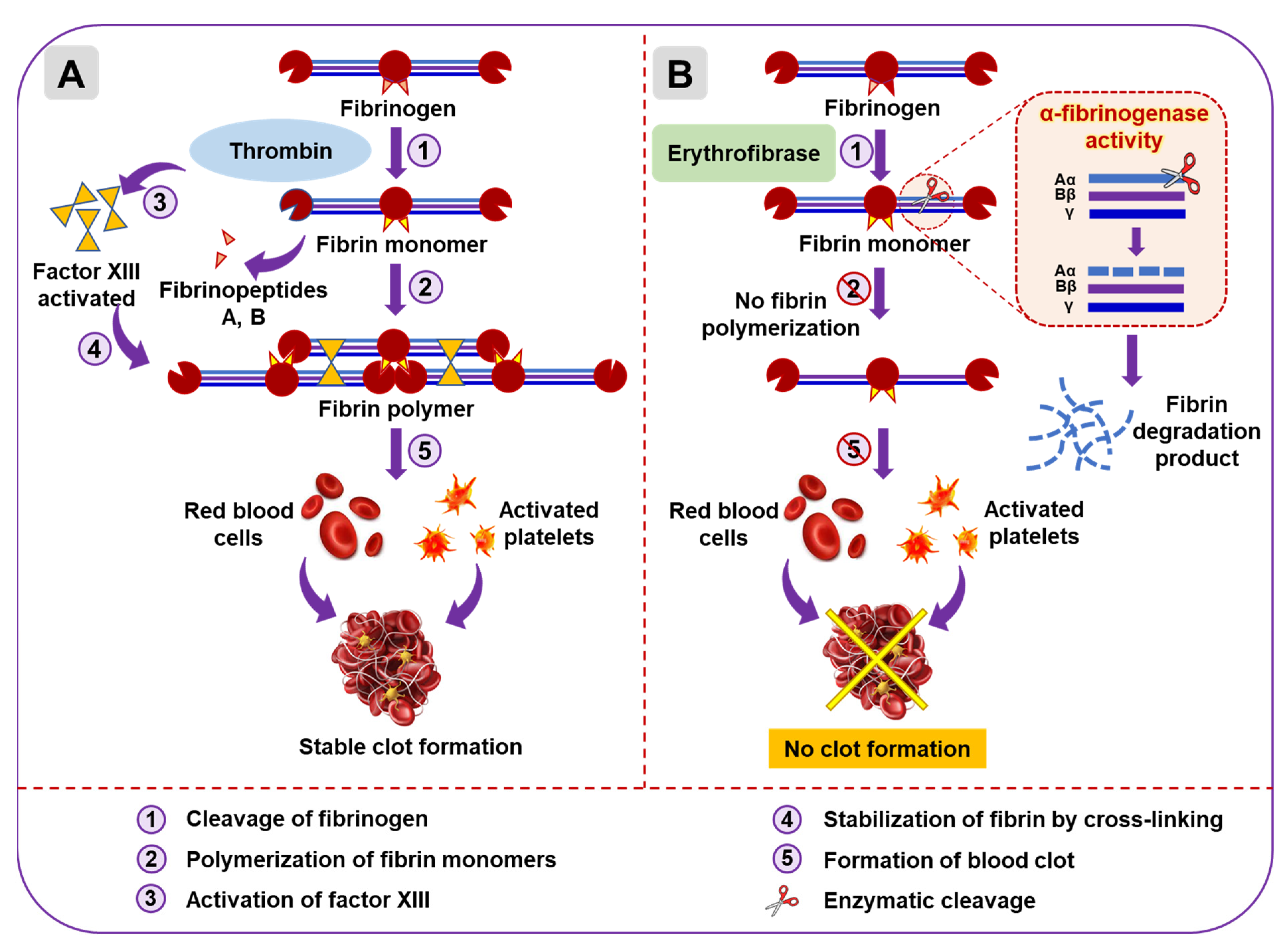
2.4. Effect of Erythrofibrase on Fibrinogenolytic and Fibrinolytic System
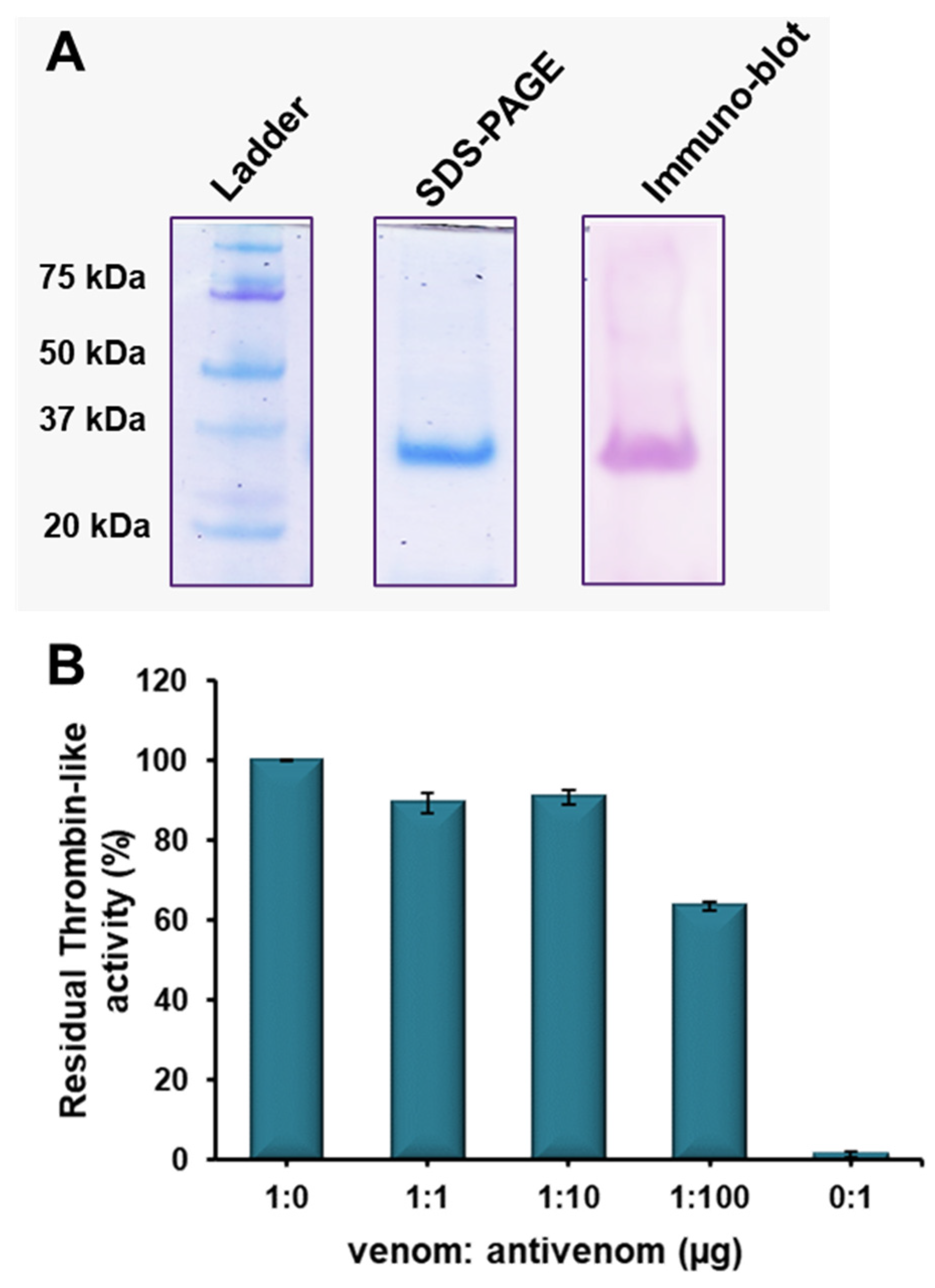
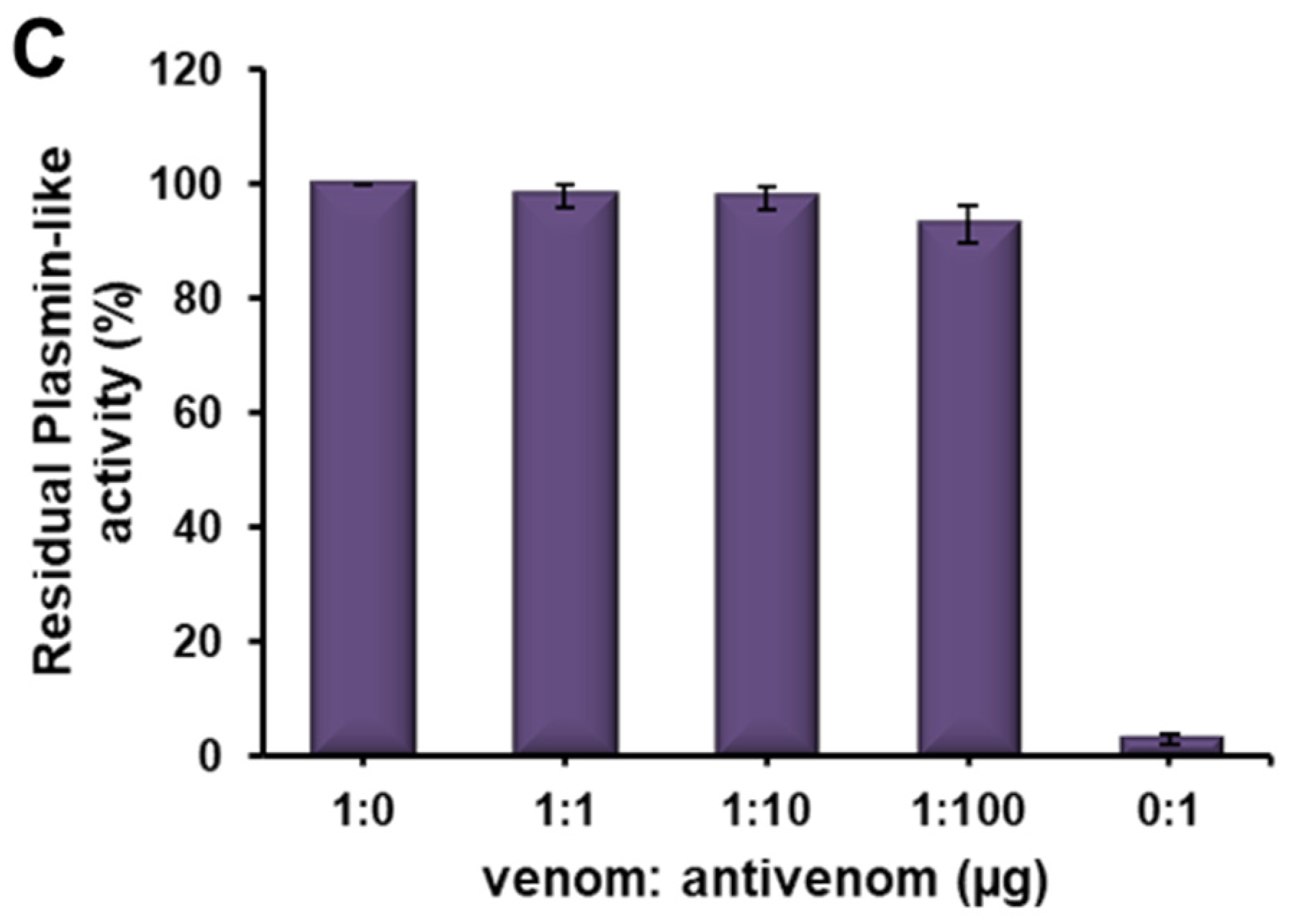
2.5. Immuno-Reactivity of Erythrofibrase with Antivenom
3. Discussion
4. Conclusions
5. Materials and Methodology
5.1. Venom and Antivenom
5.2. Purification of Erythrofibrase
5.3. LC-MS/MS of Purified Protein
5.4. Multiple Sequence Alignment
5.5. Functional Characterization of Erythrofibrase
5.5.1. Amidolytic Activity Assay
5.5.2. Clotting Assay
5.5.3. Plasminogen Activation Assay
5.5.4. Fibrinogenolytic Activity
5.5.5. Fibrinolytic Activity
5.5.6. Plasma Clot Formation/Dissolution Activity
5.6. Immuno-Reactivity of Erythrofibrase with Antivenom
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaidi, A.; Green, L. Physiology of haemostasis. Anaesth. Intensive Care Med. 2019, 20, 152–158. [Google Scholar] [CrossRef]
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant proteins from snake venoms. Pathophysiol. Haemost. Thromb. 2001, 31, 218–224. [Google Scholar] [CrossRef]
- Ullah, A.; Masood, R.; Ali, I.; Ullah, K.; Ali, H.; Akbar, H.; Betzel, C. Thrombin-like enzymes from snake venom: Structural characterization and mechanism of action. Int. J. Biol. Macromol. 2018, 114, 788–811. [Google Scholar] [CrossRef]
- Pradniwat, P.; Rojnuckarin, P. Snake venom thrombin-like enzymes. Toxin Rev. 2014, 33, 16–22. [Google Scholar] [CrossRef]
- You, W.-K.; Choi, W.-S.; Koh, Y.-S.; Shin, H.-C.; Jang, Y.; Chung, K.-H. Functional characterization of recombinant batroxobin, a snake venom thrombin-like enzyme, expressed from Pichia pastoris. FEBS Lett. 2004, 571, 67–73. [Google Scholar] [CrossRef]
- Sweetnam, P.; Thomas, H.; Yarnell, J.; Beswick, A.; Baker, I.; Elwood, P.C. Fibrinogen, viscosity and the 10-year incidence of ischaemic heart disease: The Caerphilly and Speedwell Studies. Eur. Heart J. 1996, 17, 1814–1820. [Google Scholar] [CrossRef]
- Sherman, D.G. Ancrod. Curr. Med. Res. Opin. 2002, 18, S48. [Google Scholar]
- Kakati, H.; Giri, S.; Patra, A.; Taye, S.J.; Agarwalla, D.; Boruah, H.; Choudhary, G.; Kalita, B.; Mukherjee, A.K. A retrospective analysis of epidemiology, clinical features of envenomation, and in-patient management of snakebites in a model secondary hospital of Assam, North-east India. Toxicon 2023, 230, 107175. [Google Scholar] [CrossRef]
- Blessmann, J.; Nguyen, T.P.N.; Bui, T.P.A.; Krumkamp, R.; Nguyen, H.L. Incidence of snakebites in 3 different geographic regions in Thua Thien Hue province, central Vietnam: Green pit vipers and cobras cause the majority of bites. Toxicon 2018, 156, 61–65. [Google Scholar] [CrossRef]
- Thein, M.M.; Rogers, C.A.; White, J.; Mahmood, M.A.; Weinstein, S.A.; Nwe, M.T.; Thwin, K.T.; Zaw, A.; Thant, M.; Oo, S.S.L. Characteristics and significance of “green snake” bites in Myanmar, especially by the pit vipers Trimeresurus albolabris and Trimeresurus erythrurus. Toxicon 2021, 203, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rathnayaka, R.N.; Ranathunga, P.; Kularatne, S. Epidemiology and clinical features of Green pit viper (Trimeresurus trigonocephalus) envenoming in Sri Lanka. Toxicon 2017, 137, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Sarmin, S.; Amin, M.R.; Al-Mamun, H.; Rahman, R.; Faiz, M.A. Clinical aspects of green pit viper bites in Bangladesh: A study on 40 patients. Asia Pac. J. Med. Toxicol. 2013, 2, 96–100. [Google Scholar]
- Thakur, S.; Malhotra, A.; Giri, S.; Lalremsanga, H.; Bharti, O.K.; Santra, V.; Martin, G.; Doley, R. Venom of several Indian green pit vipers: Comparison of biochemical activities and cross-reactivity with antivenoms. Toxicon 2022, 210, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Das, D.; Iyer, J.K.; Kini, R.M.; Doley, R. Unveiling the complexities of Daboia russelii venom, a medically important snake of India, by tandem mass spectrometry. Toxicon 2015, 107, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Mackessy, S.P.; Mukherjee, A.K. Proteomic analysis reveals geographic variation in venom composition of Russell’s Viper in the Indian subcontinent: Implications for clinical manifestations post-envenomation and antivenom treatment. Expert Rev. Proteom. 2018, 15, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Blotra, A.; Vasudevan, K.; Malhotra, A.; Lalremsanga, H.T.; Santra, V.; Doley, R. Proteome decomplexation of Trimeresurus erythrurus venom from Mizoram, India. J. Proteome Res. 2022, 22, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Rojnuckarin, P.; Intragumtornchai, T.; Sattapiboon, R.; Muanpasitporn, C.; Pakmanee, N.; Khow, O.; Swasdikul, D. The effects of green pit viper (Trimeresurus albolabris and Trimeresurus macrops) venom on the fibrinolytic system in human. Toxicon 1999, 37, 743–755. [Google Scholar] [CrossRef]
- Rojnuckarin, P.; Muanpasitporn, C.; Chanhome, L.; Arpijuntarangkoon, J.; Intragumtornchai, T. Molecular cloning of novel serine proteases and phospholipases A2 from green pit viper (Trimeresurus albolabris) venom gland cDNA library. Toxicon 2006, 47, 279–287. [Google Scholar] [CrossRef]
- Muanpasitporn, C.; Rojnuckarin, P. Expression and characterization of a recombinant fibrinogenolytic serine protease from green pit viper (Trimeresurus albolabris) venom. Toxicon 2007, 49, 1083–1089. [Google Scholar] [CrossRef]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef] [PubMed]
- Al-Amer, O.M. The role of thrombin in haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Batty, P.; Smith, J.G. Haemostasis. Surgery 2010, 28, 530–535. [Google Scholar] [CrossRef]
- Castro, H.; Zingali, R.; Albuquerque, M.; Pujol-Luz, M.; Rodrigues, C. Snake venom thrombin-like enzymes: From reptilase to now. Cell. Mol. Life Sci. CMLS 2004, 61, 843–856. [Google Scholar] [CrossRef] [PubMed]
- McCleary, R.J.; Kini, R.M. Snake bites and hemostasis/thrombosis. Thromb. Res. 2013, 132, 642–646. [Google Scholar] [CrossRef]
- Tan, N.H. Isolation and characterization of the thrombin-like enzyme from Cryptelytrops purpureomaculatus venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Fung, S.Y.; Yap, Y.H.Y. Isolation and characterization of the thrombin-like enzyme from Cryptelytrops albolabris (white-lipped tree viper) venom. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 161, 79–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, R.; Lee, W.-H.; Zhu, S.-W.; Xiong, Y.-L.; Wang, W.-Y. Characterization of a fibrinogen-clotting enzyme from Trimeresurus stejnegeri venom, and comparative study with other venom proteases. Toxicon 1998, 36, 131–142. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, Y.; Meng, Q.-X.; Lee, W.-H.; Li, D.-S.; Xiong, Y.-L.; Wang, W.-Y. Characterization of three fibrinogenolytic enzymes from Chinese green tree viper (Trimeresurus stejnegeri) venom. Toxicon 1998, 36, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Hwang, L.-J.; Huang, T.-F. α-Fibrinogenase from Agkistrodon rhodostoma (Malayan pit viper) snake venom. Toxicon 1983, 21, 25–33. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, X.; He, Q.; Li, H.; Li, D.; Song, X.; Wang, Y.; Wen, H.; Deng, H.; Deng, J. Expression and functional characterization of chitribrisin, a thrombin-like enzyme, in the venom of the Chinese green pit viper (Trimeresurus albolabris). Protein Expr. Purif. 2009, 67, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Pradniwat, P.; Rojnuckarin, P. The GPV-TL1, a snake venom thrombin-like enzyme (TLE) from a green pit viper (Trimeresurus albolabris), shows a strong fibrinogenolytic activity. J. Chem. Pharm. Res 2014, 6, 275–283. [Google Scholar]
- He, Q.; Li, H.; Zhou, B.; Wen, H.; Li, J.; Xiao, B.; Zhang, K.; Hodgson, W.C.; Yu, X. TA-2, a thrombin-like enzyme from the Chinese white-lipped green pitviper (Trimeresurus albolabris): Isolation, biochemical and biological characterization. Blood Coagul. Fibrinolysis 2012, 23, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.; Gowda, T.V. Detection, using antibodies, of pharmacologically active sites, apart from the catalytic site, on venom phospholipase A2. Toxicon 1990, 28, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Jenkins, T.P.; Davidsen, K.; Krause, K.E.; Martos-Esteban, A.; Engmark, M.; Rørdam Andersen, M.; Lund, O.; Laustsen, A.H. Antibody cross-reactivity in antivenom research. Toxins 2018, 10, 393. [Google Scholar] [CrossRef]
- Vivas-Ruiz, D.E.; Sandoval, G.A.; Mendoza, J.; Inga, R.R.; Gontijo, S.; Richardson, M.; Eble, J.A.; Yarleque, A.; Sanchez, E.F. Coagulant thrombin-like enzyme (barnettobin) from Bothrops barnetti venom: Molecular sequence analysis of its cDNA and biochemical properties. Biochimie 2013, 95, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Maroun, R.C. Snake venom serine proteinases: Sequence homology vs. substrate specificity, a paradox to be solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef]
- Ullah, A.; Souza, T.d.A.C.B.d.; Zanphorlin, L.M.; Mariutti, R.B.; Santana, V.; Murakami, M.T.; Arni, R. Crystal structure of Jararacussin-I: The highly negatively charged catalytic interface contributes to macromolecular selectivity in snake venom thrombin-like enzymes. Protein Sci. 2013, 22, 128–132. [Google Scholar] [CrossRef]
- Kinter, M.; Sherman, N.E. Protein Sequencing and Identification Using Tandem Mass Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 9. [Google Scholar]
- Deka, A.; Sharma, M.; Sharma, M.; Mukhopadhyay, R.; Doley, R. Purification and partial characterization of an anticoagulant PLA2 from the venom of Indian Daboia russelii that induces inflammation through upregulation of proinflammatory mediators. J. Biochem. Mol. Toxicol. 2017, 31, e21945. [Google Scholar] [CrossRef]
- Park, D.; Kim, H.; Chung, K.; Kim, D.-S.; Yun, Y. Expression and characterization of a novel plasminogen activator from Agkistrodon halys venom. Toxicon 1998, 36, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Astrup, T.; Stefsdorff, I. Fibrinolysokinase Activity in Animal and Human Tissue 1. Acta Physiol. Scand. 1956, 37, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Devi, A.; Saikia, B.; Doley, R. Expression and characterization of Flavikunin: A Kunitz-type serine protease inhibitor identified in the venom gland cDNA library of Bungarus flaviceps. J. Biochem. Mol. Toxicol. 2019, 33, e22273. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Sanz, L.; Flores-Díaz, M.; Figueroa, L.; Madrigal, M.; Herrera, M.a.; Villalta, M.; León, G.; Estrada, R.; Borges, A. Impact of regional variation in Bothrops asper snake venom on the design of antivenoms: Integrating antivenomics and neutralization approaches. J. Proteome Res. 2009, 9, 564–577. [Google Scholar] [CrossRef] [PubMed]

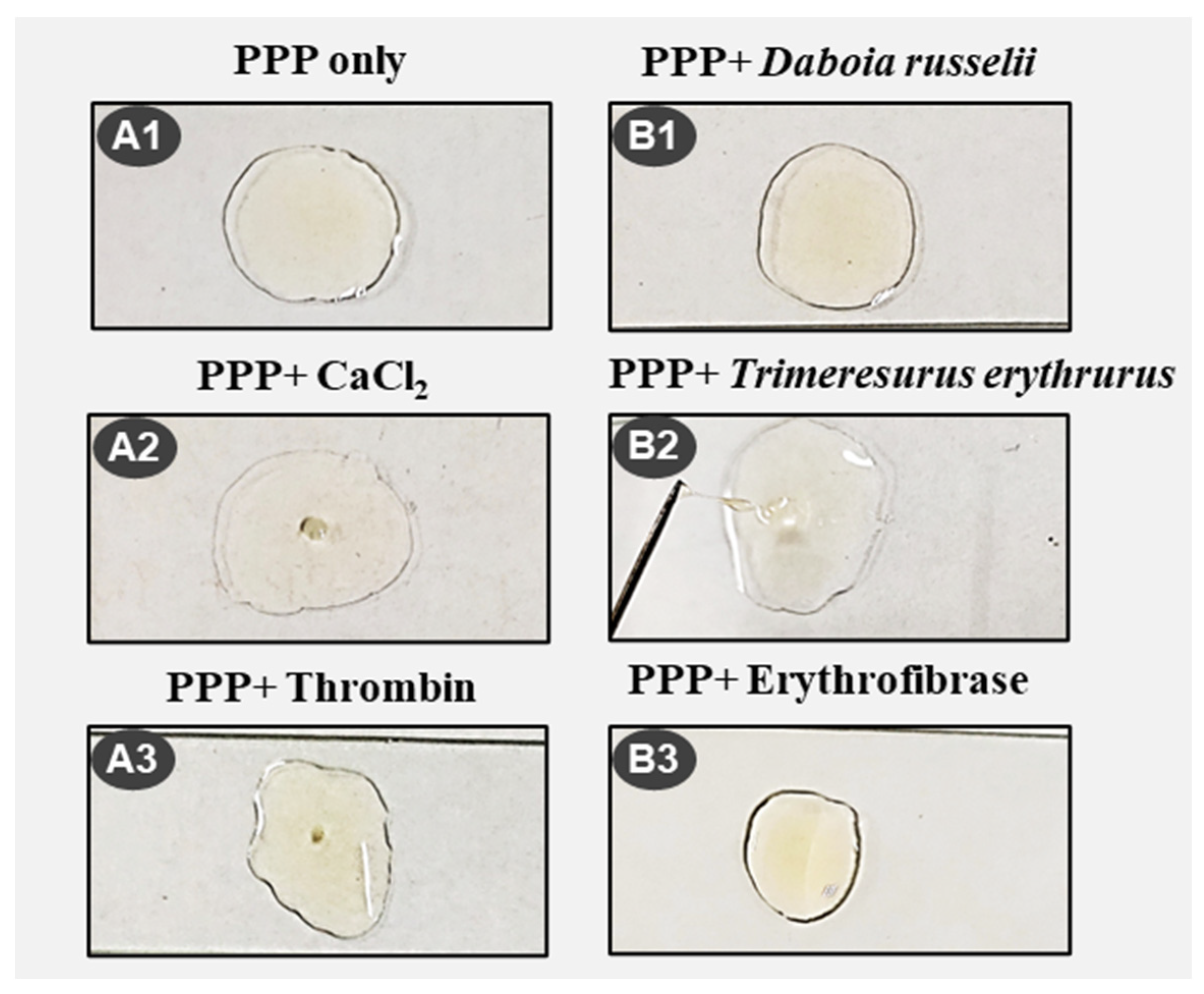
| Protein Sample | Treatment | Clotting Time | Clot Dissolution Time | Nature of Clot | Image |
|---|---|---|---|---|---|
| - | CaCl2 | 7 min | - | Thrombus | A2 |
| Daboia russelii | - | No clot | - | No clot | B1 |
| Daboia russelii | CaCl2 | 3 min | - | Thrombus | |
| Trimeresurus erythrurus | - | 25 s | 30 s | Continuous formation and dissolution of jelly like clot | B2 |
| 7 min | 15 min | ||||
| 19 min | 20 min | ||||
| 23 min | 24.5 min | ||||
| Trimeresurus erythrurus | CaCl2 | 7 min | - | Thrombus | |
| Erythrofibrase | - | 25 s | 30 s | Thin jelly like clot | B3 |
| - | Thrombin | 3 min | - | Small stable clot | A3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, S.; Yasmin, R.; Malhotra, A.; Lalremsanga, H.T.; Santra, V.; Giri, S.; Doley, R. Isolation and Functional Characterization of Erythrofibrase: An Alfa-Fibrinogenase Enzyme from Trimeresurus erythrurus Venom of North-East India. Toxins 2024, 16, 201. https://doi.org/10.3390/toxins16040201
Thakur S, Yasmin R, Malhotra A, Lalremsanga HT, Santra V, Giri S, Doley R. Isolation and Functional Characterization of Erythrofibrase: An Alfa-Fibrinogenase Enzyme from Trimeresurus erythrurus Venom of North-East India. Toxins. 2024; 16(4):201. https://doi.org/10.3390/toxins16040201
Chicago/Turabian StyleThakur, Susmita, Rafika Yasmin, Anita Malhotra, Hmar Tlawmte Lalremsanga, Vishal Santra, Surajit Giri, and Robin Doley. 2024. "Isolation and Functional Characterization of Erythrofibrase: An Alfa-Fibrinogenase Enzyme from Trimeresurus erythrurus Venom of North-East India" Toxins 16, no. 4: 201. https://doi.org/10.3390/toxins16040201