Potential Degradation of Swainsonine by Intracellular Enzymes of Arthrobacter sp. HW08
Abstract
:1. Introduction
2. Results and Discussion
2.1. Degrading SW by IEHW08


2.2. Metabolites of SW Degraded by IEHW08 Was Safe to Mice
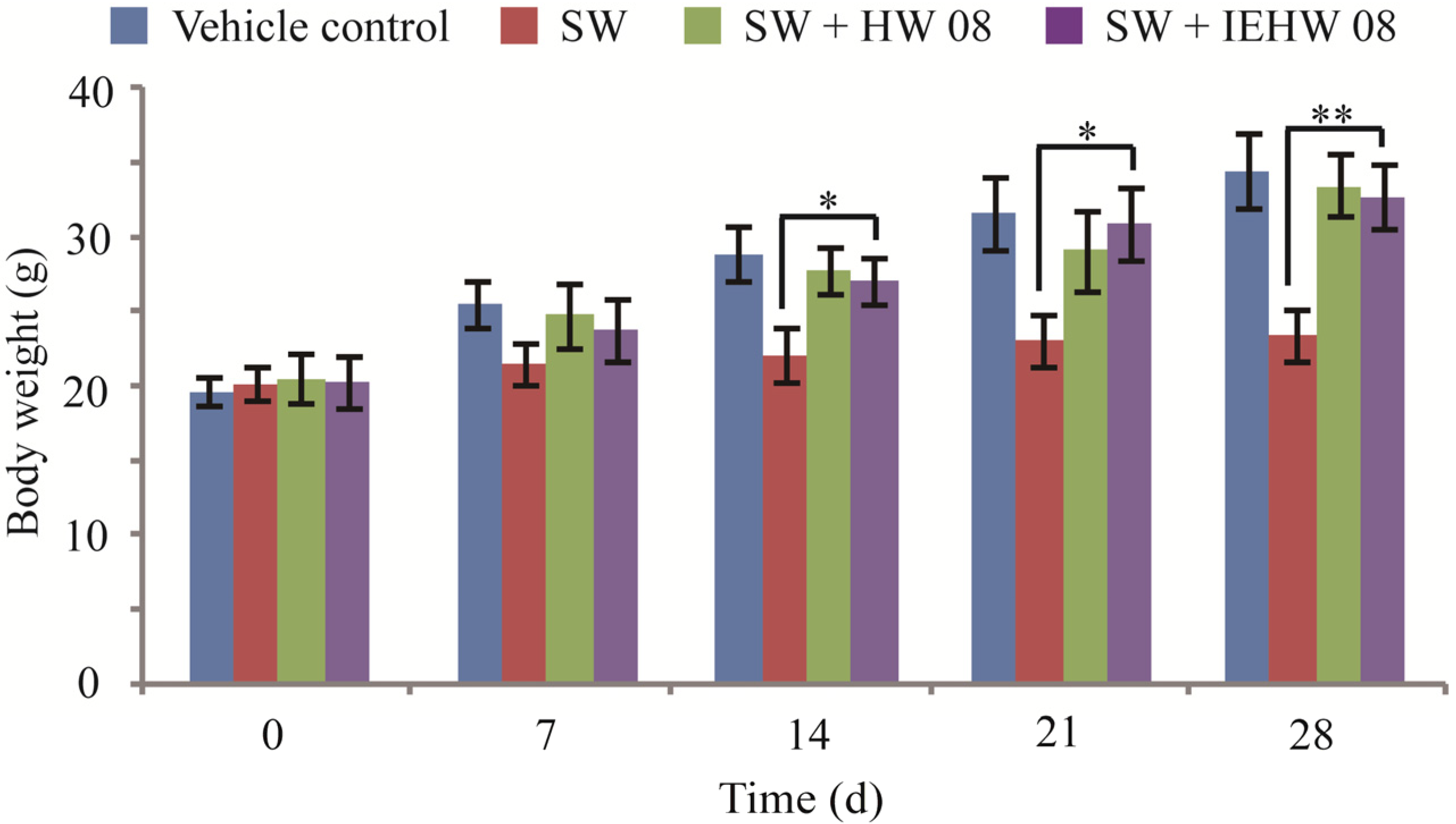

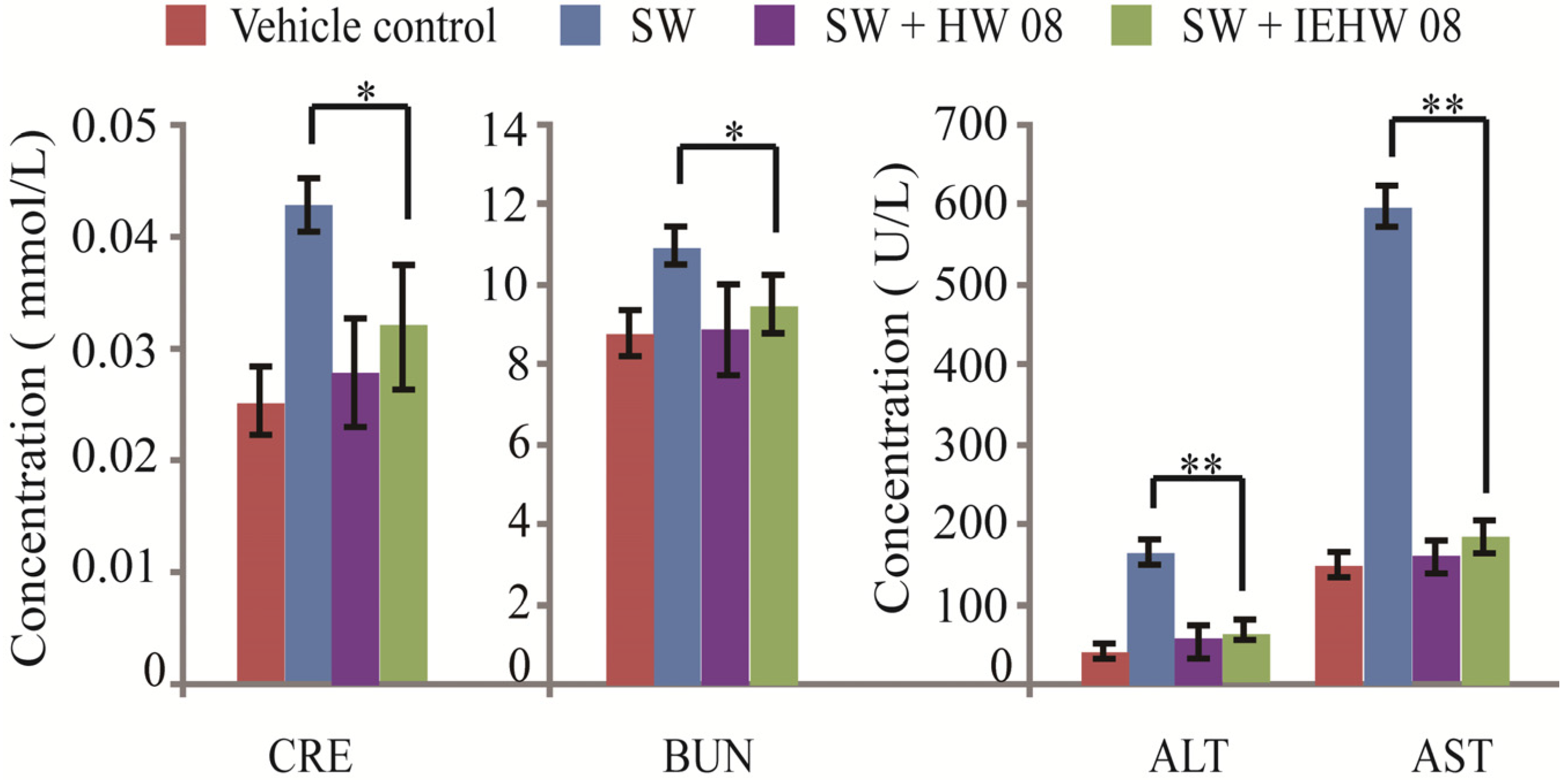



2.3. Discussion
3. Experimental Section
3.1. Preparation of HW08 Cell-Free Extract and SW
3.2. Enzyme Activity
3.3. Animal Experiment
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cook, D.; Shi, L.; Gardner, D.R.; Pfister, J.A.; Grum, D.; Welch, K.D.; Ralphs, M.H. Influence of phenological stage on swainsonine and endophyte concentrations in Oxytropis sericea. J. Chem. Ecol. 2012, 38, 195–203. [Google Scholar] [CrossRef]
- Ralphs, M.H.; Welsh, S.L.; Gardner, D.R. Distribution of locoweed toxin swainsonine in populations of Oxytropis lambertii. J. Chem. Ecol. 2002, 28, 701–707. [Google Scholar] [CrossRef]
- Ashley, A.K.; Custis, M.; Ashley, R.; Strickland, J.R. Toxicokinetic profile of swainsonine following exposure to locoweed (Oxytropis sericea) in naive and previously exposed sheep. N. Z. Vet. J. 2006, 54, 34–40. [Google Scholar] [CrossRef]
- Pfister, J.A.; Stegelmeier, B.L.; Cheney, C.D.; Ralphs, M.H.; Gardner, D.R. Conditioning taste aversions to locoweed (Oxytropis sericea) in horses. J. Anim. Sci. 2002, 80, 79–83. [Google Scholar]
- Taylor, J.B.; Strickland, J.R. Appearance and disappearance of swainsonine in serum and milk of lactating ruminants with nursing young following a single dose exposure to swainsonine (locoweed; Oxytropis sericea). J. Anim. Sci. 2002, 80, 2476–2484. [Google Scholar]
- Bachman, S.E.; Galyean, M.L.; Smith, G.S.; Hallford, D.M.; Graham, J.D. Early aspects of locoweed toxicosis and evaluation of a mineral supplement or clinoptilolite as dietary treatments. J. Anim. Sci. 1992, 70, 3125–3132. [Google Scholar]
- Dugarte-Stavanja, M.; Smith, G.S.; Edrington, T.S.; Hallford, D.M. Failure of dietary bentonite clay, Silent Herder mineral supplement, or parenteral Banamine to alleviate locoweed toxicosis in rats. J. Anim. Sci. 1997, 75, 1867–1875. [Google Scholar]
- Pfister, J.A.; Stegelmeier, B.L.; Cheney, C.D.; Gardner, D.R. Effect of previous locoweed (Astragalus and Oxytropis species) intoxication on conditioned taste aversions in horses and sheep. J. Anim. Sci. 2007, 85, 1836–1841. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Q.; Wang, J.; Wang, Y.; Song, Y.; Geng, G.; Li, Q. Swainsonine-producing fungal endophytes from major locoweed species in China. Toxicon 2010, 56, 330–338. [Google Scholar] [CrossRef]
- Zhao, X.H.; He, X.; Wang, J.N.; Song, Y.M.; Geng, G.X.; Wang, J.H. Biodegradation of Swainsonine by Acinetobacter calcoaceticus strain YLZZ-1 and its isolation and identification. Biodegradation 2009, 20, 331–338. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.C.; Wang, J.H.; Yu, Y.T.; Song, Y.M.; Yang, G.D.; Geng, G.X. Isolation and characterization of Arthrobacter sp. HW08 capable of biodegrading swainsonine. Afr. J. Microbiol. Res. 2011, 4, 1635–1638. [Google Scholar]
- Hu, Y.C.; Wang, Y.; Wang, J.N.; Yang, G.D.; Li, H.L.; Geng, G.X.; Wang, J.H. Biodegradation of swainsonine by five types of plasmid-transformants from genomic library of Arthrobacter sp. HW08. Afr. J. Microbiol. Res. 2011, 5, 1673–1681. [Google Scholar]
- Armien, A.G.; Tokarnia, C.H.; Peixoto, P.V.; Frese, K. Spontaneous and experimental glycoprotein storage disease of goats induced by Ipomoea carnea subsp fistulosa (Convolvulaceae). Vet. Pathol. 2007, 44, 170–184. [Google Scholar] [CrossRef]
- Li, Q.F.; Hao, C.J.; Xu, Y.P.; Liang, J.; Yang, K.; Cui, Z.H. Identification of a new locoweed (Oxytropis serioopetala) and its clinical and pathological features in poisoned rabbits. J. Vet. Med. Sci. 2012, 74, 989–993. [Google Scholar] [CrossRef]
- Gotardo, A.T.; Schumaher, B.H.; Pfister, J.A.; Traldi, A.S.; Maiorka, P.C.; Spinosa, H.S.; Gorniak, S.L. The use of ultrasonography to study teratogenicity in ruminants: Evaluation of Ipomoea carnea in goats. Birth Defects Res. B 2012, 95, 289–295. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; James, L.F.; Panter, K.E.; Gardner, D.R.; Pfister, J.A.; Ralphs, M.H.; Molyneux, R.J. Dose response of sheep poisoned with locoweed (Oxytropis sericea). J. Vet. Diagn. Invest. 1999, 11, 448–456. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Molyneux, R.J.; Asano, N.; Watson, A.A.; Nash, R.J. The comparative pathology of the glycosidase inhibitors swainsonine, castanospermine, and calystegines A3, B2, and C1 in mice. Toxicol. Pathol. 2008, 36, 651–659. [Google Scholar] [CrossRef]
- Ralphs, M.H.; Panter, K.E.; James, L.F. Feed preferences and habituation of sheep poisoned by locoweed. J. Anim. Sci. 1990, 68, 1354–1362. [Google Scholar]
- Stegelmeier, B.L.; James, L.F.; Gardner, D.R.; Panter, K.E.; Lee, S.T.; Ralphs, M.H.; Pfister, J.A.; Spraker, T.R. Locoweed (Oxytropis sericea)—Induced lesions in mule deer (Odocoileius hemionus). Vet. Pathol. 2005, 42, 566–578. [Google Scholar] [CrossRef]
- Srilatha, C.H.; Gopal Naidu, N.; Rama Rao, P. Pathology of Ipomoea carnea toxicity in goats. Indian J. Anim. Sci. 1997, 7, 253–254. [Google Scholar]
- Dantas, A.F.; Riet-Correa, F.; Gardner, D.R.; Medeiros, R.M.; Barros, S.S.; Anjos, B.L.; Lucena, R.B. Swainsonine-induced lysosomal storage disease in goats caused by the ingestion of Turbina cordata in Northeastern Brazil. Toxicon 2007, 49, 111–116. [Google Scholar] [CrossRef]
- Schumaher-Henrique, B.; Gorniak, S.L.; Dagli, M.L.; Spinosa, H.S. The clinical, biochemical, haematological and pathological effects of long-term administration of Ipomoea carnea to growing goats. Vet. Res. Commun. 2003, 27, 311–319. [Google Scholar] [CrossRef]
- Cholich, L.A.; Gimeno, E.J.; Teibler, P.G.; Jorge, N.L.; Acosta de Perez, O.C. The guinea pig as an animal model for Ipomoea carnea induced alpha-mannosidosis. Toxicon 2009, 54, 276–282. [Google Scholar] [CrossRef]
- De Balogh, K.K.; Dimande, A.P.; Van der Lugt, J.J.; Molyneux, R.J.; Naude, T.W.; Welman, W.G. A lysosomal storage disease induced by Ipomoea carnea in goats in Mozambique. J. Vet. Diagn. Invest. 1999, 11, 266–273. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.C.; Wang, J.H.; Liu, Z.B.; Yang, G.D.; Geng, G.X. Ultrasound-assisted solvent extraction of swainsonine from Oxytropis ochrocephala Bunge. J. Med. Plants Res. 2011, 5, 890–894. [Google Scholar]
- Zhao, B.Y.; Liu, Z.Y.; Wang, J.J.; Sun, L.S.; Wang, Z.X.; Wang, Y.C. Isolation and NMR study on swainsonine from locoweed, Astragalus strictus. Agr. Sci. China 2009, 8, 115–120. [Google Scholar] [CrossRef]
- Yang, G.D.; Kang, D.J.; Li, Y.H.; Li, J.C.; Wang, Y.; Kong, X.Y.; Li, Q.F.; Wang, J.H. Swainsonine accumulation by endophytic Undifilum fungi in liquid media and determined by means of a modified enzymatic assay. J. Anim. Vet. Adv. 2012, 11, 3876–3881. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Li, Y.; Hu, Y.; Li, J.; Yang, G.; Kang, D.; Li, H.; Wang, J. Potential Degradation of Swainsonine by Intracellular Enzymes of Arthrobacter sp. HW08. Toxins 2013, 5, 2161-2171. https://doi.org/10.3390/toxins5112161
Wang Y, Li Y, Hu Y, Li J, Yang G, Kang D, Li H, Wang J. Potential Degradation of Swainsonine by Intracellular Enzymes of Arthrobacter sp. HW08. Toxins. 2013; 5(11):2161-2171. https://doi.org/10.3390/toxins5112161
Chicago/Turabian StyleWang, Yan, Yanhong Li, Yanchun Hu, Jincheng Li, Guodong Yang, Danju Kang, Haili Li, and Jianhua Wang. 2013. "Potential Degradation of Swainsonine by Intracellular Enzymes of Arthrobacter sp. HW08" Toxins 5, no. 11: 2161-2171. https://doi.org/10.3390/toxins5112161




