Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients
Abstract
:1. Introduction
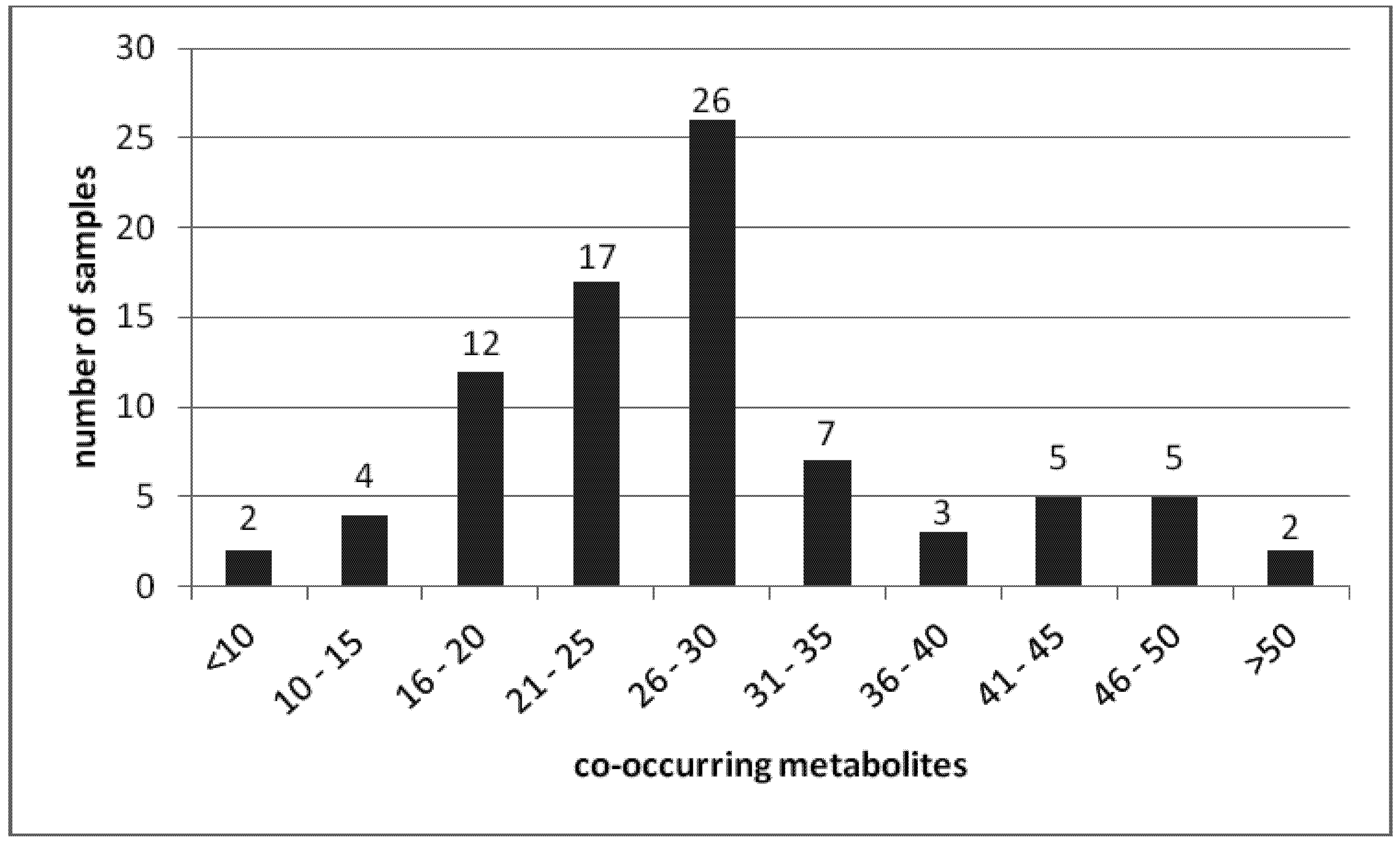
2. Results
| Mycotoxin/metabolite | n (pos) | % pos | Median (μg/kg) | Max (μg/kg) | Mycotoxin/metabolite | n (pos) | % pos | Median (μg/kg) | Max (μg/kg) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beauvericin | 81 | 98 | 6.7 | 2326 | Pestalotin | 5 | 6 | 10 | 19 | ||
| sum of Enniatins | 80 | 96 | 30 | 5441 | Tetracycline (ab) | 5 | 6 | 77 | 10,696 | ||
| Enniatin A1 | 79 | 95 | 5.5 | 2216 | Versicolorin C ** | 5 | 6 | 0.8 | 89 | ||
| Enniatin B | 76 | 92 | 11 | 780 | Amoxycillin * (ab) | 4 | 5 | NA | NA | ||
| Enniatin B1 | 76 | 92 | 14 | 2690 | Andrastin D * | 4 | 5 | NA | NA | ||
| Enniatin A | 72 | 87 | 0.8 | 1745 | Dechlorogriseofulvin | 4 | 5 | 18 | 182 | ||
| Enniatin B2 | 8 | 10 | 0.8 | 13 | Griseofulvin | 4 | 5 | 31 | 399 | ||
| Enniatin B3 | 7 | 8 | 0.01 | 0.1 | |||||||
| Deoxynivalenol | 74 | 89 | 122 | 25,928 | Linamarin (plt) | 4 | 5 | 1705 | 20,205 | ||
| Emodin | 74 | 89 | 9.8 | 1570 | Monoacetoxyscirpenol | 4 | 5 | 7.7 | 31 | ||
| Equisetin | 72 | 87 | 23 | 13,680 | Neoxaline | 4 | 5 | 3.3 | 13 | ||
| Zearalenone | 72 | 87 | 14 | 5326 | Penitrem A | 4 | 5 | 92 | 701 | ||
| Aurofusarin | 70 | 84 | 85 | 17,659 | Roquefortine C | 4 | 5 | 103 | 915 | ||
| Alternariol methyl ether | 68 | 82 | 1.4 | 733 | Secalonic acid D | 4 | 5 | 4.5 | 369 | ||
| Alternariol | 66 | 80 | 2.8 | 221 | 3-AcetylDON | 3 | 4 | 28 | 588 | ||
| Tentoxin | 66 | 80 | 3.9 | 76 | Agroclavin | 3 | 4 | 0.1 | 0.9 | ||
| Moniliformin | 63 | 76 | 45 | 12,236 | Aminodecyl octadecanol | 3 | 4 | 39 | 276 | ||
| DON-3-glucoside | 62 | 75 | 15 | 7764 | Cytochalasin J | 3 | 4 | 27 | 164 | ||
| Culmorin | 61 | 63 | 195 | 44,616 | Ergocorninine | 3 | 4 | 14 | 15 | ||
| Nivalenol | 61 | 63 | 17 | 1760 | Ergocristine | 3 | 4 | 47 | 63 | ||
| Tryptophol | 59 | 71 | 267 | 99,040 | Ergocristinin | 3 | 4 | 21 | 25 | ||
| Apicidin | 55 | 66 | 1.9 | 160 | Ergocryptine | 3 | 4 | 16 | 25 | ||
| Brevianamide F | 54 | 65 | 69 | 2043 | Ergocryptinine | 3 | 4 | 8.8 | 11 | ||
| Tenuazonic acid | 54 | 65 | 68 | 1983 | Ergosin | 3 | 4 | 27 | 52 | ||
| 15-Hydroxyculmorin | 52 | 63 | 49 | 15,620 | Ergosinin | 3 | 4 | 4.8 | 8.6 | ||
| Butenolide | 43 | 52 | 23 | 1490 | Ergotamine | 3 | 4 | 71 | 129 | ||
| ZEN-4-sulfate | 41 | 49 | 1 | 136 | Ergotaminine | 3 | 4 | 9.7 | 18 | ||
| Altertoxin-I | 35 | 42 | 1.1 | 65 | Lotaustralin (plt) | 3 | 4 | 50 | 435 | ||
| Curvularin | 29 | 35 | 14 | 484 | Ochratoxin A | 3 | 4 | 4.9 | 31 | ||
| Avenacin Y | 26 | 31 | 209 | 9948 | Paspalitrem A * | 3 | 4 | NA | NA | ||
| Macrosporin A | 26 | 31 | 3.3 | 9.1 | Rubellin D | 3 | 4 | 259 | 1188 | ||
| T-2 Toxin | 26 | 31 | 3.8 | 427 | Rubrofusarin | 3 | 4 | 2374 | 4923 | ||
| Macrosporin | 24 | 29 | 3.7 | 15 | T-2 Tetraol | 3 | 4 | 9.5 | 1655 | ||
| Monocerin | 24 | 29 | 0.9 | 2644 | sum of Aflatoxins | 2 | 2 | 0.24 a | 861 | ||
| Siccanol * | 23 | 28 | 9607 | 39850 | Aflatoxin B1 | 2 | 2 | 0.24 a | 699 | ||
| 3-Nitropropionic acid | 19 | 23 | 6 | 392 | Aflatoxin B2 | 1 | 1 | - | 63 | ||
| sum of Fumonisins | 18 | 22 | 203 | 57,667 | Aflatoxin G1 | 1 | 1 | - | 69 | ||
| Fumonisin B1 | 18 | 22 | 142 | 40,300 | Aflatoxin G2 | 1 | 1 | - | 4.4 | ||
| Fumonisin B2 | 18 | 22 | 38 | 10,372 | Aflatoxin M1 | 1 | 1 | - | 26 | ||
| Fumonisin B3 | 14 | 17 | 18 | 3859 | Cyclopiazonic acid | 2 | 2 | 37 a | 2319 | ||
| Fumonisin B4 | 7 | 8 | 112 | 3136 | Diacetoxyscirpenol | 2 | 2 | 0.04 a | 2.7 | ||
| Fusaric acid | 18 | 22 | 643 | 13,593 | Ergocornine | 2 | 2 | 19 a | 25 | ||
| HT-2 Toxin | 18 | 22 | 13 | 1910 | Ergocristam * | 2 | 2 | 17,370 a | 19230 | ||
| Chrysophanol | 17 | 20 | 8.2 | 41 | Ergocristinam * | 2 | 2 | 7181 a | 8233 | ||
| Mycophenolic acid | 17 | 20 | 40 | 21,856 | Ergovalin | 2 | 2 | 1 a | 5.4 | ||
| Averufin ** | 16 | 19 | 0.4 | 215 | Gibberellic acid | 2 | 2 | 9.7 a | 89 | ||
| β-Zearalenol | 15 | 18 | 5.1 | 174 | Methylsterigmatocystin | 2 | 2 | 0.4 a | 27 | ||
| Bikaverin * | 14 | 17 | 27510 | 51,130 | Neosolaniol | 2 | 2 | 0.7 a | 290 | ||
| Fusaproliferin | 14 | 17 | 2555 | 14,844 | Penicillic acid | 2 | 2 | 12 a | 13 | ||
| Fusarinolic acid ** | 13 | 16 | 643 | 13,965 | Radicicol | 2 | 2 | 5.3 a | 5.9 | ||
| Skyrin | 13 | 16 | 1.4 | 6853 | Terphenyllin | 2 | 2 | 16 a | 67 | ||
| Chlamydosporol | 12 | 14 | 55 | 656 | 15-AcetylDON | 1 | 1 | - | 2718 | ||
| 5-Hydroxyculmorin | 11 | 13 | 350 | 3920 | Aflatrem * | 1 | 1 | NA | NA | ||
| α-Zearalenol | 11 | 13 | 2 | 51 | Averantin | 1 | 1 | - | 20 | ||
| Ergometrinin | 11 | 13 | 0.3 | 14 | Averufanin ** | 1 | 1 | - | 29 | ||
| Kojic acid | 10 | 12 | 75 | 3172 | Calphostin C | 1 | 1 | - | 431 | ||
| Chanoclavin | 8 | 10 | 0.1 | 8.9 | Citrinin | 1 | 1 | - | 42 | ||
| Andrastin A * | 7 | 8 | 4814 | 7603 | Cycloaspeptide A | 1 | 1 | - | 4.6 | ||
| Physcion | 7 | 8 | 245 | 1162 | Cytochalasin B | 1 | 1 | - | 27 | ||
| Sterigmatocystin | 7 | 8 | 1.5 | 4.7 | Cytochalasin H | 1 | 1 | - | 77 | ||
| 15-Hydroxyculmoron | 6 | 7 | 83 | 4886 | Decalonectrin | 1 | 1 | NA | NA | ||
| Aspterric acid | 6 | 7 | 194 | 3992 | Erginie | 1 | 1 | - | 0.33 | ||
| hydrolysed FB1 | 6 | 7 | 15 | 95 | Festucalvin | 1 | 1 | - | 0.34 | ||
| Paspalin * | 6 | 7 | NA | NA | Malformin A ** | 1 | 1 | - | 8 | ||
| Sambucinol | 6 | 7 | 149 | 675 | Malformin C | 1 | 1 | - | 2.7 | ||
| Citreoviridin | 5 | 6 | 94 | 382 | Nidurufin ** | 1 | 1 | - | 9 | ||
| Ergometrin | 5 | 6 | 6.8 | 26 | Norsolorinic acid ** | 1 | 1 | - | 1.6 | ||
| Lycomycin * | 5 | 6 | NA | NA | Pyrenophorol | 1 | 1 | - | 13 | ||
| Meleagrin | 5 | 6 | 237 | 1821 | Rugulosin | 1 | 1 | - | 201,640 | ||
| Oxytetracyclin | 5 | 6 | NA | NA | T-2 Triol | 1 | 1 | - | 278 | ||
| Paxillin | 5 | 6 | 5017 | 94,860 | Versicolorin A ** | 1 | 1 | - | 27 | ||

| Mycotoxin/metabolite | Samples, concentrations in μg/kg | |||||
|---|---|---|---|---|---|---|
| 2010-1 * | 2010-2 | 2012-1 | 2012-2 | 2012-3 | 2012-4 * | |
| 15-acetyl-DON | - | - | - | - | - | 2718 |
| 15-Hydroxyculmorin | - | - | - | - | - | 4634 |
| 3-acetyl-DON | - | - | - | - | - | 588 |
| α-Zearalenol | - | - | - | - | - | 35 |
| Apicidin | 0.03 | 22 | 2 | - | - | - |
| Aurofusarin | 240 | 3380 | 1.3 | 44 | 0.9 | 17,659 |
| Avenacin Y | 1210 | - | - | - | - | - |
| Beauvericin | 1390 | 970 | 1591 | 64 | 699 | 57 |
| β-Zearalenol | - | - | - | - | - | 174 |
| Bikaverin | - | - | detected | detected | detected | detected |
| Butenolide | 167 | 1490 | 32 | 167 | - | 569 |
| Culmorin | - | - | - | - | - | 44,616 |
| Decalonectrin | - | - | - | - | - | detected |
| Deoxynivalenol | 12 | 43 | 65 | 48 | 36 | 25,928 |
| Diacetoxyscirpenol | 0.04 | 2.7 | - | - | - | - |
| DON-3-glucoside | - | 0.5 | 0.1 | - | 0.1 | 7764 |
| Enniatin A | 301 | 0.6 | - | 8.3 | - | - |
| Enniatin A1 | 1670 | 4.9 | 0.4 | 83 | 0.3 | 0.5 |
| Enniatin B | 780 | 3.9 | 0.6 | 64 | 0.3 | 0.3 |
| Enniatin B1 | 2690 | 9.8 | 1 | 166 | 0.7 | 0.8 |
| Equisetin | - | - | - | - | 4.4 | 1.5 |
| Fusaproliferin | 1970 | 46 | 14,844 | 975 | 5029 | - |
| Fusaric acid | - | - | 2146 | 1155 | 1426 | 85 |
| Fusarinolic acid | - | - | 9405 | 440 | 3960 | 1936 |
| Gibberellic acid | - | - | 9.7 | - | - | - |
| Moniliformin | 5750 | 1650 | 2809 | 244 | 1,779 | 231 |
| Monoacetoxyscirpenol | 0.64 | 6.1 | - | - | - | - |
| Nivalenol | 147 | 1760 | 2.3 | - | - | 41 |
| Pestalotin | - | - | 2.2 | 19 | 10 | - |
| Sambucinol | - | - | - | - | - | 181 |
| Siccanol | - | - | detected | detected | detected | detected |
| Skyrin | - | 10 | - | - | - | - |
| Tentoxin | - | - | 0.5 | 2.8 | - | - |
| Tenuazonic acid | - | - | 499 | 134 | - | 133 |
| Zearalenone | - | - | - | - | - | 5326 |
| ZEN-4-sulfate | - | - | - | - | - | 136 |
3. Discussion
| Metabolite | Produced by | Effects |
|---|---|---|
| Alternariol | Alternaria sp. A. alternata A. triticina A. arborescens | conflicting results regarding mutagenic activity (strongly mutagenic in Bacillus subtilis rec assay and E. coli ND 160 reverse mutation assay; weak to no mutagenic activity in Ames test) genotoxic (causes DNA strand breaks by interacting with mammalian topoisomerases); implicated as risk factor for oesophageal cancer (reviewed in [33]) chicken embryos: no mortality or teratogenic effects in chicken embryos at doses up to 1000 mg/egg [34] |
| Alternariol methyl ether | Alternaria alternata, A. triticina A. cucumerina A. dauci A. kikuchiana A. solani A. arborescens | genotoxic (causes DNA strand breaks by interacting with mammalian topoisomerase IIα) (reviewed in [33]); brain haemorrhages and bleeding in cerebral ventricles; effect on progesterone synthesis in pigs; therefore, postulated effect on reproductive performance in pig and other mammalian species [35] mice: precancerous changes in oesophageal mucosa of mice fed 100 mg/kg bw/day for 10 months, also causing weight loss, lower food consumption (less toxic effect than tenuazonic acid (25 mg/kg bw/day for 10 months)) [36] chicken embryos: no mortality or teratogenic effects in chicken embryos at doses up to 500 mg/egg; [34] |
| Apicidin | Fusarium sp. F. pallidoreseum (F. semitectum) | histone deacetylase inhibitor, broad spectrum antiprotozoal activity [37]; antiproliferative [38] and apoptotic [39] in various cancer cell lines brine shrimp larvae: LD50: 40 mg/mL; [40] rats: died with 0.05% apicidin supplementation of diet; apicidin causes body weight loss, haemorrhage in the stomach, intestines and bladder [40] |
| Aurofusarin | F. graminearum F. acuminatum F. avenaceum F. crookwellens F. culmorum F. graminearum F. poae F. sambucinum F. tricinctum | major effect on poultry are changes in egg yolk colour from yellow-orange to dark-brown [41,42]; decrease of protein and fat content in chicken meat [43] quail eggs: 26.4 mg/kg feed influenced quality of eggs—decrease in vitamins E, A, total carotenoid, lutein and zeaxanthin concentrations and significantly increased egg yolk susceptibility to lipid peroxidation [44] breeding chickens: 26.4 mg/kg feed compromised the immune system of the progeny [45] |
| Beauvericin | Beaveria bassiana F. moniliforme F. avenaceum F. subglutianans F. proliferatum F. tricinctum F. poae [46,47] | antibacterial, insecticidal, cytotoxic, (reviewed in [29,48]); ionophoric properties cause dysfunction of mitochondria [49] and induces apoptosis (reviewed in [29,48]); co-occurs with enniatin A [50]; bioactivities are different to enniatins due to 3 N-methylphenylalanyl residues [48] acute toxicity (summarised in [29]) mice: LD50: ≥10 mg/kg bw, intraperitoneal; ≥100 mg/kg bw oral low/no acute toxicity in: duckling (100 mg/kg bw, gastric intubation) turkey (2.5 mg/kg feed) broiler (12 mg/kg feed) |
| Brevianamide F | Penicillium brevicompactum Aspergillus versicolor A. fumigatus | antibacterial, [51] precursor of the fumitremorgins and the tryprostatins [51,52] also produced by Streptomyces sp. [53] |
| Butenolide | Fusarium crookwellense F. culmorum F. graminearum F. poae F. moniliformin | myocardial oxidative damage; [54] oxidative injuries in chick embryonic livers and kidneys [55]; growth retardation and differentiation inhibition in vitro in rat embryos [56]; probably implicated in fescue foot disease [57]; anti-marine-fouling compound—toxic to zebra fish embryos by inducing apoptosis [58] mice: LD50: 44 mg/kg bw, intraperitoneal; 275 mg/kg bw oral [59] steers: died after 68 and 39 mg/kg for 2 and 3 days oral dosage; with smaller dosage, development of petechial haemorrhages and acute inflammation of gastric compartments ([60] in [59]) |
| Culmorin | F. culmorum, F. graminearum F. poae F. langsethiae F. cerealis | low toxicity in several in vitro assays, Ames test negative [61], co-occurrence with DON [62] swine: some minor non-significant effects on growth and feed consumption (2 mg/kg feed) with and without DON (6 mg/kg feed) [63] |
| 15-Hydroxy-culmorin | F. graminearum F. culmorum F. poae | co-occurrence with culmorin and DON [64] no evidence of acute toxicity to mammals so far [65] |
| Emodin | A. wentii A. flavus A. ocharaceus Plants:rhubarb root | possibly genotoxic [66]; cytotoxic, antitumour, antibacterial, anti-inflammatory, immunosuppressive [30] active ingredient of various Chinese herbs, potential drug for therapy of type-2 diabetes [32] mice: induces embryonic toxicity in mouse blastocysts through apoptosis; [30] 1-day-old cockerels: LD50: 3.7 mg/kg bw [67] clinical symptoms in cockerels included loss of appetite, accumulation of faecal material with acute epidermal irritation around the cloaca, general debilitation and mortality within 5 days of ingestion; zebrafish embryos: 0.25 μg/mL negatively affected embryo survival and hatching success; toxic to larvae at relatively low concentrations [68] |
| Enniatin A Enniatin A1 Enniatin B Enniatin B1 | F. moniliforme F. avenaceum F. roseum F. solani F. nivale F. acuminatum | antibacterial, antifungal, herbicidal, insecticidal, ionophore, induction of apoptosis [29,49,69,70] enniatin B often occurs together with enniatin B1 and enniatin A with beauvericin [50] mice: 10–40 mg/kg bw, intraperitoneal, every 8 h: mice died within 2–5 days; doses <10 mg/kg bw resulted in reduced body weight [71] brine shrimp: 50 μg enniatin B/mL killed almost all after 24 h; ranking in brine shrimps: enniatin B > B1 > A1 > A, mixture of all four most toxic [72] |
| Equisetin | F. equiseti F. semitectum | antibiotic activity against Gram-positive bacteria [73]; potent inhibitor of HIV-1 integrase enzyme [74] mice: LD50: 63.0 mg/kg bw, intraperitoneal [73] |
| Moniliformin | F. avenaceum F. subglutinans F. proliferatum F. oxysporum F. fusariodes | 1-day-old chicks: LD50: 5.4 mg/kg bw oral [75] 7-weeks-old broilers: LD50: 1.38 mg/kg bw, intravenous [76] broilers: 50 mg/kg feed toxic for broilers fed to market age, resulting in lower body weight gain, less efficient feed converting rate, higher mortality [77]; additive effects with aflatoxin [78], as well as with deoxynivalenol [79] turkeys: 37.5 mg/kg feed hepatotoxic; 25 mg/kg feed cardiotoxic [77] swine:100 mg/kg feed for 28 days in growing barrows reduced body weight, body weight gain and feed consumption and affected serum biochemical analytes; 50 mg/kg feed influence on haematologic values [80]; additive effects with fumonisin B1[81] sheep: 10 mg/kg bw, intubation caused death after 18 h, also degeneration of the proximal tubules of kidneys [82] |
| Tentoxin | Alternaria alternata | induces chlorosis in germinating seedlings of many dicotyledonous plants [64] |
| Tenuazonic acid | Alternaria sp. A. alternata A. triticina A. tenuis A. arborescens | mice: LD50: 81 mg/kg bw oral, female; 186 mg/kg bw oral, male [83]; risk for oesophageal cancer: toxic effect on oesophageal mucosa of mice (25 mg/kg bw/day oral for 10 months) stronger than with alternariol methyl ether (100 mg/kg bw/day oral for 10 months)—resulting in weight loss and lower feed consumption [36] rats: LD50: 168 mg/kg bw oral, female; 180 mg/kg bw oral, male [83] young chicken: LD50: 37.5 mg/kg bw oral [84] 1.25–1.50 mg/kg bw/day (3-weeks oral) induced microscopic and macroscopic lesions in various tissues and significant weight loss [84] |
| Tryptophol | Candida albicans Acremonium lolii | in vitro: cytotoxic, cytostatic, genotoxic effects in lymphocytes [85]; 2 mM (24h) damages DNA in HepG2, A549 and THP-1 cells (comet-assay) [86]; induction of apoptosis in leukaemic blood monocytes cell line U937 [87] mice: LD50: 351 mg/kg bw, intraperitoneal [88] |
4. Experimental Section
4.1. Samples
4.2. Analysis
5. Conclusions
Conflict of Interest
References
- Frisvad, J.C.; Thrane, U.; Samson, R.A.; Pitt, J.I. Important mycotoxins and the fungi which produce them. Adv. Exp. Med. Biol. 2006, 571, 3–31. [Google Scholar] [PubMed]
- Binder, E.M. Managing the risk of mycotoxins in modern feed production. Anim. Feed Sci. Technol. 2007, 133, 149–166. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Berthiller, F.; Sulyok, M.; Krska, R.; Schuhmacher, R. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 2007, 119, 33–37. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef]
- Sulyok, M.; Krska, R.; Schuhmacher, R. Application of an LC-MS/MS based multi-mycotoxin method for the semi-quantitative determination of mycotoxins occurring in different types of food infected by moulds. Food Chem. 2010, 119, 408–416. [Google Scholar] [CrossRef]
- Vishwanath, V.; Sulyok, M.; Labuda, R.; Bicker, W.; Krska, R. Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 395, 1355–1372. [Google Scholar] [CrossRef]
- Berthiller, F.; Schuhmacher, R.; Adam, G.; Krska, R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009, 395, 1243–1252. [Google Scholar] [CrossRef]
- Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. Cleavage of zearalenone-glycoside, a “masked” mycotoxin, during digestion in swine. J. Vet. Med. 1990, 37, 236–240. [Google Scholar] [CrossRef]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microbiol. 1991, 57, 146–150. [Google Scholar] [PubMed]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC-MS-MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Borutova, R.; Acosta Aragon, Y.; Nährer, K.; Berthiller, F. Co-occurrence and statistical correlations between mycotoxins in feedstuffs collected in the Asia-Oceania Region in 2010. Anim. Feed Sci. Technol. 2012, 178, 190–197. [Google Scholar] [CrossRef]
- Rodrigues, I.; Chin, L.J. A comprehensive survey on the occurrence of mycotoxins in maize dried distillers’ grain and solubles sourced worldwide. World Mycotoxin J. 2012, 5, 83–88. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Addit. Contam. B 2011, 4, 168–179. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Griessler, K.; Rodrigues, I.; Handl, J.; Hofstetter, U. Occurrence of mycotoxins in Southern Europe. World Mycotoxin J. 2010, 3, 301–309. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. Prevalence of mycotoxins in feedstuffs and feed surveyed worldwide in 2009 and 2010. Phytopathol. Mediterr. 2012, 51, 175–192. [Google Scholar]
- Ezekiel, C.N.; Bandyopadhyay, R.; Sulyok, M.; Warth, B.; Krska, R. Fungal and bacterial metabolites in commercial poultry feed from Nigeria. Food Addit. Contam. A 2012, 29, 1288–1299. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of mycotoxins in food and feed from burkina faso and mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef] [PubMed]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Bottalico, A. Fusarium diseases of cereals: Species complex and related mycotoxin profiles in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- European Commission. Commission Regulation (EU) No 165/2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins. In Commission Regulation (EU) No 178/2010; European Union: Brussels, Belguim, 2010; pp. 1–5.
- European Union. European Commission (2006/576/EU) of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Off. J. Eur. Un. 2006, L229, 7–9.
- De Boevre, M.; di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesaert, G.; de Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.-D.; Knasmüller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012, 213, 367–373. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Chang, M.-H.; Huang, F.-J.; Chan, W.-H. Emodin induces embryonic toxicity in mouse blastocysts through apoptosis. Toxicology 2012, 299, 25–32. [Google Scholar] [CrossRef]
- Wei, W.T.; Chen, H.; Ni, Z.L.; Liu, H.B.; Tong, H.F.; Fan, L.; Liu, A.; Qiu, M.X.; Liu, D.L.; Guo, H.C.; et al. Antitumor and apoptosis-promoting properties of emodin, an anthraquinone derivative from Rheum officinale Baill, against pancreatic cancer in mice via inhibition of Akt activation. Int. J. Oncol. 2011, 39, 1381–1390. [Google Scholar] [PubMed]
- Feng, Y.; Huang, S.-L.; Dou, W.; Zhang, S.; Chen, J.-H.; Shen, Y.; Shen, J.-H.; Leng, Y. Emodin, a natural product, selectively inhibits 11β-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice. Brit. J. Pharmacol. 2010, 161, 113–126. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstufs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Griffin, G.F.; Chu, F.S. Toxicity of the Alternaria metabolites alternariol, alternariol methyl ether, altenuene, and tenuazonic acid in the chicken embryo assay. Appl. Environ. Microbiol. 1983, 46, 1420–1422. [Google Scholar] [PubMed]
- Tiemann, U.; Tomek, W.; Schneider, F.; Müller, M.; Pöhland, R.; Vanselow, J. The mycotoxins alternariol and alternariol methyl ether negatively affect progesterone synthesis in porcine granulosa cells in vitro. Toxicol. Lett. 2009, 186, 139–145. [Google Scholar] [CrossRef]
- Yekeler, H.; Bitmiş, K.; Ozçelik, N.; Doymaz, M.Z.; Çalta, M. Analysis of toxic effects of Alternaria toxins on esophagus of mice by light and electron microscopy. Toxicol. Pathol. 2001, 29, 492–497. [Google Scholar] [CrossRef]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A.; et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef]
- Han, J.-W.; Ahn, S.H.; Park, S.H.; Wang, S.Y.; Bae, G.-U.; Seo, D.-W.; Kwon, H.-K.; Hong, S.; Lee, H.Y.; Lee, Y.-W.; Lee, H.-W. Apicidin, a histone deacetylase inhibitor, inhibits proliferation of tumor cells via induction of p21WAF1/Cip1 and gelsolin. Cancer Res. 2000, 60, 6068–6074. [Google Scholar] [PubMed]
- Cheong, J.-W.; Chong, S.Y.; Kim, J.Y.; Eom, J.I.; Jeung, H.K.; Maeng, H.Y.; Lee, S.T.; Min, Y.H. Induction of apoptosis by apicidin, a histone deacetylase inhibitor, via the activation of mitochondria-dependent caspase cascades in human Bcr-Abl-positive leukemia cells. Clin. Cancer Res. 2003, 9, 5018–5027. [Google Scholar] [PubMed]
- Park, J.-S.; Lee, K.-R.; Kim, J.-C.; Lim, S.-H.; Seo, J.-A.; Lee, Y.-W. A hemorrhagic factor (apicidin) produced by toxic Fusarium isolates from soybean seeds. Appl. Environ. Microbiol. 1999, 65, 126–130. [Google Scholar] [PubMed]
- Kotyk, A.N.; Trufanova, V.; Breslavets, V.A. A syndrome of changing egg quality. Bull. Ukr. Poult. Res. Insitute Kharkov 1990, 29, 41–42. [Google Scholar]
- Kotyk, A.N.; Trufanova, V.; Brestlavets, V.A.; Metasheva, Z.T. Egg Quality in Rhode Island Red Hens Fed by Fusarium graminearum Culture. In Proceedings of 6th European Symposium on the Quality of Eggs and Egg products, Zaragoza, Spain, 1995; pp. 263–266.
- Dvorska, J.E. Effect of Dimeric Naphthoquinone Aurofusarin on Chicken Meat Quality. In Proceedings of 21st World’s Poultry Congress, Montreal, Canada, 2000; pp. 46–49.
- Dvorska, J.E.; Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Effect of the mycotoxin aurofusarin on the antioxidant composition and fatty acid profile of quail eggs. Brit. Poult. Sci. 2001, 42, 643–649. [Google Scholar] [CrossRef]
- Sakhatsky, I.M.; Trufanova, V.O. Effect of Fusarium Mycotoxins Present in Laying Hens Ration on Immune System Function in Progeny Chicks. In Proceedings of 21st World’s Poultry Congress, Montreal, Canada, 2000.
- Logrieco, A.; Moretti, A.; Castella, G.; Kostecki, M.; Golinski, P.; Ritieni, A.; Chelkowski, J. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 1998, 64, 3084–3088. [Google Scholar] [PubMed]
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of beauvericin and enniatins in wheat affected by Fusarium avenaceum head blight. Appl. Environ. Microbiol. 2002, 68, 82–85. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef]
- Tonshin, A.A.; Teplova, V.V.; Andersson, M.A.; Salkinoja-Salonen, M.S. The Fusarium mycotoxins enniatins and beauvericin cause mitochondrial dysfunction by affecting the mitochondrial volume regulation, oxidative phosphorylation and ion homeostasis. Toxicology 2010, 276, 49–57. [Google Scholar] [CrossRef]
- Morrison, E.; Kosiak, B.; Ritieni, A.; Aastveit, A.H.; Uhlig, S.; Bernhoft, A. Mycotoxin production by Fusarium avenaceum strains isolated from norwegian grain and the cytotoxicity of rice culture extracts to porcine kidney epithelial cells. J. Agric. Food Chem. 2002, 50, 3070–3075. [Google Scholar] [CrossRef]
- Finefield, J.M.; Frisvad, J.C.; Sherman, D.H.; Williams, R.M. Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids. J. Nat. Prod. 2012, 75, 812–833. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Rank, C.; Nielsen, K.F.; Larsen, T.O. Metabolomics of Aspergillus fumigatus. Med. Mycol. 2009, 47, S53–S71. [Google Scholar] [CrossRef]
- Amar, M.B.; Elleuch, L.; Abd-Alla, H.I.; Najah, S.; Chakchouk, A.; Damak, M.; Ben Salem, R.; Shaaban, M.; Mellouli, L. The new Streptomyces sp. TN605 strain secretes simultaneously three active compounds and a high level of the interesting pharmaceutical industry intermediate: 2-hydroxyphenylacetic acid. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 48–56. [Google Scholar]
- Yang, H.-Y.; Wang, Y.-M.; Peng, S.-Q. Metallothionein-I/II null cardiomyocytes are sensitive to Fusarium mycotoxin butenolide-induced cytotoxicity and oxidative DNA damage. Toxicon 2010, 55, 1291–1296. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Wang, H.-J.; Peng, S.-Q. In ovo exposure of a Fusarium mycotoxin butenolide induces hepatic and renal oxidative damage in chick embryos, and antioxidants provide protections. Toxicol. in Vitro 2009, 23, 1354–1359. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.-S.; Wang, Y.-M.; Yan, C.-H.; Huang, W.-P.; Wu, J.; Yuan, H.-T.; Lin, B.-W.; Shen, J.-L.; Peng, S.-Q. Study of embryotoxicity of Fusarium mycotoxin butenolide using a whole rat embryo culture model. Toxicol. in Vitro 2011, 25, 1727–1732. [Google Scholar] [CrossRef]
- Kosuri, N.R.; Grove, M.D.; Yates, S.G.; Tallent, W.H.; Ellis, J.J.; Wolff, I.A.; Nichols, R.E. Response of cattle to mycotoxins of Fusarium tricinctum isolated from corn and fescue. J. Am. Vet. Med. Assoc. 1970, 157, 938–940. [Google Scholar] [PubMed]
- Zhang, Y.-F.; Xiao, K.; Chandramouli, K.H.; Xu, Y.; Pan, K.; Wang, W.-X.; Qian, P.-Y. Acute toxicity of the antifouling compound butenolide in non-target organisms. PLoS One 2011, 6, e23803. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, H.R.; Grove, M.D.; Kwolek, W.F. Moniliformin and butenolide: Effect on mice of high-level, long-term oral intake. Appl. Environ. Microbiol. 1980, 40, 1142–1144. [Google Scholar] [PubMed]
- Tookey, H.L.; Yates, S.G.; Ellis, J.J.; Grove, M.D.; Nichols, R.E. Toxic effects of a butenolide mycotoxin and of Fusarium tricinctum cultures in cattle. J. Am. Vet. Med. Assoc. 1972, 160, 1522–1526. [Google Scholar] [PubMed]
- Pedersen, P.B.; Miller, D.J. The fundal metabolite culmorin and related compounds. Nat. Toxins 1999, 7, 305–309. [Google Scholar] [CrossRef]
- Ghembremeskel, M.; Langseth, W. The occurrence of culmorin and hydroxy-culmorins in cereals. Mycopathologia 2000, 152, 103–108. [Google Scholar] [CrossRef]
- Rotter, R.G.; Thompson, B.K.; Trenholm, H.L.; Prelusky, D.B.; Hartin, K.E.; Miller, J.D. A preliminary examination of potential interactions between deoxynivalenol (DON) and other selected Fusarium metabolites in growing pigs. Can. J. Anim. Sci. 1992, 72, 102–116. [Google Scholar]
- Duke, S.O.; Dayan, F.E. Modes of action of microbially-produced phytotoxins. Toxins 2011, 3, 1038–1064. [Google Scholar] [CrossRef]
- Jestoi, M.; Kokkonen, M.; Uhlig, S. What about the “other” Fusarium mycotoxins? World Mycotoxin J. 2009, 2, 181–192. [Google Scholar] [CrossRef]
- Duerksen-Hughes, P.J.; Yang, J.; Ozcan, O. p53 Induction as a genotoxic test for twenty-five chemicals undergoing in vivo carcinogenicity testing. Environ. Health Perspect. 1999, 107, 805–812. [Google Scholar] [CrossRef]
- Wells, J.M.; Cole, R.J.; Kirksey, J.W. Emodin, a toxic metabolite of Aspergillus wentii isolated from weevil-damaged chestnuts. Appl. Microbiol. 1975, 30, 26–28. [Google Scholar] [PubMed]
- He, Q.; Liu, K.; Wang, S.; Hou, H.; Yuan, Y.; Wang, X. Toxicity induced by emodin on zebrafish embryos. Drug Chem. Toxicol. 2012, 35, 149–154. [Google Scholar] [CrossRef]
- Santini, A.; Meca, G.; Uhlig, S.; Ritieni, A. Fusaproliferin, beauvericin and enniatins: Occurrence in food—A review. World Mycotoxin J. 2012, 5, 71–81. [Google Scholar] [CrossRef]
- Tedjiotsop Feudjio, F.; Dornetshuber, R.; Lemmens, M.; Hoffmann, O.; Lemmens-Gruber, R.; Berger, W. Beauvericin and enniatin: Emerging toxins and/or remedies? World Mycotoxin J. 2010, 3, 415–430. [Google Scholar] [CrossRef]
- McKee, T.C.; Bokesch, H.R.; McCormick, J.L.; Rashid, M.A.; Spielvogel, D.; Gustafson, K.R.; Alavanja, M.M.; Cardellina, J.H.; Boyd, M.R. Isolation and characterization of new anti-HIV and cytotoxic leads from plants, marine, and microbial organisms1. J. Nat. Prod. 1997, 60, 431–438. [Google Scholar] [CrossRef]
- Tan, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Sivasithamparam, K.; Barbetti, M.J. Toxigenicity of enniatins from western Australian Fusarium species to brine shrimp (Artemia franciscana). Toxicon 2011, 57, 817–825. [Google Scholar] [CrossRef]
- Burmeister, H.R. Antibiotic Equisetin and Methods of Production. US3959468A 6 May 1974. [Google Scholar]
- Burke, L.T.; Dixon, D.J.; Ley, S.V.; Rodríguez, F. Total synthesis of the Fusarium toxin equisetin. Org. Biomol. Chem. 2005, 3, 274–280. [Google Scholar] [CrossRef]
- Burmeister, H.R.; Ciegler, A.; Vesonder, R.F. Moniliformin, a metabolite of Fusarium moniliforme NRRL 6322: Purification and toxicity. Appl. Environ. Microbiol. 1979, 37, 11–13. [Google Scholar] [PubMed]
- Allen, N.K.; Burmeister, H.R.; Weaver, G.A.; Mirocha, C.J. Toxicity of dietary and intravenously administered moniliformin to broiler chickens. Poult. Sci. 1981, 60, 1415–1417. [Google Scholar] [CrossRef]
- Broomhead, J.; Ledoux, D.R.; Bermudez, A.J.; Rottinghaus, G.E. Chronic effects of moniliformin in broilers and turkeys fed dietary treatments to market age. Avian Dis. 2002, 46, 901–908. [Google Scholar] [CrossRef]
- Kubena, L.F.; Harvey, R.B.; Buckley, S.A.; Edrington, T.S.; Rottinghaus, G.E. Individual and combined effects of moniliformin present in Fusarium fujikuroi culture material and aflatoxin in broiler chicks. Poult. Sci. 1997, 76, 265–270. [Google Scholar] [PubMed]
- Harvey, R.B.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Casper, H.H.; Buckley, S.A. Moniliformin from Fusarium fujikuroi culture material and deoxynivalenol from naturally contaminated wheat incorporated into diets of broiler chicks. Avian Dis. 1997, 41, 957–963. [Google Scholar] [CrossRef]
- Harvey, R.B.; Edrington, T.S.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Genovese, K.J.; Nisbet, D.J. Toxicity of moniliformin from Fusarium fujikuroi culture material to growing barrows. J. Food Prot. 2001, 64, 1780–1784. [Google Scholar] [PubMed]
- Harvey, B.; Edrington, T.S.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Genovese, K.J.; Ziprin, R.L.; Nisbet, D.J. Toxicity of fumonisin from Fusarium verticillioides culture material and moniliformin from Fusarium fujikuroi culture material when fed singly and in combination to growing barrows. J. Food Prot. 2002, 65, 373–377. [Google Scholar] [PubMed]
- Lamprecht, S.C.; Marasas, W.F.O.; Thiel, P.G.; Schneider, D.J.; Knox-Davies, P.S. Incidence and toxigenicity of seedborne Fusarium species from annual Medicago species in South Africa. Phytopathology 1986, 76, 1040–1042. [Google Scholar] [CrossRef]
- Pero, R.W.; Posner, H.; Blois, M.; Harvan, D.; Spalding, J.W. Toxicity of metabolites produced by the “Alternaria”. Environ. Health Perspect. 1973, 4, 87–94. [Google Scholar] [CrossRef]
- Giambrone, J.J.; Davis, N.D.; Diener, U.L. Effect of tenuazonic acid on young chickens. Poult. Sci. 1978, 57, 1554–1558. [Google Scholar] [CrossRef]
- Kosalec, I.; Safranic, A.; Pepeljnjak, S.; Bacun-Druzina, V.; Ramic, S.; Kopjar, N. Genotoxicity of tryptophol in a battery of short-term assays on human white blood cells in vitro. Basic Clin. Pharmacol. Toxicol. 2008, 102, 443–452. [Google Scholar] [CrossRef]
- Kosalec, I.; Ramić, S.; Jelić, D.; Antolović, R.; Pepeljnjak, S.; Kopjar, N. Assessment of tryptophol genotoxicity in four cell lines in vitro: A pilot study with alkaline comet assay. Arch. Ind. Hyg. Toxicol. 2011, 62, 41–49. [Google Scholar]
- Inagaki, S.; Morimura, S.; Tang, Y.; Akutagawa, H.; Kida, K. Tryptophol induces death receptor (DR) 5-mediated apoptosis in U937 cells. Biosci. Biotechnol. Biochem. 2007, 71, 2065–2068. [Google Scholar] [CrossRef] [PubMed]
- Bosin, T.R.; Campaigne, E.; Dinner, A.; Rogers, R.B.; Maickel, R.P. Comparative toxicological studies of indole, benzo[b]thiophene, and 1-methylindole derivatives. J. Toxicol. Environ. Health 1976, 1, 515–520. [Google Scholar] [CrossRef]
- Bräse, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef]
- Scott, P.M. Analysis of agricultural commodities and foods for alternaria mycotoxins. J. AOAC Int. 2001, 84, 1809–1817. [Google Scholar] [PubMed]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risk. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Weidenbörner, M. Encyclopedia of Food Mycotoxins; Springer: Berlin, Germany, 2001; p. 249. [Google Scholar]
- Battilani, P.; Costa, L.G.; Dossena, A.; Gullino, M.L.; Marchelli, R.; Galaverna, G.; Pietri, A.; Dall’Asta, C.; Giorni, P.; Spadaro, D.; Gualla, A. CFP/EFSA/CONTAM/2008/01—Scientific Information on Mycotoxins and Natural Plant Toxicants. Available online: http://www.efsa.europa.eu/en/supporting/pub/24e.htm (accessed on 17 April 2012).
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407.
- Ledoux, D.R.; Bermudez, A.J.; Roinghaus, G.E.; Broomhead, J.; Bennet, G.A. Effects of feeding Fusarium fujikuori culure material containing known levels of moniliformin in young broiler chicks. Poult. Sci. 1995, 74, 297–305. [Google Scholar] [CrossRef]
- Malachova, A.; Beltran, E.; Krska, R.; Sulyok, M. Validation of an LC-MS/MS multi-toxin method covering 329 fungal and bacterial metabolites for four commodities from different matrix groups specified in SANCO 12495/2011. J. Chromatogr. A 2013. to be submitted. [Google Scholar]
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Verstegen, M.W.A.; Gerrits, W.J.J. The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisin in diets on growing pigs and poultry. Nutr. Res. Rev. 2003, 16, 223–239. [Google Scholar] [CrossRef]
- Trenholm, H.L.; Foster, B.C.; Charmley, L.L.; Thompson, B.K.; Hartin, K.E.; Coopock, R.W.; Albassam, M.A. Effects of feeding diets containing Fusarium (naturally) contaminated wheat or pure deoxynivalenol (DON) in growing pigs. Can. J. Anim. Sci. 1994, 74, 361–369. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504-523. https://doi.org/10.3390/toxins5030504
Streit E, Schwab C, Sulyok M, Naehrer K, Krska R, Schatzmayr G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins. 2013; 5(3):504-523. https://doi.org/10.3390/toxins5030504
Chicago/Turabian StyleStreit, Elisabeth, Christina Schwab, Michael Sulyok, Karin Naehrer, Rudolf Krska, and Gerd Schatzmayr. 2013. "Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients" Toxins 5, no. 3: 504-523. https://doi.org/10.3390/toxins5030504
APA StyleStreit, E., Schwab, C., Sulyok, M., Naehrer, K., Krska, R., & Schatzmayr, G. (2013). Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins, 5(3), 504-523. https://doi.org/10.3390/toxins5030504





