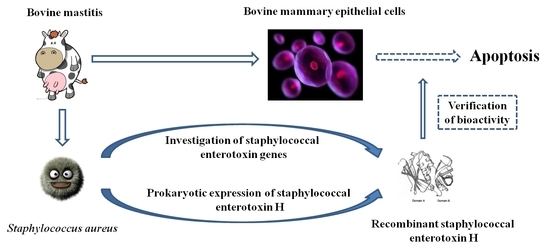
Staphylococcal Enterotoxin H Induced Apoptosis of Bovine Mammary Epithelial Cells in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Prevalence of SE Genes
| Genes | No. of Isolates | % (n = 116) |
|---|---|---|
| seh | 42 | 36.2 |
| sei | 14 | 12.1 |
| seg | 13 | 11.2 |
| ser | 5 | 4.3 |
| sec | 4 | 3.4 |
| sea | 3 | 2.6 |
| sed | 2 | 1.7 |
| seb, see, set | None | 0 |
2.2. Sequence Analysis and Expression of the Gene seh

2.3. Bioactivity of rSEH

2.4. Effect of rSEH on the Viability of bMECs

2.5. Apoptosis of bMECs Measured by Hoechst-PI Staining Fluorescence Imaging
2.6. Apoptosis of bMECs Examined by Flow Cytometry

3. Experimental Section
3.1. Detection of SE Genes
3.2. Cloning and Expression of the seh Gene
3.3. Native Purification and Preparation of rSEH
3.4. Bioactivity Analysis of rSEH in Vitro
| Groups | Compositions |
|---|---|
| Zero adjustment | Culture solution |
| Blank | Culture solution, splenic lymphocyte |
| Negative control | Culture solution, splenic lymphocyte, 10 μg/mL PPC |
| Positive control | Culture solution, splenic lymphocyte, 10 μg/mL ConA |
| Test | Culture solution, splenic lymphocyte, rSEH at different concentrations (0.01, 0.1, 1 μg/mL) |
| Inhibition Test | Culture solution, splenic lymphocyte, 1 μg/mL rSEH, and 10 μg/mL anti-rSEH IgGs |
3.5. Culture and Treatment of Bovine Mammary Epithelial Cells (bMECs)
3.6. Cell Viability Assay
3.7. Examination of rSEH-Induced Apoptosis by Hoechst-PI Staining Fluorescence Imaging
3.8. Examination of rSEH-Induced Apoptosis by Flow Cytometry
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, W.; Zerbe, H.; Petzl, W.; Brunner, R.M.; Günther, J.; Draing, C.; Seyfert, H.M. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-κB in mammary epithelial cells and to quickly induce TNFα and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 2008, 45, 1385–1397. [Google Scholar] [CrossRef]
- Zecconi, A.; Piccinini, R.; Fox, L.K. Epidemiologic study of intramammary infections with Staphylococcus aureus during a control program in nine commercial dairy herds. JAVMA 2003, 223, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Moore, C.E.; Justice, A.; Kantzanou, M.; Story, L.; Mackie, K.; Day, N.P. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 2002, 70, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Kerro, D.O.; van Dijk, J.E.; Nederbragt, H. Factors involved in the early pathogenesis of bovine Staphylococcus aureus mastitis with emphasis on bacterial adhesion and invasion. A review. Vet. Quart. 2002, 24, 181–198. [Google Scholar] [CrossRef]
- Becker, K.; Friedrich, A.W.; Lubritz, G.; Weilert, M.; Peters, G.; von Eiff, C. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J. Clin. Microbiol. 2003, 41, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Bohach, G.A.; Nair, S.P.; Hiramatsu, K.; Jouvin-Marche, E.; Mariuzza, R. Standard nomenclature for the superantigens expressed by Staphylococcus. J. Infect. Dis. 2004, 189, 2334–2336. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Wu, C.M.; Xia, S.C.; Qi, Y.H.; Xia, L.N.; Shen, J.Z. Distribution of superantigenic toxin genes in Staphylococcus aureus isolates from milk samples of bovine subclinical mastitis cases in two major diary production regions of China. Vet. Microbiol. 2009, 137, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.K.; Omoe, K.; Imanishi, K.I.; Iwakabe, Y.; Hu, D.L.; Kato, H.; Shinagawa, K. Identification and characterization of two novel staphylococcal enterotoxins, types S and T. Infect. Immun. 2008, 76, 4999–5005. [Google Scholar] [CrossRef] [PubMed]
- Zschöck, M.; Kloppert, B.; Wolter, W.; Hamann, H.P.; Lämmler, C.H. Pattern of enterotoxin genes seg, seh, sei and sej positive Staphylococcus aureus isolated from bovine mastitis. Vet. Microbiol. 2005, 108, 243–249. [Google Scholar] [CrossRef]
- Xu, S.X.; McCormick, J.K. Staphylococcal superantigens in colonization and disease. Front. Cell. Infect. Microbiol. 2012, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Cesaris, L.; Liandris, E.; Daprà, V.; Piccinini, R. Role of several Staphylococcus aureus virulence factors on the inflammatory response in bovine mammary gland. Microb. Pathog. 2006, 40, 177–183. [Google Scholar] [CrossRef]
- Günaydın, B.; Aslantaş, Ö.; Demir, C. Detection of superantigenic toxin genes in Staphylococcus aureus strains from subclinical bovine mastitis. Trop. Anim. Health Prod. 2011, 43, 1633–1637. [Google Scholar] [CrossRef]
- Aydin, A.; Sudagidan, M.; Muratoglu, K. Prevalence of staphylococcal enterotoxins, toxin genes and genetic-relatedness of foodborne Staphylococcus aureus strains isolated in the Marmara Region of Turkey. Int. J. Food Microbiol. 2011, 148, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.; Philpott, D.J. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 2005, 18, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Chen, C.L.; Huang, W.C.; Cheng, Y.L.; Hsieh, C.Y.; Wang, C.Y.; Hong, M.Y. Different types of cell death induced by enterotoxins. Toxins 2010, 2, 2158–2176. [Google Scholar] [CrossRef] [PubMed]
- Schuberth, H.J.; Krueger, C.; Zerbe, H.; Bleckmann, E.; Leibold, W. Characterization of leukocytotoxic and superantigen-like factors produced by Staphylococcus aureus isolates from milk of cows with mastitis. Vet. Microbiol. 2001, 82, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Larsen, H.D.; Aarestrup, F.M.; Jensen, N.E. Geographical variation in the presence of genes encoding superantigenic exotoxins and β-hemolysin among Staphylococcus aureus isolated from bovine mastitis in Europe and USA. Vet. Microbiol. 2002, 85, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, W.B.; Melles, D.C.; Alaidan, A.; al-Ahdal, M.; Boelens, H.A.; Snijders, S.V.; van Belkum, A. Host-and tissue-specific pathogenic traits of Staphylococcus aureus. J. Bacteriol. 2005, 187, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
- Rall, V.L.M.; Vieira, F.P.; Rall, R.; Vieitis, R.L.; Fernandes, A., Jr.; Candeias, J.M.G.; Araújo, J.P., Jr. PCR detection of staphylococcal enterotoxin genes in Staphylococcus aureus strains isolated from raw and pasteurized milk. Vet. Microbiol. 2008, 132, 408–413. [Google Scholar] [CrossRef]
- Jørgensen, H.J.; Mørk, T.; Høgåsen, H.R.; Rørvik, L.M. Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. J. Appl. Microbiol. 2005, 99, 158–166. [Google Scholar] [CrossRef]
- Ortega, E.; Abriouel, H.; Lucas, R.; Gálvez, A. Multiple roles of Staphylococcus aureus enterotoxins: Pathogenicity, superantigenic activity, and correlation to antibiotic resistance. Toxins 2010, 2, 2117–2131. [Google Scholar] [CrossRef]
- Ren, K.; Bannan, J.D.; Pancholi, V.; Cheung, A.L.; Robbins, J.C.; Fischetti, V.A.; Zabriskie, J.B. Characterization and biological properties of a new staphylococcal exotoxin. J. Exp. Med. 1994, 180, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Wong, A.C. Identification and purification of a new staphylococcal enterotoxin H. Appl. Environ. Microb. 1995, 61, 1438–1443. [Google Scholar]
- Omoe, K.; Ishikawa, M.; Shimoda, Y.; Hu, D.L.; Ueda, S.; Shinagawa, K. Detection of seg, seh, and sei genes in Staphylococcus aureus isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, seh, or sei genes. J. Clin. Microbiol. 2002, 40, 857–862. [Google Scholar] [CrossRef] [PubMed]
- McLauchlin, J.; Narayanan, G.L.; Mithani, V.; O’Neill, G. The detection of enterotoxins and toxic shock syndrome toxin genes in Staphylococcus aureus by polymerase chain reaction. J. Food Protect. 2000, 63, 479–488. [Google Scholar]
- Pereira, M.L.; do Carmo, L.S.; Santos, E.J.D.; Pereira, J.L.; Bergdoll, M.S. Enterotoxin H in staphylococcal food poisoning. J. Food Protect. 1996, 59, 559–561. [Google Scholar]
- Jørgensen, H.J.; Mathisen, T.; Løvseth, A.; Omoe, K.; Qvale, K.S.; Loncarevic, S. An outbreak of staphylococcal food poisoning caused by enterotoxin H in mashed potato made with raw milk. FEMS Microbiol. Lett. 2005, 252, 267–272. [Google Scholar] [CrossRef]
- Ikeda, T.; Tamate, N.; Yamaguchi, K.; Makino, S.I. Mass outbreak of food poisoning disease caused by small amounts of staphylococcal enterotoxins A and H. Appl. Environ. Microb. 2005, 71, 2793–2795. [Google Scholar] [CrossRef]
- Carson, M.; Johnson, D.H.; McDonald, H.; Brouillette, C.; DeLucas, L.J. His-tag impact on structure. Acta Crystallogr. D 2007, 63, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chruszcz, M.; Wlodawer, A.; Minor, W. Determination of protein structures-a series of fortunate events. Biophys. J. 2008, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bonkovsky, H.L.; Guo, J. Structural analysis of heme proteins: Implications for design and prediction. BMC Struct. Biol. 2011, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.G.; Guo, J. Systematic analysis of short internal indels and their impact on protein folding. BMC Struct. Biol. 2010, 10, 24. [Google Scholar] [CrossRef]
- Na, S.J.; Chae, S.Y.; Lee, S.; Park, K.; Kim, K.; Park, J.H.; Lee, K.C. Stability and bioactivity of nanocomplex of TNF-related apoptosis-inducing ligand. Int. J. Pharm. 2008, 363, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ballestriero, F.; Thomas, T.; Burke, C.; Egan, S.; Kjelleberg, S. Identification of compounds with bioactivity against the nematode Caenorhabditis elegans by a screen based on the functional genomics of the marine bacterium Pseudoalteromonas tunicata D2. Appl. Environ. Microb. 2010, 76, 5710–5717. [Google Scholar] [CrossRef]
- Janeway, C.J.; Fischer, L.K.; Hammerling, U. The Mls locus: New clues to a lingering mystery (news). Trends Immunol. 1988, 9, 125–126. [Google Scholar] [CrossRef]
- White, J.; Herman, A.; Pullen, A.M.; Kubo, R.; Kappler, J.W.; Marrack, P. The Vβ-specific superantigen staphylococcal enterotoxin B: Stimulation of mature T cells and clonal deletion in neonatal mice. Cell 1989, 56, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Alva-Murillo, N.; Ochoa-Zarzosa, A.; López-Meza, J.E. Effects of sodium octanoate on innate immune response of mammary epithelial cells during Staphylococcus aureus internalization. BioMed Res. Int. 2013, 2013, 927643. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granié, C.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Bayley, H.; Valeva, A.; Walev, I.; Walker, B.; Weller, U.; Palmer, M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: Prototypes of pore-forming bacterial cytolysins. Arch. Microbiol. 1996, 165, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Rosado, C.J.; Kondos, S.; Bull, T.E.; Kuiper, M.J.; Law, R.H.; Buckle, A.M.; Dunstone, M.A. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 2008, 10, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, L.; Cooper, A.; Fantino, C.; Altmann, D.M.; Sriskandan, S. The mechanism of superantigen-mediated toxic shock: Not a simple Th1 cytokine storm. J. Immunol. 2005, 175, 6870–6877. [Google Scholar] [CrossRef] [PubMed]
- Ionin, B.; Hammamieh, R.; Shupp, J.W.; Das, R.; Pontzer, C.H.; Jett, M. Staphylococcal enterotoxin B causes differential expression of Rnd3 and RhoA in renal proximal tubule epithelial cells while inducing actin stress fiber assembly and apoptosis. Microb. Pathog. 2008, 45, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ferreri, M.; Yu, F.; Liu, X.; Chen, L.; Su, J.; Han, B. Molecular types and antibiotic resistance of Staphylococcus aureus isolates from bovine mastitis in a single herd in China. Vet. J. 2012, 192, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.M.; Smith, J.A.; Struhl, K. Current Protocols in Molecular Biology; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Chiang, Y.C.; Chang, L.T.; Lin, C.W.; Yang, C.Y.; Tsen, H.Y. PCR primers for the detection of staphylococcal enterotoxins K, L, and M and survey of staphylococcal enterotoxin types in Staphylococcus aureus isolates from food poisoning cases in Taiwan. J. Food Protect. 2006, 69, 1072–1079. [Google Scholar]
- Johnson, W.M.; Tyler, S.D.; Ewan, E.P.; Ashton, F.E.; Pollard, D.R.; Rozee, K.R. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J. Clin. Microbiol. 1991, 29, 426–430. [Google Scholar] [PubMed]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Vandenesch, F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Bania, J.; Dabrowska, A.; Bystron, J.; Korzekwa, K.; Chrzanowska, J.; Molenda, J. Distribution of newly described enterotoxin-like genes in Staphylococcus aureus from food. Int. J. Food Microbiol. 2006, 108, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Liao, W.W.; Fan, C.M.; Pai, W.Y.; Chiou, C.S.; Tsen, H.Y. PCR detection of Staphylococcal enterotoxins (SEs) N, O, P, Q, R, U, and survey of SE types in Staphylococcus aureus isolates from food-poisoning cases in Taiwan. Int. J. Food Microbiol. 2008, 121, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jarraud, S.; Peyrat, M.A.; Lim, A.; Tristan, A.; Bes, M.; Mougel, C.; Lina, G. A highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 2001, 166, 669–677. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Shirzad-Wasei, N.; van Oostrum, J.; Bovee-Geurts, P.H.; Wasserman, M.; Bosman, G.J.; DeGrip, W.J. Large scale expression and purification of mouse melanopsin-L in the baculovirus expression system. Protein Expr. Purif. 2013, 91, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Salek-Ardakani, S.; Stuart, A.D.; Arrand, J.E.; Lyons, S.; Arrand, J.R.; Mackett, M. High level expression and purification of the Epstein-Barr virus encoded cytokine viral interleukin 10: Efficient removal of endotoxin. Cytokine 2002, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, B.; Iturralde, M. Binding of a surface protein of Staphylococcus aureus to cultured ovine mammary gland epithelial cells. Vet. Microbiol. 2001, 82, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Ying, Y.B.; Pan, Y.Q.; Li, D.X.; Sun, H.Y.; Chen, S.Q. Expression and bioactivity analysis of staphylococcal enterotoxin C2. Acta Pharmacol. Sin. 2006, 41, 406–411. [Google Scholar]
- Anaya-López, J.L.; Contreras-Guzmán, O.E.; Cárabez-Trejo, A.; Baizabal-Aguirre, V.M.; Lopez-Meza, J.E.; Valdez-Alarcon, J.J.; Ochoa-Zarzosa, A. Invasive potential of bacterial isolates associated with subclinical bovine mastitis. Res. Vet. Sci. 2006, 81, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Ge, Z.Q.; Li, J.C.; Wu, J.C.; Hu, Z.D. Differentiation of apoptotic and necrotic cells in suspension cultures of Taxus cuspidata by the combined use of fluorescent dying and histochemical staining methods. Biotechnol. Lett. 2002, 24, 71–76. [Google Scholar] [CrossRef]
- Brady, H.J.M. Apoptosis Methods and Protocols; Humana Press: Totowa, NJ, USA, 2004; Volume 282. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, W.; Ali, T.; Alkasir, R.; Yin, J.; Liu, G.; Han, B. Staphylococcal Enterotoxin H Induced Apoptosis of Bovine Mammary Epithelial Cells in Vitro. Toxins 2014, 6, 3552-3567. https://doi.org/10.3390/toxins6123552
Liu Y, Chen W, Ali T, Alkasir R, Yin J, Liu G, Han B. Staphylococcal Enterotoxin H Induced Apoptosis of Bovine Mammary Epithelial Cells in Vitro. Toxins. 2014; 6(12):3552-3567. https://doi.org/10.3390/toxins6123552
Chicago/Turabian StyleLiu, Yongxia, Wei Chen, Tariq Ali, Rashad Alkasir, Jinhua Yin, Gang Liu, and Bo Han. 2014. "Staphylococcal Enterotoxin H Induced Apoptosis of Bovine Mammary Epithelial Cells in Vitro" Toxins 6, no. 12: 3552-3567. https://doi.org/10.3390/toxins6123552
APA StyleLiu, Y., Chen, W., Ali, T., Alkasir, R., Yin, J., Liu, G., & Han, B. (2014). Staphylococcal Enterotoxin H Induced Apoptosis of Bovine Mammary Epithelial Cells in Vitro. Toxins, 6(12), 3552-3567. https://doi.org/10.3390/toxins6123552