Cabinet of Curiosities: Venom Systems and Their Ecological Function in Mammals, with a Focus on Primates
Abstract
:1. Introduction
1.1. The Definition of Venom
1.2. Venom in Mammals—An Unused Resource
1.3. Layout of this Review
2. Why Is Venom Use in Mammals Rare?
3. The Venom System and Its Functions in Mammals
3.1. Chiroptera
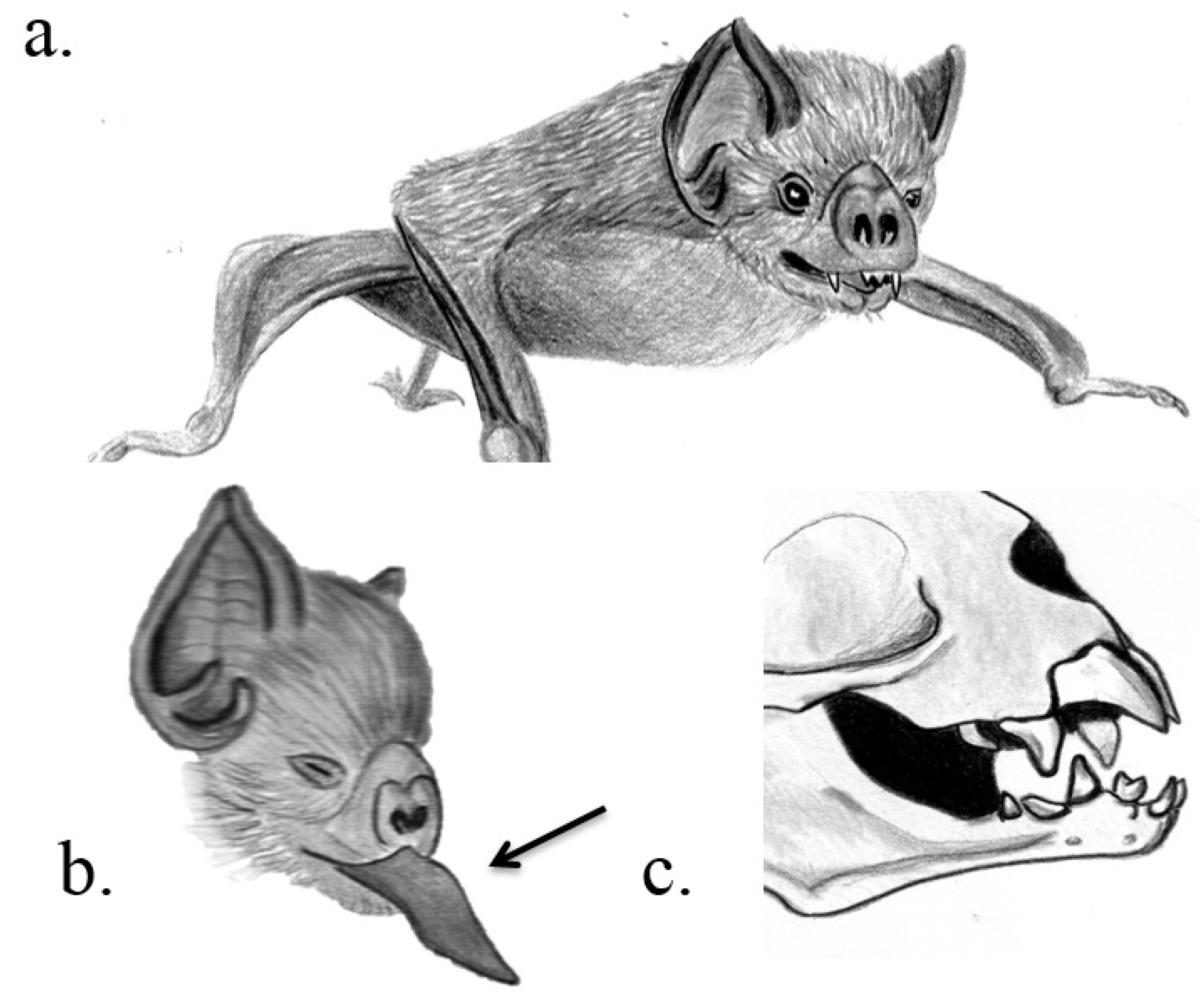
| Order, Family | English Name | Scientific Name | VDA | Venom Gland Position | Ecological Function | References |
|---|---|---|---|---|---|---|
| Chiroptera, Phyllostomidae | Hairy-legged vampire bat, white-winged vampire bat, common vampire bat | Diphylla ecaudata, Diaemus youngi, Desmodus rotundus | Razor-like upper and lower incisors | Principal submaxillary gland | Facilitation of feeding | Low et al. 2013 |
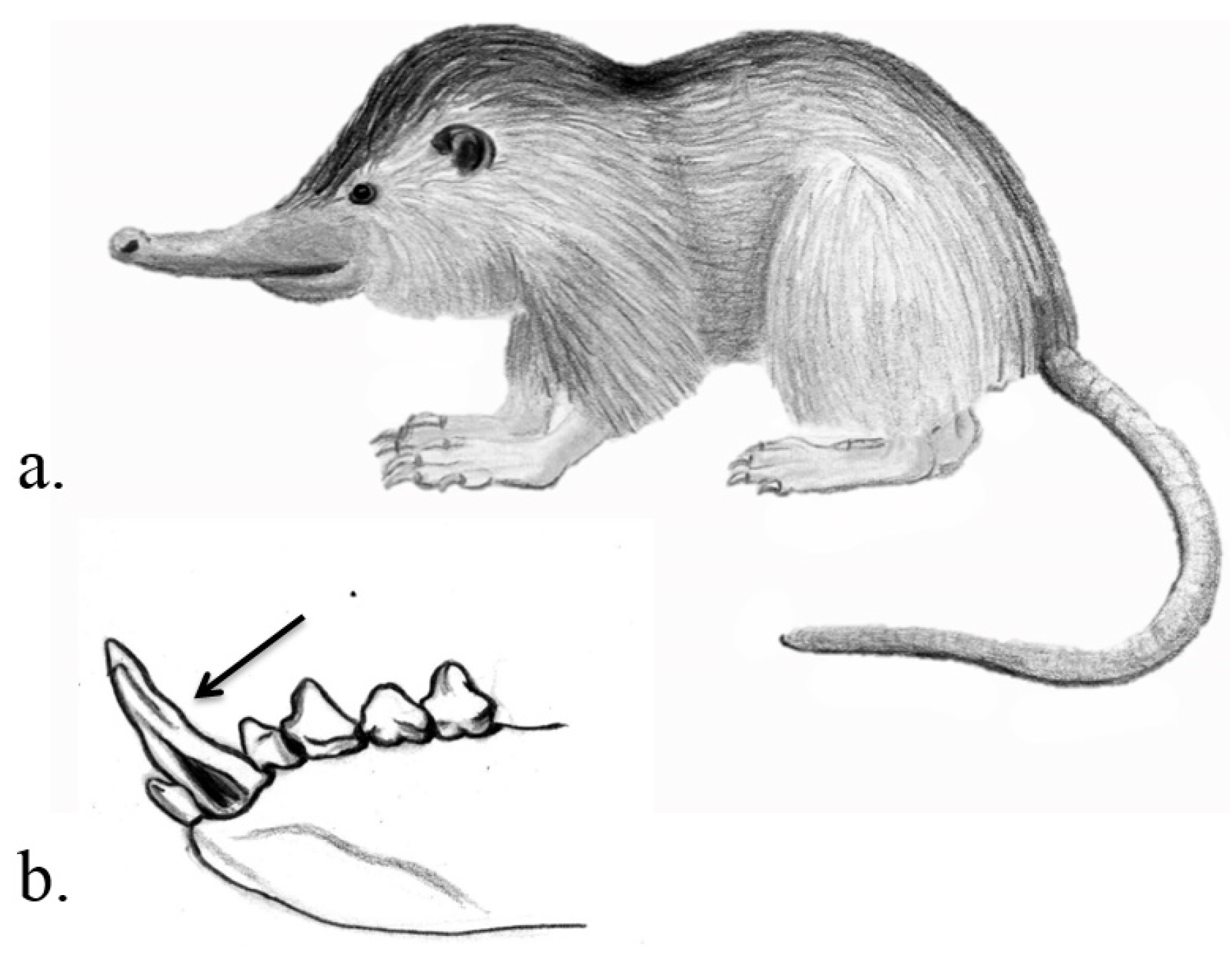
| Soricomorpha, Soricidae | American short-tailed Shrew, European water shrew, Mediterranean water shrew | Blarina brevicauda, Neomys fodiens, N. anomalus | Sharp and large incisors and canines | Significantly enlarged and granular submaxillary salivary glands | Unclear Possible: PC, prey immobilising agent, digestive aid | Tomasi et al. 1978, Martin 1981, Lopez-Jurado & Mateo 1996, Kita et al. 2004, Dufton 1992 |
| Soricomorpha, Solenodontidae | Hispaniolan solenodon, Cuban solenodon | Solenodon paradoxus, S. cubanus | Enlarged and modified lower second incisors with almost tube-like deep groove | Submaxillary glands near base of the tubular lower second incisors | Unclear Possible: PC, IC | Orr 2007, Folinsbee et al. 2007 |
| Monotremata, Ornithorhynchidae | Platypus | Ornithorhynchus anatinus | “Crural system”: Hollow keratinised spurs on hindlegs connected by a duct to the venom gland | “Crural glands”: Specialised venom glands in thigh area | IC (sexual competition during mating season), PD | Temple-Smith 1973, Whittington & Belov 2007, Krause 2009, Grant & Temple-Smith 1998 |
| Primates, Lorisidae | Slow and pygmy lorises | Nycticebus spp. | Needle-like toothcomb (incisors and canines of lower jaw) | “Brachial gland”: Venom gland on the ventral side of the upper arm, submaxillary saliva gland | Unclear Possible: PC, PD, IC and/or ectoparasite defence | Nekaris et al. 2013, Hagey et al. 2007, Krane et al. 2003, Alterman 1995 |
3.2. Eulipotyphla

3.3. Monotremata

3.4. Primates

3.4.1. Intraspecific Competition
3.4.2. Predator Defense
3.4.3. (Ecto-) Parasite Defense
3.4.4. Prey Capture
3.5. Arguably Venomous Species
4. Looking Forward
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Ann. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; van der Weerd, L.; Kochva, E.; Renjifo, C. Evolution and diversification of the Toxicofera reptile venom system. J. Proteomics 2009, 72, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Mackessy, S.P.; Fry, B.G.; Bhatia, M.; Mourier, G.; Fruchart-Gaillard, C.; Servent, D.; Ménez, R.; Stura, E.; Ménez, A. Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J. Biol. Chem. 2006, 281, 29030–29041. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Mackessy, S.P.; Sixberry, N.M.; Stura, E.A.; le Du, M.H.; Ménez, R.; Foo, C.S.; Ménez, A.; Nirthanan, S.; Kini, R.M. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009, 23, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Low, D.H.; Sunagar, K.; Undheim, E.A.; Ali, S.A.; Alagon, A.C.; Ruder, T.; Jackson, T.N.; Pineda Gonzalez, S.; King, G.F.; Jones, A. Dracula’s children: Molecular evolution of vampire bat venom. J. Proteomics 2013, 89, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.; Morgenstern, D.; Mofiz, E.; Gombert, S.; Morris, K.M.; Temple-Smith, P.; Renfree, M.B.; Whittington, C.M.; King, G.F.; Warren, W.C.; et al. Proteomics and deep sequencing comparison of seasonally active venom glands in the platypus reveals novel venom peptides and distinct expression profiles. Mol. Cell. Proteomics 2012, 11, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.W.; Nicol, S.; Warren, W.C.; Belov, K. Echidna venom gland transcriptome provides insights into the evolution of monotreme venom. PLoS ONE 2013, 8, e79092. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.R.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an arsenal structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell. Proteomics 2008, 7, 215–246. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.W.; Belov, K. Venom evolution through gene duplications. Gene 2012, 496, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ligabue-Brown, R.; Verli, H.; Carlini, C.R. Venomous mammals: A review. Toxicon 2012, 59, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Dufton, M.J. Venomous mammals. Pharmacol. Ther. 1992, 53, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Nijman, V.; Nekaris, K.A.I. Traditions, taboos and trade in slow lorises in Sundanese communities in southern Java, Indonesia. Endanger. Species Res. 2014. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened Species. Version 2014.1. Available online: http://www.iucnredlist.org (accessed on 2 June 2014).
- Jackson, K. The evolution of venom-delivery systems in snakes. Zool. J. Linn. Soc. 2003, 137, 337–354. [Google Scholar] [CrossRef]
- Smith, W.L.; Wheeler, W.C. Venom evolution widespread in fishes: A phylogenetic road map for the bioprospecting of piscine venoms. J. Hered. 2006, 97, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Folinsbee, K.E.; Müller, J.; Reisz, R.R. Canine grooves: Morphology, function, and relevance to venom. J. Vertebr. Paleontol. 2007, 27, 547–551. [Google Scholar] [CrossRef]
- Wilson, D.; Reeder, D. Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, ML, USA, 2005. [Google Scholar]
- Dos Reis, M.; Inoue, J.; Hasegawa, M.; Asher, R.J.; Donoghue, P.C.; Yang, Z. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc. R. Soc. B Biol. Sci. 2012. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.M.; Delezene, L.K.; Scott, J.E.; Tocheri, M.W.; Schwartz, G.T. The comparative method and the inference of venom-delivery systems in fossil mammals. J. Vertebr. Paleontol. 2007, 27, 541–546. [Google Scholar] [CrossRef]
- Fox, R.C.; Scott, C.S. First evidence of a venom delivery apparatus in extinct mammals. Nature 2005, 435, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Bescós, G.; Rofes, J. First evidence of poisonous shrews with an envenomation apparatus. Naturwissenschaften 2007, 94, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Greenhall, A.M.; Smith, U. Natural History of Vampire Bats; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Apitz-Castro, R.; Beguin, S.; Tablante, A.; Bartoli, F.; Holt, J.C.; Hemker, H.C. Purification and partial characterization of draculin, the anticoagulant factor present in the saliva of vampire bats (Desmodus rotundus). Thromb. Haemost. 1995, 73, 94–100. [Google Scholar] [PubMed]
- Hawkey, C. Plasminogen activator in saliva of the vampire bat Desmodus rotundus. Nature 1966, 211, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Krätzschmar, J.; Haendler, B.; Langer, G.; Boidol, W.; Bringmann, P.; Alagon, A.; Donner, P.; Schleuning, W.D. The plasminogen activator family from the salivary gland of the vampire bat Desmodus rotundas: Cloning and expression. Gene 1991, 105, 229–237. [Google Scholar] [CrossRef]
- Schondube, J.E.; Herrera-M, L.G.; Martínez del Rio, C. Diet and the evolution of digestion and renal function in phyllostomid bats. Zoology 2001, 104, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Delpietro, H.A.; Russo, R.G. Acquired resistance to saliva anticoagulants by prey previously fed upon by vampire bats (Desmodus rotundus): Evidence for immune response. J. Mammal. 2009, 90, 1132–1138. [Google Scholar] [CrossRef]
- Lopez-Jurado, L.F.; Mateo, J.A. Evidence of venom in the Canarian shrew (Crocidura canariensis): Immobilizing effects on the Atlantic lizard (Gallotia atlantica). J. Zool. 1996, 239, 394–395. [Google Scholar] [CrossRef]
- Kita, M.; Nakamura, Y.; Okumura, Y.; Ohdachi, S.D.; Oba, Y.; Yoshikuni, M.; Kido, H.; Uemura, D. Blarina toxin, a mammalian lethal venom from the short-tailed shrew Blarina brevicauda: Isolation and characterization. Proc. Natl. Acad. Sci. USA 2004, 101, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, T.E. Function of venom in the short-tailed shrew, Blarina brevicauda. J. Mammal. 1978, 59, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.G. Venom of the short-tailed shrew (Blarina brevicauda) as an insect immobilizing agent. J. Mammal. 1981, 62, 189–192. [Google Scholar] [CrossRef]
- Pucek, M. Chemistry and pharmacology of insectivore venoms. In Venomous Animals and Their Venoms; Academic Press: New York, NY, USA, 1968; Volume 1, pp. 43–50. [Google Scholar]
- Rabb, G.B. Toxic salivary glands in the primitive insectivore Solenodon. Nat. Hist. Misc. 1959, 170, 1–3. [Google Scholar]
- Kita, M.; Yuushi, O.; Satoshi, D.O.; Yuichi, O.; Michiyasu, Y.; Yasuo, N.; Hiroshi, K.; Daisuke, U. Purification and characterisation of blarinasin, a new tissue kallikrein-like protease from the short-tailed shrew Blarina brevicauda: Comparative studies with blarina toxin. Biol. Chem. 2005, 386, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.F. Winter survival adaptations of the short-tailed shrew (Blarina brevicauda) in an Appalachian montane forest. J. Mammal. 1986, 67, 450–464. [Google Scholar] [CrossRef]
- Furió, M.; Agustí, J.; Mouskhelishvili, A.; Sanisidro, Ó.; Santos-Cubedo, A. The paleobiology of the extinct venomous shrew Beremendia (Soricidae, Insectivora, Mammalia) in relation to the geology and paleoenvironment of Dmanisi (Early Pleistocene, Georgia). J. Vertebr. Paleontol. 2010, 30, 928–942. [Google Scholar] [CrossRef]
- Wolk, K. The winter food of the European water shrew. Acta Therioly 1976, 21, 117–129. [Google Scholar] [CrossRef]
- Haberl, W. Food storage, prey remains and notes on occasional vertebrates in the diet of the Eurasian water shrew, Neomys fodiens. Folia Zool. Praha 2002, 51, 93–102. [Google Scholar]
- Pucek, M. The effect of the venom of the European water shrew (Neomys fodiens fodiens Pennant) on certain experimental animals. Acta Theriol. 1959, 3, 93–104. [Google Scholar] [CrossRef]
- Grant, T.R.; Temple-Smith, P.D. Field biology of the platypus (Ornithorhynchus anatinus): Historical and current perspectives. Philos. Trans. R. Soc. Lond. Ser. B 1998, 353, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Casewell, N.R.; Wüster, W.; Vidal, N.; Young, B.; Jackson, T.N. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.R. The Platypus; UNSW Press: Sydney, Australia, 1995. [Google Scholar]
- Whittington, C.; Belov, K. Platypus venom: A review. Aust. Mammal. 2007, 29, 57–62. [Google Scholar] [CrossRef]
- Fenner, P.J.; Williamson, J.A.; Myers, D. Platypus envenomation—A painful learning experience. Med. J. Aust. 1992, 157, 829–832. [Google Scholar] [PubMed]
- Whittington, C.M.; Papenfuss, T.; Bansal, P.; Torres, A.M.; Wong, E.S.W.; Deakin, J.E.; Graves, T.; Alsop, A.; Schatzkamer, K.; Kremitzki, C.; Ponting, C.P.; et al. Defensins and the convergent evolution of platypus and reptile venom genes. Genome Res. 2008, 18, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Whittington, C.M.; Papenfuss, A.T.; Locke, D.P.; Mardis, E.R.; Wilson, R.K.; Abubucker, S.; Mitreva, M.; Wong, E.S.W.; Hsu, A.L.; Kuchel, P.W.; et al. Novel venom gene discovery in the platypus. Genome Biol. 2010, 11, R95. [Google Scholar] [CrossRef] [PubMed]
- Kellaway, C.H.; LeMessurier, D.H. The venom of the platypus (Ornithorhynchus anatinus). Aust. J. Exp. Biol. Med. Sci. 1935, 13, 205–221. [Google Scholar] [CrossRef]
- Martin, C.J.; Tidswell, F. Observations on the femoral gland of Ornithorhynchus and its secretion; together with an experimental enquiry concerning its supposed toxic action. Proc. Linn. Soc. N. S. W. 1895, 9, 471–500. [Google Scholar]
- Temple-Smith, P.D. Seasonal Breeding Biology of the Platypus, Ornithorhynchus anatinus Shaw 1799, with Special Reference to the Male. Ph.D. Thesis, Australian National University, Canberra, Australia, 1793. [Google Scholar]
- Hagey, L.; Fry, B.; Fitch-Snyder, H. Talking defensively, a dual use for the brachial gland exudate of slow and pygmy lorises. In Primate Anti-Predator Strategies; Gursky, S., Nekaris, K.A.I., Eds.; Springer: New York, NY, USA, 2007; pp. 253–272. [Google Scholar]
- Alterman, L. Toxins and toothcombs: Potential allospecific chemical defenses in Nycticebus and Perodicticus. In Creatures of the Dark: The Nocturnal Prosimians; Alterman, L., Doyle, G.A., Izard, M.K., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 413–424. [Google Scholar]
- Wilde, H. Anaphylactic shock following bite by a “slow loris”, Nycticebus coucang. Am. J. Trop. Med. Hyg. 1972, 21, 592. [Google Scholar] [PubMed]
- Streicher, U. Aspects of Ecology and Conservation of the Pygmy Loris Nycticebus pygmaeus in Vietnam. Ph.D. Thesis, Ludwig-Maximilian-Universität München, Munich, Germany, 2004. [Google Scholar]
- Klotz, J.H.; Klotz, S.A.; Pinnas, J.L. Animal bites and stings with anaphylactic potential. J. Emerg. Med. 2009, 36, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Prameswari, W.; Sanchez, K.L.; Moore, R.S. Treatment of ulcerative lesions caused by slow loris venomous bites in rescued slow lorises (Nycticebus spp.). In Proceedings of International Primatological Society Congress, Hanoi, Vietnam, 11–17 August 2014.
- Madani, G.; Nekaris, K.A.I. Anaphylactic shock following the bite of a wild Kayan slow loris (Nycticebus kayan): Implications for slow loris conservation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Grow, N.B.; Wirdateti, M.; Nekaris, K.A.I. Does toxic defence in Nycticebus spp. relate to ectoparasites? The lethal effects of slow loris venom on arthropods. Toxicon 2015, 95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Krane, S.; Itagaki, Y.; Nakanishi, K.; Weldon, P.J. “Venom” of the slow loris: Sequence similarity of prosimian skin gland protein and Fel d 1 cat allergen. Naturwissenschaften 2003, 90, 60–62. [Google Scholar] [PubMed]
- Nekaris, K.A.I.; Moore, R.S.; Rode, J.; Fry, B.G. Mad, bad and dangerous to know: The biochemistry, ecology and evolution of slow loris venom. J. Venom. Anim. Toxins Incl. Trop. Dis 2013. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.; Wirdateti, M.; Nijman, V.; Nekaris, K.A.I. Eagles’ responses to a venomous mammal—Do chemical cues in the venom of slow lorises repel avian predators? IBIS 2015. in review. [Google Scholar]
- Takeshita, F.; Wada, S. Morphological comparison of the second gnathopod in males of four Caprellidspecies (Amphipoda: Caprellidae). J. Crustac. Biol. 2012, 32, 673–676. [Google Scholar] [CrossRef]
- Olivera, B.M.; Showers-Corneli, P.; Watkins, M.; Fedosov, A. Biodiversity of cone snails and other venomous marine gastropods: Evolutionary success through neuropharmacology. Ann. Rev. Anim. Biosci. 2014, 2, 487–513. [Google Scholar] [CrossRef] [PubMed]
- Sutherland-Smith, M.; Stalis, I. Health. In Management of Lorises in Captivity. A Husbandry Manual for Asian Lorisines; Fitch-Snyder, H., Schulze, H., Larson, L., Eds.; Center for Reproduction of Endangered Species, Zoological Society of San Diego: San Diego, CA, USA, 2001. [Google Scholar]
- Wiens, F.; Zitzmann, A.; Hussein, N.A. Fast food for slow lorises: Is low metabolism related to secondary compounds in high-energy plant diet? J. Mammal. 2006, 87, 790–798. [Google Scholar] [CrossRef]
- Fuller, G.; Lukas, K.E.; Kuhar, C.; Dennis, P.M. A retrospective review of mortality in lorises and pottos in North American zoos, 1980–2010. Endanger. Species Res. 2014, 23, 205–217. [Google Scholar] [CrossRef]
- Wiens, F.; Zitzmann, A. Social structure of the solitary slow loris Nycticebus coucang (Lorisidae). J. Zool. 2003, 261, 35–46. [Google Scholar] [CrossRef]
- Nekaris, K.A.I. Extreme primates: Ecology and evolution of Asian lorises. Evol. Anthropol. 2014, 23, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Crompton, R.H.; Sellers, W.I.; Gunther, M.M. Energetic efficiency and ecology as selective factors in the saltatory adaptation of prosimian primates. Proc. R. Soc. Lond. Ser. B 1993, 254, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.I.; Bearder, S.K. The lorisiform primates of Asia and mainland Africa: Diversity shrouded in darkness. In Primates in Perspective, 2nd ed.; Campbell, C.J., Fuentes, A., MacKinnon, K.C., Bearder, S.K., Stumpf, R.M., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 34–54. [Google Scholar]
- Fitch-Snyder, H.; Schulze, H. Management of Lorises in Captivity: A Husbandry Manual for Asian Lorisines (Nycticebus & Loris Spp.); Center for Reproduction of Endangered Species, Zoological Society of San Diego: San Diego, CA, USA, 2001. [Google Scholar]
- Weldon, J. Defensive anointing: Extended chemical phenotype and unorthodox ecology. Chemoecology 2004, 14, 1–4. [Google Scholar] [CrossRef]
- Clucas, B.; Rowe, M.P.; Owings, D.H.; Arrowood, P.C. Snake scent application in ground squirrels, Spermophilus spp.: A novel form of antipredator behaviour? Anim. Behav. 2008, 75, 299–307. [Google Scholar] [CrossRef]
- Dumbacher, J.P.; Menon, G.K.; Daly, J.W. Skin as a toxin storage organ in the endemic New Guinean genus Pitohui. Auk 2009, 126, 520–530. [Google Scholar] [CrossRef]
- Dumbacher, J.P.; Wako, A.; Derrickson, S.R.; Samuelson, A.; Spande, T.F.; Daly, J.W. Melyrid beetles (Choresine): A putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds. Proc. Natl. Acad. Sci. USA 2004, 101, 15857–15860. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Brodie, E.D., Jr.; Brodie, E.D., III. Coevolution of deadly toxins and predator resistance: Self-assessment of resistance by garter snakes leads to behavioral rejection of toxic newt prey. Herpetologica 2003, 59, 155–163. [Google Scholar] [CrossRef]
- Rode-Margono, E.J.; Nekaris, K.A.I. Impact of climate and moonlight on a venomous mammal, the Javan slow loris (Nycticebus javanicus Geoffroy, 1812). Contrib. Zool. 2014, 83, 217–225. [Google Scholar]
- Hart, D. Predation on primates: A biogeographical analysis. In Primate Anti-Predator Strategies; Gursky, S.J., Nekaris, K.A.I., Eds.; Springer Press: New York, NY, USA, 2007; pp. 27–59. [Google Scholar]
- Combes, C. Parasitism: The Ecology and Evolution of Intimate Interactions; University of Chicago Press: Chicago, IL, USA, 2001. [Google Scholar]
- Forbey, J.S.; Harvey, A.L.; Huffman, M.A.; Provenza, F.D.; Sullivan, R.; Tasdemir, D. Exploitation of secondary metabolites by animals: A response to homeostatic challenges. Integr. Comp. Biol. 2009, 49, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.A. Current evidence for self-medication in primates: A multidisciplinary perspective. Am. J. Phys. Anthropol. 1997, 104, 171–200. [Google Scholar] [CrossRef]
- Lozano, G.A. Parasitic stress and self-medication in wild animals. Adv. Stud. Behav. 1998, 27, 291–317. [Google Scholar]
- Spruijt, B.M.; van Hooff, J.A.; Gispen, W.H. Ethology and neurobiology of grooming behavior. Physiol. Rev. 1992, 72, 825–852. [Google Scholar] [PubMed]
- Douglas, H.D., III. Prenuptial perfume: Alloanointing in the social rituals of the crested auklet (Aethia cristatella) and the transfer of arthropod deterrents. Naturwissenschaften 2008, 95, 45–53. [Google Scholar]
- Wiens, F. Behavior and Ecology of Wild Slow Lorises (Nycticebus coucang): Social Organization, Infant Care System, and Diet. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2002. [Google Scholar]
- Wright, P.C.; Arrigo-Nelson, S.J.; Hogg, K.L.; Bannon, B.; Morelli, T.L.; Wyatt, J.; Harivelo, A.L.; Ratelolahy, F.; Huffman, M.A.; Chapman, C.A. Habitat disturbance and seasonal fluctuations of lemur parasites in the rain forest of Ranomafana National Park, Madagascar. In Primate Parasite Ecology. The Dynamics and Study of Host-Parasite Relationships; Chapman, C., Huffman, M.A., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 311–330. [Google Scholar]
- Gray, A.; Wirdateti; Nekaris, K.A.I. Use of exudate-based enrichment to improve the welfare of captive slow lorises (Nycticebus spp.) rescued from the illegal pet trade in Indonesia. Endanger. Species Res. 2015, 27, 21–29. [Google Scholar] [CrossRef]
- Brodie, E.D. Hedgehogs use toad venom in their own defence. Nature 1977, 268, 627–628. [Google Scholar] [CrossRef]
- Kingdon, J.; Agwanda, B.; Kinnaird, M.; O’Brien, T.; Holland, C.; Gheysens, T.; Boulet-Audet, M.; Vollrath, F. A poisonous surprise under the coat of the African crested rat. Proc. R. Soc. B 2011, 279, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M. Sur la mattiere cristallisee active de fleches empoisonnee des somalis extradite du bois d’Ovabio. C. R. Acad. Sci. 1988, 106, 1011–1162. [Google Scholar]
- Schmelzer, G.H.; Gurib-Fakim, A. Medical Plants 1; Backhuys Publishers: Wageningen, The Netherlands, 2008. [Google Scholar]
- Fürstenwerth, H. Ouabain—The insulin of the heart. Int. J. Clin. Pract. 2010, 64, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Manunta, P.; Ferrandi, M.; Bianchi, G.; Hamlyn, J.M. Endogenous ouabain in cardiovascular function and disease. J. Hypertens. 2009, 27, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, K. Ecological function of venom in Varanus, with a compilation of dietary records from the literature. Biawak 2009, 3, 46–56. [Google Scholar]
- Whittington, C.M.; Belov, K. Tracing monotreme venom evolution in the genomics era. Toxins 2014, 6, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Guércio, R.A.P.; Shevchenko, A.; Shevchenko, A.; López-Lozano, J.L.; Paba, J.; Sousa, M.V.; Ricart, C.A.O. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteome Sci. 2006, 4. [Google Scholar] [CrossRef] [PubMed]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rode-Margono, J.E.; Nekaris, K.A.-I. Cabinet of Curiosities: Venom Systems and Their Ecological Function in Mammals, with a Focus on Primates. Toxins 2015, 7, 2639-2658. https://doi.org/10.3390/toxins7072639
Rode-Margono JE, Nekaris KA-I. Cabinet of Curiosities: Venom Systems and Their Ecological Function in Mammals, with a Focus on Primates. Toxins. 2015; 7(7):2639-2658. https://doi.org/10.3390/toxins7072639
Chicago/Turabian StyleRode-Margono, Johanna E., and K. Anne-Isola Nekaris. 2015. "Cabinet of Curiosities: Venom Systems and Their Ecological Function in Mammals, with a Focus on Primates" Toxins 7, no. 7: 2639-2658. https://doi.org/10.3390/toxins7072639
APA StyleRode-Margono, J. E., & Nekaris, K. A.-I. (2015). Cabinet of Curiosities: Venom Systems and Their Ecological Function in Mammals, with a Focus on Primates. Toxins, 7(7), 2639-2658. https://doi.org/10.3390/toxins7072639






