Viperid Envenomation Wound Exudate Contributes to Increased Vascular Permeability via a DAMPs/TLR-4 Mediated Pathway
Abstract
:1. Introduction
2. Results
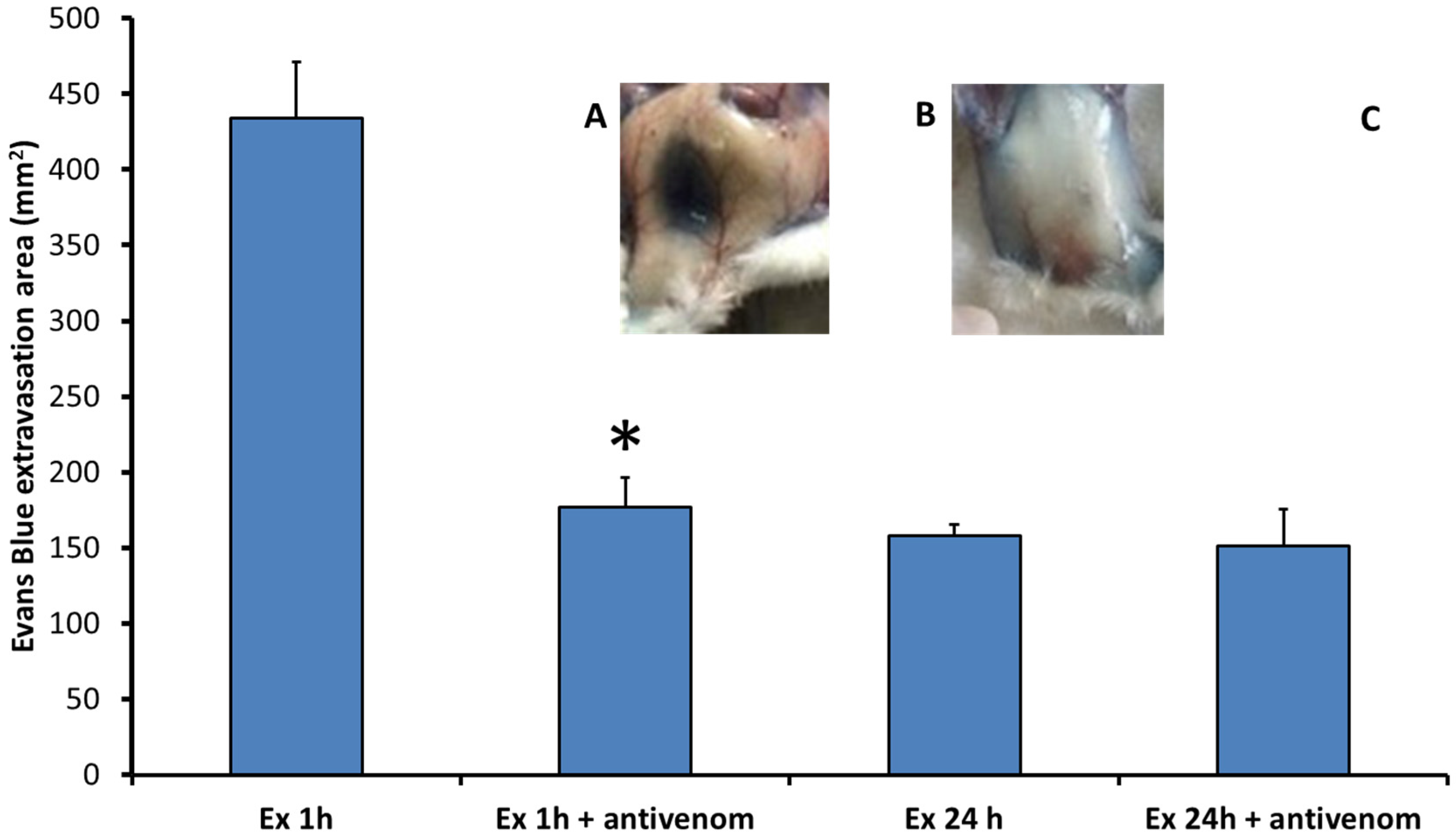
2.1. Exudates Collected from Mice Injected with B. asper Venom Increase Vascular Permeability
2.2. Exudates Contain High Concentrations of Inflammatory Mediators
2.3. Abundant DAMPs Are Identified in the Proteomes of Exudates
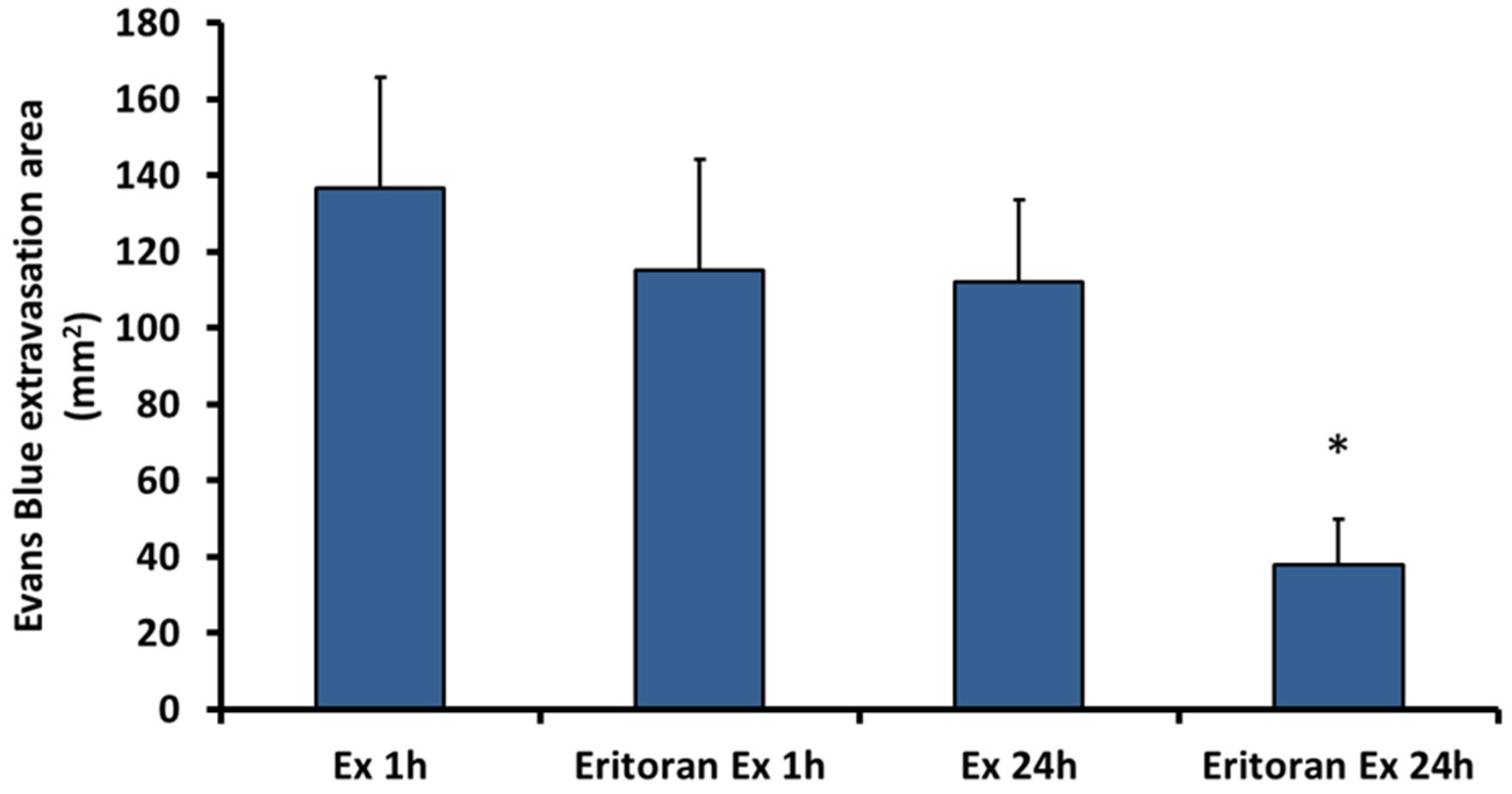
2.4. Eritoran, an Inhibitor of TLR4, Inhibits the Vascular Permeability Effect Induced by Exudate
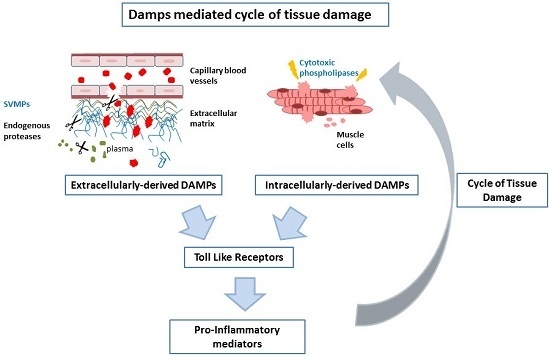
3. Discussion
4. Materials and Methods
4.1. Venom
4.2. Exudate Collection
4.3. Increase in Vascular Permeability
4.4. Effect of Eritoran in Exudate-Induced Vascular Permeability
4.5. Quantification of Inflammatory Mediators in Exudates by Luminex Assays
4.6. Complete Proteomic Analysis of Wound Exudates and Identification of DAMPs
4.7. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Warrell, D.A. Snakebites in Central and South America: Epidemiology, clinical features, and clinical management. In The Venomous Reptiles of the Western Hemisphere; Campbell, J.A., Lamar, W.W., Eds.; Cornell University Press: Ithaca, NY, USA, 2004; pp. 709–761. [Google Scholar]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Fox, J.W.; Bjarnason, J.B. New proteases from Crotalus atrox venom. J. Toxicol. Toxin Rev. 1983, 2, 161–204. [Google Scholar] [CrossRef]
- Bjarnason, J.B.; Fox, J.W. Hemorrhagic toxins from snake venoms. J. Toxicol. Toxin Rev. 1988, 7, 121–209. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Lomonte, B.; Angulo, Y.; Fox, J.W. Tissue pathology induced by snake venoms: How to understand a complex pattern of alterations from a systems biology perspective? Toxicon 2010, 55, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.G.; Bao, Y.; Serrano, S.M.T.; Kamiguti, A.S.; Theakston, R.D.G.; Fox, J.W. Use of microarrays for investigating the subtoxic effects of snake venoms: Insights into venom-induced apoptosis in human umbilical vein endothelial cells. Toxicon 2003, 41, 429–440. [Google Scholar] [CrossRef]
- Baramova, E.N.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Identification of the cleavage sites by a hemorrhagic metalloproteinase in type IV collagen. Matrix 1990, 10, 91–97. [Google Scholar] [CrossRef]
- Baramova, E.N.; Shannon, J.D.; Fox, J.W.; Bjarnason, J.B. Proteolytic digestion of non-collagenous basement membrane proteins by the hemorrhagic metalloproteinase Ht-e from Crotalus atrox venom. Biomed. Biochim. Acta 1991, 50, 763–768. [Google Scholar] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Ortiz, N.; Rucavado, A.; Sanchez, E.F.; Richardson, M.; Fox, J.W.; Gutiérrez, J.M. Role of collagens and perlecan in microvascular stability: Exploring the mechanism of capillary vessel damage by snake venom metalloproteinases. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.; Bao, Y.; Serrano, S.M.T.; Laing, G.D.; Theakston, R.D.G.; Gutiérrez, J.M.; Escalante, T.; Zigrino, P.; Moura-Da-Silva, A.M.; Nischt, R.; et al. Role of the snake venom toxin jararhagin in proinflammatory pathogenesis: In vitro and in vivo gene expression analysis of the effects of the toxin. Arch. Biochem. Biophys. 2005, 441, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Cury, Y.; Moreira, V.; Picolo, G.; Chaves, F. Inflammation induced by Bothrops asper venom. Toxicon 2009, 54, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rucavado, A.; Pinto, A.F.M.; Terra, R.M.S.; Gutiérrez, J.M.; Fox, J.W. Wound exudate as a proteomic window to reveal different mechanisms of tissue damage by snake venom toxins. J. Proteome Res. 2009, 8, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Escalante, T.; Shannon, J.D.; Ayala-Castro, C.N.; Villalta, M.; Gutiérrez, J.M.; Fox, J.W. Efficacy of IgG and F(ab′) 2 antivenoms to neutralize snake venom-induced local tissue damage as assessed by the proteomic analysis of wound exudate. J. Proteome Res. 2012, 11, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Escalante, T.; Shannon, J.; Gutiérrez, J.M.; Fox, J.W. Proteomics of wound exudate in snake venom-induced pathology: Search for biomarkers to assess tissue damage and therapeutic success. J. Proteome Res. 2011, 10, 1987–2005. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.-Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003, 101, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shen, Q.; Pivetti, C.D.; Lee, E.S.; Wu, M.H.; Yuan, S.Y. Molecular mechanisms of endothelial hyperpermeability: Implications in inflammation. Expert Rev. Mol. Med. 2009, 11. [Google Scholar] [CrossRef] [PubMed]
- Khakpour, S.; Wilhelmsen, K.; Hellman, J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun. 2015, 21, 827–846. [Google Scholar] [CrossRef] [PubMed]
- Park-Windhol, C.; D’Amore, P.A. Disorders of vascular permeability. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.; Scherbaum, C.R.; Darisipudi, M.N.; Mulay, S.R.; Hägele, H.; Lichtnekert, J.; Hagemann, J.H.; Rupanagudi, K.V.; Ryu, M.; Schwarzenberger, C.; et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J. Am. Soc. Nephrol. 2012, 23, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.; Teixeira, C.; Borges da Silva, H.; D’Império Lima, M.R.; Dos-Santos, M.C. The crucial role of the MyD88 adaptor protein in the inflammatory response induced by Bothrops atrox venom. Toxicon 2013, 67, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, K.C.; Leiguez, E.; Moreira, V.; Nascimento, N.G.; Lomonte, B.; Gutiérrez, J.M.; Lopes de Melo, R.; Teixeira, C. A Lys49 phospholipase A2, isolated from Bothrops asper snake venom, induces lipid droplet formation in macrophages which depends on distinct signaling pathways and the C-terminal region. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef]
- Moreira, V.; Teixeira, C.; Borges da Silva, H.; D’Império Lima, M.R.; Dos-Santos, M.C. The role of TLR2 in the acute inflammatory response induced by Bothrops atrox snake venom. Toxicon 2016, 118, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A 2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.P.; Zamunér, S.R.; Zuliani, J.P.; Fernandes, C.M.; Cruz-Hofling, M.A.; Fernandes, I.; Chaves, F.; Gutiérrez, J.M. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve 2003, 28, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Saravia-Otten, P.; Robledo, B.; Escalante, T.; Bonilla, L.; Rucavado, A.; Lomonte, B.; Hernández, R.; Flock, J.I.; Gutiérrez, J.M.; Gastaldello, S. Homogenates of skeletal muscle injected with snake venom inhibit myogenic differentiation in cell culture. Muscle Nerve 2013, 47, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Macêdo, J.K.A.; Feoli, A.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M.; Fox, J.W. Muscle tissue damage induced by the venom of Bothrops asper: Identification of early and late pathological events through proteomic analysis. PLoS Negl. Trop. Dis. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Chaves, F.; Cerdas, L. Inflammatory infiltrate in skeletal muscle injected with Bothrops asper venom. Rev. Biol. Trop. 1986, 34, 209–214. [Google Scholar] [PubMed]
- Mahdavian Delavary, B.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H.J. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Brancato, S.K.; Albina, J.E. Wound macrophages as key regulators of repair: Origin, phenotype, and function. Am. J. Pathol. 2011, 178, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef] [PubMed]
- Vénéreau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from cell death to new life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wei, F.; Tewary, P.; Howard, O.M.Z.; Oppenheim, J.J. Alarmin-induced cell migration. Eur. J. Immunol. 2013, 43, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J. Mol. Cell. Cardiol. 2016, 94, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Zornetta, I.; Caccin, P.; Fernandez, J.; Lomonte, B.; Gutierrez, J.M.; Montecucco, C. Envenomations by Bothrops and Crotalus snakes induce the release of mitochondrial alarmins. PLoS Negl. Trop. Dis. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Cintra-Francischinelli, M.; Caccin, P.; Chiavegato, A.; Pizzo, P.; Carmignoto, G.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Montecucco, C. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14140–14145. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, A.; Tomita, T.; Ohto, U.; Takemura, K.; Kitao, A.; Akashi-Takamura, S.; Miyake, K.; Maru, Y. Eritoran inhibits S100A8-mediated TLR4/MD-2 activation and tumor growth by changing the immune microenvironment. Oncogene 2016, 35, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Trowbridge, J.M.; Rudisill, J.A.; Termeer, C.C.; Simon, J.C.; Gallo, R.L. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 2004, 279, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Voelcker, V.; Gebhardt, C.; Averbeck, M.; Saalbach, A.; Wolf, V.; Weih, F.; Sleeman, J.; Anderegg, U.; Simon, J. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp. Dermatol. 2008, 17, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-Y.; Segovia, J.A.; Chang, T.-H.; Morris, I.R.; Berton, M.T.; Tessier, P.A.; Tardif, M.R.; Cesaro, A.; Bose, S. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: Role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.B.; Brunn, G.J.; Kodaira, Y.; Platt, J.L. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 2002, 168, 5233–5239. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Watari, M.; Jerud, E.S.; Young, D.W.; Ishizaka, S.T.; Rose, J.; Chow, J.C.; Strauss, J.F. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001, 276, 10229–10233. [Google Scholar] [CrossRef] [PubMed]
- Smiley, S.T.; King, J.A.; Hancock, W.W. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 2001, 167, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Oliveira, E.L.; Ferreira da Silva, R.; Correa Leite, P.E.; Cogo, J.C.; Quirico-Santos, T.; Lagrota-Candido, J. TLR4 signaling protects from excessive muscular damage induced by Bothrops jararacussu snake venom. Toxicon 2012, 60, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Leiguez, E.; Giannotti, K.C.; Moreira, V.; Matsubara, M.H.; Gutiérrez, J.M.; Lomonte, B.; Rodríguez, J.P.; Balsinde, J.; Teixeira, C. Critical role of TLR2 and MyD88 for functional response of macrophages to a group IIA-secreted phospholipase A2 from snake venom. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamaji, N.; Yasunaga, K.; Saito, T.; Matsumoto, S.; Katoh, M.; Kobayashi, S.; Masuho, Y. The fibronectin extra domain A activates matrix metalloproteinase gene expression by an interleukin-1-dependent mechanism. J. Biol. Chem. 1999, 274, 30756–30763. [Google Scholar] [CrossRef] [PubMed]
- Kelsh, R.M.; McKeown-Longo, P.J. Topographical changes in extracellular matrix: Activation of TLR4 signaling and solid tumor progression. Trends Cancer Res. 2013, 9, 1–13. [Google Scholar] [PubMed]
- Järveläinen, H.; Sainio, A.; Wight, T.N. Pivotal role for decorin in angiogenesis. Matrix Biol. 2015, 43, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, H.; Chen, X.; Jiang, Y.; Huang, Q. Functional characterization of S100A8 and S100A9 in altering monolayer permeability of human umbilical endothelial cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Tauseef, M.; Knezevic, N.; Chava, K.R.; Smith, M.; Sukriti, S.; Gianaris, N.; Obukhov, A.G.; Vogel, S.M.; Schraufnagel, D.E.; Dietrich, A.; et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 2012, 209, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Proteome Software. Available online: http://www.proteomesoftware.com (accessed on 18 April 2016).

| Analytes (pg/mL) | Exudate 1 h | Exudate 24 h | Fold change * |
|---|---|---|---|
| CCL11 (EOTAXIN) | 220.0 | 982.4 | 4.5 |
| CSF-3 (G-CSF) | 1670.0 | >11,610.0 | >6.9 |
| CSF-2 (GM-CSF) | 21.2 | 219.0 | 10.3 |
| IFNy | 3.2 | 37.8 | 11.8 |
| IL-10 | 436.3 | 3419.0 | 7.8 |
| IL-12p40 | 10.3 | 37.5 | 3.6 |
| IL-12p70 | 5.2 | 23.8 | 4.6 |
| IL-13 | 268.4 | 1217.0 | 4.5 |
| IL-15 | 25.0 | 117.3 | 4.7 |
| IL-17 | <2.9 | 12.0 | >4.1 |
| IL-1a | 228.3 | 5952.0 | 26.1 |
| IL-1b | 8.0 | 843.1 | 105.4 |
| IL-2 | 4.8 | 9.2 | 1.9 |
| IL-3 | <2.4 | 10.2 | >4.2 |
| IL-4 | <1.4 | 4.7 | >1.9 |
| IL-5 | 15.9 | 68.1 | 4.3 |
| IL-6 | 7901.0 | >17,536.0 | >2.2 |
| IL-7 | 3.8 | 10.5 | 2.8 |
| IL-9 | 324.0 | 509.9 | 1.6 |
| CXCL10 (IP-10) | 52.6 | 2424.0 | 46.1 |
| CXCL1/GRO alpha (KC) | 4514.0 | 15,957.0 | 3.5 |
| LIF | 24.6 | 2252.0 | 91.5 |
| CXCL5 (LIX) | 1494.0 | 3817.0 | 2.5 |
| CCL2 (MCP-1) | 938.8 | >18,874.0 | >20.1 |
| CSF-1 (M-CSF) | 26.1 | 529.8 | 20.3 |
| CXCL9 (MIG) | 228.6 | 3034.0 | 13.3 |
| CCL3 (MIP-1a) | 27.9 | >14,741.0 | >528.3 |
| CCL4 (MIP-1b) | 80.7 | >14,663.0 | >181.7 |
| CXCL2 (MIP-2) | 4623.0 | 12,954.0 | 2.8 |
| CCL5 (RANTES) | 5.0 | 307.3 | 61.4 |
| TNF-a | 9.0 | 799.2 | 88.8 |
| VEGF | <1.3 | 89.3 | >68.7 |
| Identified Proteins | Accession Number | Molecular Weight | Quantitative Value | Fold Change * | |
|---|---|---|---|---|---|
| 1 h | 24 h | ||||
| Hemoglobin subunit beta-2 | P02089 | 16 kDa | 745 | 1329 | 1.8 |
| Fibronectin | P11276 | 273 kDa | 274 | 290 | 1.0 |
| Fibrinogen gamma chain | Q8VCM7 | 49 kDa | 49 | 145 | 2.9 |
| Heat shock cognate 71 kDa protein | P63017 | 71 kDa | 50 | 17 | 2.9 |
| Fibrinogen beta chain | Q8K0E8 | 55 kDa | 12 | 107 | 8.9 |
| Heat shock protein HSP 90-beta | P11499 | 83 kDa | 41 | 26 | 1.6 |
| Basement membrane-specific heparan sulfate proteoglycan core protein | B1B0C7 | 469 kDa | 83 | 0 | >83 |
| Serum amyloid P-component | P12246 | 26 kDa | 27 | 65 | 2.4 |
| Histone H4 | P62806 | 11 kDa | 55 | 46 | 1.2 |
| Histone H2B type 1-M | P10854 | 14 kDa | 46 | 29 | 1.6 |
| Proteoglycan 4 | E9QQ17 | 111 kDa | 18 | 12 | 1.5 |
| Protein S100-A9 | P31725 | 13 kDa | 1 | 21 | 21 |
| Myosin light chain 1/3, skeletal muscle isoform | P05977 | 21 kDa | 10 | 59 | 5.9 |
| Myosin-9 | Q8VDD5 | 226 kDa | 64 | 30 | 2.1 |
| Serum amyloid A-4 protein | P31532 | 15 kDa | 13 | 11 | 1.1 |
| Myosin-10 | Q3UH59 | 233 kDa | 1 | 23 | 23 |
| 60 kDa heat shock protein | P63038 | 61 kDa | 18 | 0 | >18 |
| 40S ribosomal protein S19 | Q9CZX8 | 16 kDa | 0 | 34 | >34 |
| Decorin | P28654 | 40 kDa | 1 | 22 | 22 |
| Chondroitin sulfate proteoglycan 4 | Q8VHY0 | 252 kDa | 0 | 11 | >11 |
| Isoform 2 of Myosin-11 | O08638-2 | 223 kDa | 1 | 34 | 34 |
| Myosin regulatory light chain 12B | Q3THE2 | 20 kDa | 18 | 57 | 3.1 |
| Endoplasmin | P08113 | 92 kDa | 1 | 45 | 45 |
| Heat shock protein beta-1 | P14602 | 23 kDa | 37 | 45 | 1.2 |
| Calreticulin | P14211 | 48 kDa | 0 | 22 | >22 |
| Protein S100-A8 | P27005 | 10 kDa | 0 | 80 | >80 |
| Isoform Smooth muscle of Myosin | Q60605-2 | 17 kDa | 1 | 12 | 12 |
| Myosin light chain 3 | P09542 | 22 kDa | 0 | 14 | >14 |
| Heat shock protein beta-2 | Q99PR8 | 20 kDa | 27 | 0 | >27 |
| Biglycan | P28653 | 42 kDa | 0 | 22 | >22 |
| Serum amyloid A-1 protein | P05366 | 14 kDa | 0 | 16 | >16 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rucavado, A.; Nicolau, C.A.; Escalante, T.; Kim, J.; Herrera, C.; Gutiérrez, J.M.; Fox, J.W. Viperid Envenomation Wound Exudate Contributes to Increased Vascular Permeability via a DAMPs/TLR-4 Mediated Pathway. Toxins 2016, 8, 349. https://doi.org/10.3390/toxins8120349
Rucavado A, Nicolau CA, Escalante T, Kim J, Herrera C, Gutiérrez JM, Fox JW. Viperid Envenomation Wound Exudate Contributes to Increased Vascular Permeability via a DAMPs/TLR-4 Mediated Pathway. Toxins. 2016; 8(12):349. https://doi.org/10.3390/toxins8120349
Chicago/Turabian StyleRucavado, Alexandra, Carolina A. Nicolau, Teresa Escalante, Junho Kim, Cristina Herrera, José María Gutiérrez, and Jay W. Fox. 2016. "Viperid Envenomation Wound Exudate Contributes to Increased Vascular Permeability via a DAMPs/TLR-4 Mediated Pathway" Toxins 8, no. 12: 349. https://doi.org/10.3390/toxins8120349
APA StyleRucavado, A., Nicolau, C. A., Escalante, T., Kim, J., Herrera, C., Gutiérrez, J. M., & Fox, J. W. (2016). Viperid Envenomation Wound Exudate Contributes to Increased Vascular Permeability via a DAMPs/TLR-4 Mediated Pathway. Toxins, 8(12), 349. https://doi.org/10.3390/toxins8120349