Insight into the Mode of Action of Haedoxan A from Phryma leptostachya
Abstract
:1. Introduction
2. Results
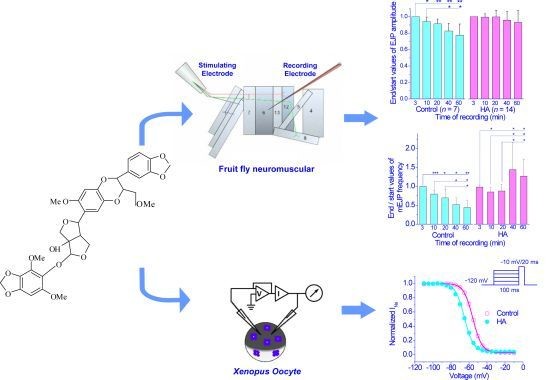
2.1. HA Delayed the Decay Rate of Evoked EJPs at the Drosophila NMJ
2.2. HA Increased the Frequency of mEJPs at the Drosophila NMJ
2.3. HA Altered the Voltage Dependence of Inactivation of Insect Sodium Channels Expressed in Xenopus oocytes
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Insects
4.3. Measurement of Synaptic Activity by Using Current Clamp
4.4. Expression of Insect Sodium Channels in Xenopus Laevis Oocytes and Electrophysiological Recording
4.5. Data Collection and Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, T.; Ting, Z.; Zhao, S. Modern Compendium of Materia Medica; China Medical Science Press: Beijing, China, 2001; p. 934. [Google Scholar]
- Lee, S.; Min, B.; Kho, Y. Brine shrimp lethality of the compounds from Phryma leptostachya L. Arch. Pharm. Res. 2002, 25, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, E.; Oshima, Y. Phrymarolin-I, a novel lignan from Phryma leptostachya L. Agric. Biol. Chem. 1972, 36, 1018–1025. [Google Scholar] [CrossRef]
- Taniguchi, E.; Oshima, Y. Structure of phrymarolin-II. Agric. Biol. Chem. 1972, 36, 1489–1496. [Google Scholar] [CrossRef]
- Taniguchi, E.; Imamura, K.; Ishibashi, F.; Matsui, T.; Nishio, A. Structure of the novel insecticidal sesquilignan, haedoxan A. Agric. Biol. Chem. 1989, 53, 631–643. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, E. Syntheses of (±)-haedoxan A, D, E and their Stereoisomers. Agric. Biol. Chem. 1989, 53, 1565–1573. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, E. Synthesis and absolute configuration of the insecticidal sesquilignan (+)-Haedoxan A. Phytochemistry 1998, 49, 613–622. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, E. Syntheses of (±)-phrymarolin II and its stereoisomers. Agric. Biol. Chem. 1986, 50, 3119–3122. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, E. Synthesis and absolute configuration of the acetalic lignan (+)-phrymarolin I. Bull. Chem. Soc. Jpn. 1988, 61, 4361–4366. [Google Scholar] [CrossRef]
- Yamauchi, S.; Taniguchi, E. Synthesis and insecticidal activity of lignan analogs (I). Agric. Biol. Chem. 1991, 55, 3075–3084. [Google Scholar] [CrossRef]
- Yamauchi, S.; Taniguchi, E. Synthesis and insecticidal activity of lignan analogs (II). Agric. Biol. Chem. 1992, 56, 412–417. [Google Scholar] [CrossRef]
- Okazaki, M.; Ishibashi, F.; Shutout, Y.; Taniguchi, E. Total synthesis of (+)-phrymarolin I from (+)-malic acid. Biosci. Biotechnol. Biochem. 1997, 61, 660–663. [Google Scholar] [CrossRef]
- Park, I.; Shin, S.; Kim, C.; Lee, H.; Choi, W.; Ahn, Y. Larvicidal activity of lignans identified in Phryma leptostachya var. asiatica roots against three mosquito species. J. Agric. Food Chem. 2005, 53, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Park, I. Larvicidal activity of medicinal plant extracts and lignan identified in Phryma leptostachya var. asiatica roots against housefly (Musca domestica L.). Parasitol. Res. 2012, 110, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, Z.; Shi, B.; Wei, S.; Wu, W. Larvicidal activity of lignans from Phryma leptostachya L. against Culex pipiens pallens. Parasitol. Res. 2012, 110, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, Z.; Ji, Z.; Shi, B.; Wei, S.; Wu, W. Isolation, structure identification and bioactivity of active ingredients from Phryma leptostachya. Chin. J. Pestic. Sci. 2012, 14, 583–586. [Google Scholar]
- Xiao, X.; Ji, Z.; Zhang, J.; Shi, B.; Wei, S.; Wu, W. A new lignan from Phryma leptostachya L. Chem. Nat. Compd. 2013, 49, 21–23. [Google Scholar] [CrossRef]
- Ozoe, Y; Hasegawa, H.; Mochida, K.; Satoh, H.; Iwabuchi, J.; Kurozumi, A.; Taniguchi, E. Sesquilignan haedoxans: Interaction with the GABAA receptor in rat brain. Biosci. Biotechnol. Biochem. 1994, 58, 760–761. [Google Scholar]
- Collins, C.A.; DiAntonio, A. Synaptic development: Insights from Drosophila. Curr. Opin. Neurobiol. 2007, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Krans, J.L.; Parfitt, K.D.; Gawera, K.D.; Rivlin, P.K.; Hoy, R.R. The resting membrane potential of Drosophila melanogaster larval muscle depends strongly on external calcium concentration. J. Insect Physiol. 2010, 56, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.M.; Kavalali, E.T. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr. Opin. Neurobiol. 2011, 21, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Imlach, W.L.; Jiao, W.; Wolfram, V.; Wu, Y.; Grbic, M.; Cela, C.; Baines, R.; Nitabach, M.N.; McCabe, B.D. Miniature neurotransmission regulates Drosophila synaptic structural maturation. Neuron 2014, 82, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.; Rahman, M.M.; Vassanelli, S. Na+ Channels at postsynaptic muscle membrane affects synaptic transmission at neuromuscular junction: A simulation study. In Proceedings of the 34th Annual International Conference of the IEEE EMBS, San Diego, CA, USA, 28 August–1 September 2012; pp. 3616–3619.
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Flucher, R.E.; Danielst, M.P. Distribution of Na+ channels and Ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd Protein. Neuron 1989, 3, 163–175. [Google Scholar] [CrossRef]
- Boudier, J.L.; le Treut, T.; Jover, E. Autoradiographic localization of voltage-dependent sodium channels on the mouse neuromuscular junction using 12SI-cy-scorpion toxin. II. Sodium channel distribution on postsynaptic membranes. J. Neurosci. 1992, 12, 454–466. [Google Scholar] [PubMed]
- Lupa, M.T.; Krzemien, D.M.; Schaller, K.L.; Caldwell, J.H. Aggregation of sodium channels during development and maturation of the neuromuscular junction. J. Neurosci. 1993, 13, 1326–1336. [Google Scholar] [PubMed]
- Peled, E.S.; Newman, Z.L.; Isacoff, E.Y. Evoked and spontaneous transmission favored by distinct sets of synapses. Curr. Biol. 2014, 24, 484–293. [Google Scholar] [CrossRef] [PubMed]
- Rees, D. The effect of metabolic inhibitors on the cockroach nerve-muscle synapse. J. Exp. Biol. 1974, 61, 331–343. [Google Scholar] [PubMed]
- Salgado, V.L.; Irving, S.N.; Miller, T.A. Depolarization of motor nerve terminals by pyrethroid in susceptible and kdr-resistant house flies. Pestic. Biochem. Physiol. 1983, 20, 100–l14. [Google Scholar] [CrossRef]
- Ulbricht, W. Sodium channel inactivation: molecular determinants and modulation. Physiol. Rev. 2005, 85, 1271–1301. [Google Scholar] [CrossRef] [PubMed]
- Motomura, H.; Narahashi, T. Temperature dependence of pyrethroid modification of single sodium channels in rat hippocampal neurons. J. Membr. Biol. 2000, 177, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Pugsley, M.K.; Yu, E.J.; Goldin, A.L. Spiradoline, a kappa opioid receptor agonist, produces tonic- and use-dependent block of sodium channels expressed in Xenopus oocytes. Gen. Pharmacol. 2000, 34, 417–427. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Kem, Q.; Wang, S.Y.; Auktor, K.; Yang, Y.; Wang, G.K.; Morgan, J.P.; Leaf, A. Single point mutations affect fatty acid block of human myocardial sodium channel a subunit Na+ channels. Proc. Natl. Acad. Sci. USA 2001, 98, 3606–3611. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. 1994, 175, 179–191. [Google Scholar] [CrossRef]
- Stewart, B.A.; Schuster, C.M.; Goodman, C.S.; Atwood, H.L. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J. Neurosci. 1996, 16, 3877–3886. [Google Scholar] [PubMed]
- Stimson, D.T.; Estes, P.S.; Smith, M.; Kelly, L.E.; Ramaswami, M. A product of the Drosophila stoned locus regulates neurotransmitter release. J. Neurosci. 1998, 18, 9638–9649. [Google Scholar] [PubMed]
- Ormerod, K.G.; Hadden, J.K.; Deady, L.D.; Mercier, A.J.; Krans, J.L. Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae. J. Neurophysiol. 2013, 110, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, Z.; Nomura, Y.; Goldin, A.L.; Dong, K. Alternative splicing of an insect sodiumchannel gene generates pharmacologically distinct sodium channels. J. Neurosci. 2002, 22, 5300–5309. [Google Scholar] [PubMed]
- Olson, R.O.; Liu, Z.; Nomura, Y.; Song, W.; Dong, K. Molecular and functional characterization of voltage-gated sodium channel variants from Drosophila melanogaster. Insect Biochem. Mol. Biol. 2008, 38, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Nomuraa, Y.; Satar, G.; Hu, Z.; Nauen, R.; He, S.Y.; Zhorove, B.S.; Dong, K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc. Natl. Acad. Sci. USA 2013, 110, 11785–11790. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Nomura, Y.; Luo, N.; Liu, Z.; Lee, J.E.; Khambay, B.; Dong, K. Molecular determinants on the insect sodium channel for the specific action of type II pyrethroid insecticides. Toxicol. Appl. Pharmacol. 2009, 234, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, Z.; Wang, R.; Huang, Z.Y.; Chen, A.C.; Gurevitz, M.; Dong, K. Identification of amino acid residues in the insect sodium channel critical for pyrethroid binding. Mol. Pharmacol. 2005, 67, 513–522. [Google Scholar] [CrossRef] [PubMed]






| Na+ Channel Type | Activation | Inactivation | ||||||
|---|---|---|---|---|---|---|---|---|
| Toxin-Free | HA | Toxin-Free | HA | |||||
| V0.5 | k | V0.5 | k | V0.5 | k | V0.5 | k | |
| BgNav1-1a | −31.5 ± 0.1 | 4.9 ± 0.1 | −29.5 ± 0.2 | 6.1 ± 0.1 | −53.5 ± 1.5 | 4.7 ± 0.1 | −64.3 ± 1.0 * | 5.4 ± 0.2 |
| DmNav22 | −30.8 ± 1.9 | 5.3 ± 0.2 | −30.5 ± 0.1 | 4.6 ± 0.7 | −46.2 ± 0.1 | 5.5 ± 0.2 | −54.1 ± 0.5 * | 6.7 ± 0.3 |
| AaNav1-1 | −30.1 ± 0.8 | 5.2 ± 0.5 | −30.1 ± 0.7 | 5.7 ± 0.5 | −52.8 ± 1.8 | 4.9 ± 0.2 | −61.3 ± 0.9 * | 5.6 ± 0.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Du, Y.; Xiao, X.; Dong, K.; Wu, W. Insight into the Mode of Action of Haedoxan A from Phryma leptostachya. Toxins 2016, 8, 53. https://doi.org/10.3390/toxins8020053
Hu Z, Du Y, Xiao X, Dong K, Wu W. Insight into the Mode of Action of Haedoxan A from Phryma leptostachya. Toxins. 2016; 8(2):53. https://doi.org/10.3390/toxins8020053
Chicago/Turabian StyleHu, Zhaonong, Yuzhe Du, Xinmin Xiao, Ke Dong, and Wenjun Wu. 2016. "Insight into the Mode of Action of Haedoxan A from Phryma leptostachya" Toxins 8, no. 2: 53. https://doi.org/10.3390/toxins8020053