Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms
Abstract
:1. Introduction
2. Results
2.1. Isolation of Major Toxins from the Venom of Naja kaouthia
2.2. Isolation of Major Toxins from the Venom of Hydrophis schistosus
2.3. Protein Concentration of Antivenom and Neutralization of Lethality
2.4. Lethality Profile and Toxicity Scoring of Purified Toxins Isolated from the Venoms of Naja kaouthia (Thailand) and Hydrophis schistosus (Malaysia)
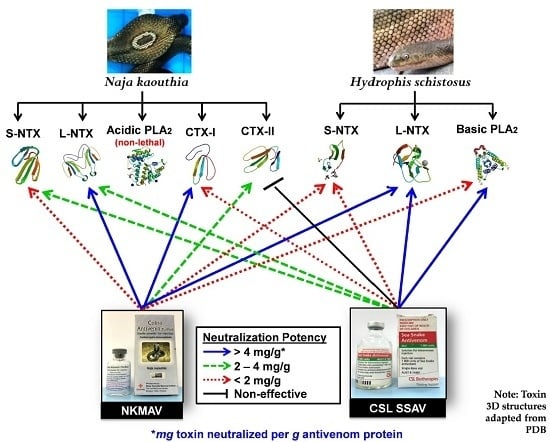
2.5. Antivenom Neutralization of Purified Toxins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venoms and Antivenoms
5.2. Animals and Ethics Clearance
5.3. Chemicals and Materials
5.4. Estimation of Protein Concentration in Antivenom
5.5. Isolation and Purification of Major Toxins from the Venom of Naja kaouthia and Hydrophis schistosus
5.5.1. Fractionation of N. kaouthia Venom Using Resource S Ion-Exchange Chromatography
5.5.2. Purification of Venom Toxins Using Reverse-Phase HPLC
5.6. SDS-PAGE and In-Solution Tryptic Digestion of Purified Toxins
5.7. Protein Identification by Liquid Chromatography-Tandem Mass Spectrometry
5.8. Estimation of the Relative Protein Abundance of Purified Toxin
5.9. Determination of Lethality of Venoms and Their Purified Toxins
5.10. Neutralization of Venoms and Purified Toxins in a Mouse Model
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Nk-T | Naja kaouthia of Thailand |
| Hs-M | Hydrophis schistosus of Malaysia |
| NKMAV | Naja kaouthia Monovalent Antivenom |
| SSAV | Sea Snake Antivenom |
| SNTX | Short neurotoxin |
| LNTX | Long neurotoxin |
| CTX | Cytotoxin |
| PLA2 | Phospholipase A2 |
| LD50 | Median lethal dose |
| ED50 | Dose at which 50% of animals survived (µL) |
| ER50 | Median effective ratio, ratio of the amount of venom (mg) to the volume dose of antivenom (mL) at which 50% of mice survived |
| P | Potency |
| T.S. | Toxicity score |
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Management of Snake-Bites; World Health Organization: Regional Office for South-East Asia: New Delhi, India, 2010. [Google Scholar]
- World Health Organization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Williams, D.J.; Gutierrez, J.M.; Calvete, J.J.; Wuster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Warrell, D.A.; Williams, D.J.; Jensen, S.; Brown, N.; Calvete, J.J.; Harrison, R.A. The need for full integration of snakebite envenoming within a global strategy to combat the neglected tropical diseases: The way forward. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malasit, P.; Warrell, D.A.; Chanthavanich, P.; Viravan, C.; Mongkolsapaya, J.; Singhthong, B.; Supich, C. Prediction, prevention, and mechanism of early (anaphylactic) antivenom reactions in victims of snake bites. Br. Med. J. 1986, 292, 17–20. [Google Scholar] [CrossRef]
- Leong, P.K.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of common southeast Asian viperid venoms by a Thai polyvalent snake antivenom (Hemato polyvalent snake antivenom). Acta Trop. 2014, 132, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro polyvalent snake antivenom). PLoS Negl. Trop. Dis. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Fung, S.Y.; Tan, C.H.; Sim, S.M.; Tan, N.H. Immunological cross-reactivity and neutralization of the principal toxins of Naja sumatrana and related cobra venoms by a thai polyvalent antivenom (neuro polyvalent snake antivenom). Acta Trop. 2015, 149, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, R.; Isbister, G.K.; O’Leary, M.A.; Mirtschin, P.; Dunstan, N.; Hodgson, W.C. Cross-neutralisation of the neurotoxic effects of egyptian cobra venom with commercial tiger snake antivenom. Basic Clin. Pharmacol. Toxicol. 2013, 112, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Leong, P.K.; Fung, S.Y.; Sim, S.M.; Ponnudurai, G.; Ariaratnam, C.; Khomvilai, S.; Sitprija, V.; Tan, N.H. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop. 2011, 117, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of southeast asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, K.Y.; Lim, S.E.; Tan, N.H. Venomics of the beaked sea snake, Hydrophis schistosus: A minimalist toxin arsenal and its cross-neutralization by heterologous antivenoms. J. Proteom. 2015, 126, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, N.H.; Tan, K.Y.; Kwong, K.O. Antivenom cross-neutralization of the venoms of Hydrophis schistosus and Hydrophis curtus, two common sea snakes in malaysian waters. Toxins 2015, 7, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Gutiérrez, J.M.; Lohse, B.; Rasmussen, A.R.; Fernández, J.; Milbo, C.; Lomonte, B. Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon 2015, 99, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, C.H. Symptomatology, pathology and treatment of the bites of elapid snakes. In Snake Venoms; Lee, C.-Y., Ed.; Springer: Berlin, Germany; Heidelberg, Germany, 1979; Volume 52, pp. 898–921. [Google Scholar]
- Tan, C.H.; Tan, N.H. Toxinology of snake venoms: The Malaysian context. In Snake Venoms; Gopalakrishnakone, P., Inagaki, H., Mukherjee, A.K., Rahmy, T.R., Vogel, C.-W., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–37. [Google Scholar]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Osipov, A.V.; Kasheverov, I.E.; Makarova, Y.V.; Starkov, V.G.; Vorontsova, O.V.; Ziganshin, R.; Andreeva, T.V.; Serebryakova, M.V.; Benoit, A.; Hogg, R.C.; et al. Naturally occurring disulfide-bound dimers of three-fingered toxins: A paradigm for biological activity diversification. J. Biol. Chem. 2008, 283, 14571–14580. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Tan, C.H. Pharmacokinetics of Naja sumatrana (equatorial spitting cobra) venom and its major toxins in experimentally envenomed rabbits. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K. Correlation between the phospholipids domains of the target cell membrane and the extent of Naja kaouthia PLA2-induced membrane damage: Evidence of distinct catalytic and cytotoxic sites in PLA2 molecules. Biochim. Biophys. Acta 2007, 1770, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Khow, O.; Chanhome, L.; Omori-Satoh, T.; Sitprija, V. Effectiveness of Thai cobra (Naja kaouthia) antivenom against sea snake (Lapemis hardwickii) venom: Verification by affinity purified F(ab′)2 fragments. J. Nat. Toxins 2001, 10, 249–253. [Google Scholar] [PubMed]
- Minton, S.A., Jr. Paraspecific protection by elapid and sea snake antivenins. Toxicon 1967, 5, 47–55. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Schneider, J.J.; Krishnan, B.P.; Lavis, C.; McKendry, A.; Ong, L.K.; Isbister, G.K. Cross-neutralisation of Australian brown and tiger snake venoms with commercial antivenoms: Cross-reactivity or antivenom mixtures? Toxicon 2007, 50, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Chetty, N.; Du, A.; Hodgson, W.C.; Winkel, K.; Fry, B.G. The in vitro neuromuscular activity of Indo-pacific sea-snake venoms: Efficacy of two commercially available antivenoms. Toxicon 2004, 44, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, C.H.; Tan, N.H. Venom and purified toxins of the spectacled cobra (Naja naja) from Pakistan: Insights into toxicity and antivenom neutralization. Am. J. Trop. Med. Hyg. 2016. [Google Scholar] [CrossRef]
- Sriprapat, S.; Aeksowan, S.; Sapsutthipas, S.; Chotwiwatthanakun, C.; Suttijitpaisal, P.; Pratanaphon, R.; Khow, O.; Sitprija, V.; Ratanabanangkoon, K. The impact of a low dose, low volume, multi-site immunization on the production of therapeutic antivenoms in Thailand. Toxicon 2003, 41, 57–64. [Google Scholar] [CrossRef]
- Chotwiwatthanakun, C.; Pratanaphon, R.; Akesowan, S.; Sriprapat, S.; Ratanabanangkoon, K. Production of potent polyvalent antivenom against three elapid venoms using a low dose, low volume, multi-site immunization protocol. Toxicon 2001, 39, 1487–1494. [Google Scholar] [CrossRef]
- Brade, V.; Vogt, W. Immunization against cobra venom. Experientia 1971, 27, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.-H. Improvement of malayan cobra (Naja naja sputatrix) antivenin. Toxicon 1983, 21, 75–79. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Fung, S.Y.; Tan, N.H. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genom. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Howard-Jones, N. A CIOMS ethical code for animal experimentation. WHO Chron. 1985, 39, 51–56. [Google Scholar] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge University Press: London, UK, 1952. [Google Scholar]
- Morais, V.; Ifran, S.; Berasain, P.; Massaldi, H. Antivenoms: Potency or median effective dose, which to use? J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 191–193. [Google Scholar] [CrossRef]



| Protein Fraction | % | Protein ID | MS/MS Derived Sequence | Matched Peptide | Matched MH+ | MH+ Error (ppm) | Accession No. (Species) | Protein Score |
|---|---|---|---|---|---|---|---|---|
| F1a | 17.0 | Acidic PLA2 1 | CCQVHDNCYNEAEK | 1 | 1828.70 | −0.3 | P00596 (N. kaouthia) | 187 |
| CCQVHDNCYNEAEK | 1 | 1827.69 | −2.1 | |||||
| CWPYFK | 1 | 901.42 | 0.9 | |||||
| CWPYFKTYSYECSQGTLTCK | 1 | 2581.12 | −1.0 | |||||
| CWPYFKTYSYECSQGTLTCK | 1 | 2580.11 | 0.0 | |||||
| GDNDACAAAVCDCDR | 1 | 1670.61 | 0.5 | |||||
| GDNDACAAAVCDCDR | 1 | 1671.62 | 1.0 | |||||
| LAAICFAGAPYNNNNYNIDLK | 3 | 2357.14 | −1.3 | |||||
| LAAICFAGAPYNNNNYNIDLK | 1 | 2358.15 | −1.4 | |||||
| LAAICFAGAPYNNNNYNIDLK | 4 | 2358.15 | 0.4 | |||||
| LAAICFAGAPYNNNNYNIDLK | 1 | 2358.15 | −0.6 | |||||
| LAAICFAGAPYNNNNYNIDLK | 1 | 2359.16 | 1.0 | |||||
| LAAICFAGAPYNNNNYNIDLKAR | 1 | 2585.29 | −0.2 | |||||
| NMIQCTVPNR | 1 | 1249.59 | −0.8 | |||||
| NMIQCTVPNR | 1 | 1234.61 | 5.4 | |||||
| SWWDFADYGCYCGR | 1 | 1843.71 | 0.6 | |||||
| SWWDFADYGCYCGR | 2 | 1844.72 | 0.5 | |||||
| SWWDFADYGCYCGR | 1 | 1843.71 | −0.6 | |||||
| SWWDFADYGCYCGR | 1 | 1843.71 | −0.4 | |||||
| TYSYECSQGTLTCK | 1 | 1698.72 | 0.6 | |||||
| TYSYECSQGTLTCK | 1 | 1699.73 | 2.2 | |||||
| TYSYECSQGTLTCK | 1 | 1698.72 | 0.1 | |||||
| F2a | 4.6 | Cobrotoxin-c | LECHNQQSSQAPTTK | 1 | 1730.81 | 1.1 | P59276 (N. kaouthia) | 64 |
| LECHNQQSSQAPTTKTCSGETNCYK | 1 | 2932.27 | −1.9 | |||||
| LECHNQQSSQAPTTKTCSGETNCYK | 1 | 2931.26 | −2.1 | |||||
| VKPGVNLNCCR | 1 | 1317.66 | 0.6 | |||||
| F2b | 30.9 | Alpha-elapitoxin Nk2a | CFITPDITSK | 5 | 1182.60 | 1.6 | P01391 (N. kaouthia) | 101 |
| RVDLGCAATCPTVK | 1 | 1549.81 | 15.3 | |||||
| TGVDIQCCSTDNCNPFPTR | 1 | 2242.94 | −0.2 | |||||
| TGVDIQCCSTDNCNPFPTR | 1 | 2243.95 | 2.1 | |||||
| TGVDIQCCSTDNCNPFPTR | 1 | 2244.96 | 2.7 | |||||
| TGVDIQCCSTDNCNPFPTRK | 1 | 2372.04 | 0.9 | |||||
| TGVDIQCCSTDNCNPFPTRK | 1 | 2371.03 | −2.0 | |||||
| TWCDAFCSIR | 2 | 1316.57 | 1.5 | |||||
| TWCDAFCSIR | 1 | 1317.57 | 2.7 | |||||
| TWCDAFCSIR | 1 | 1316.56 | 1.0 | |||||
| VDLGCAATCPTVK | 1 | 1393.68 | 2.2 | |||||
| F3a | 19.2 | Cytotoxin 2 | GCIDVCPKNSLLVK | 1 | 1603.84 | 0.1 | P01445 (N. kaouthia) | 82 |
| LIPLAYK | 1 | 818.53 | 0.6 | |||||
| LIPLAYK | 5 | 818.53 | 0.9 | |||||
| LIPLAYK | 1 | 818.53 | 3.8 | |||||
| LIPLAYKTCPAGK | 1 | 1433.82 | 3.5 | |||||
| NSLLVKYVCCNTDR | 1 | 1742.85 | 1.7 | |||||
| YVCCNTDR | 1 | 1088.44 | 0.4 | |||||
| F4a | 4.6 | Cytotoxin | CNKLVPLFYKTCPAGK | 1 | 1897.99 | −7.6 | Q02454 (N. sputatrix) | 146 |
| MFMVATPKVPVK | 1 | 1349.76 | −4.5 | |||||
| LKCNKLVPLFYK | 1 | 1523.88 | −3.0 | |||||
| SSLLVKYVCCNTDR | 1 | 1715.83 | −2.2 | |||||
| YVCCNTDR | 1 | 1088.44 | −0.8 | |||||
| GCIDVCPKSSLLVK | 1 | 1576.83 | −0.5 | |||||
| NLCYKMFMVATPK | 1 | 1603.79 | 0.4 | |||||
| SSLLVKYVCCNTDR | 1 | 1716.84 | 0.6 | |||||
| LVPLFYKTCPAGK | 1 | 1495.83 | 0.8 | |||||
| MFMVATPK | 2 | 925.48 | 1.7 | |||||
| LVPLFYK | 6 | 880.54 | 2.6 |
| Venom | Toxin Abundance in Venom (%) | i.v. LD50 (µg/g) | Toxicity Score (g/µg) |
|---|---|---|---|
| Naja kaouthia (Thailand) | 0.18 (0.12–0.27) # | ||
| F1a (Nk-T acidic PLA2) | 17.0 | >5.00 | <5 |
| F2a (Nk-T SNTX) | 4.6 | 0.12 (0.11–0.14) | 38 |
| F2b (Nk-T LNTX) | 30.9 | 0.09 (0.06–0.14) | 343 |
| F3a (Nk-T CTX-I) | 19.2 | 1.41 (1.08–1.85) | 14 |
| F4a (Nk-T CTX-II) | 4.6 | 1.75 (1.68–1.83) | 3 |
| Hydrophis schistosus (Malaysia) | 0.07 (0.05–0.09) @ | ||
| H1 (Hs-M SNTX) | 52.2 * | 0.07 (0.05–0.09) * | 746 |
| H2 (Hs-M LNTX) | 11.9 * | 0.18 (0.16–0.20) * | 66 |
| H3 (Hs-M basic PLA2) | 19.2 * | 0.08 (0.06–0.10) * | 240 |
| Antivenom | Protein Concentration (mg/mL) | Neutralization of Naja kaouthia Venom | |||
|---|---|---|---|---|---|
| ED50 (µL) | ER50 (mg/mL) | P (mg/mL) | Normalized P (mg/g) | ||
| NKMAV | 45.0 ± 0.6 | 18.75 # | 1.15 # (0.77–1.73) | 0.92 # | 20.44 |
| SSAV | 217.2 ± 3.0 | 11.24 | 2.00 (1.33–3.00) | 1.60 | 7.37 |
| Venom Toxin | i.v. LD50 (µg/g) | NKMAV | SSAV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Challenge | ED50 (µL) | ER50 (mg/mL) | P (mg/mL) | Normalized P (mg/g) | Challenge | ED50 (µL) | ER50 (mg/mL) | P (mg/mL) | Normalized P (mg/g) | ||
| Naja kaouthia (Thailand) | |||||||||||
| F2a (Nk-T SNTX) | 0.12 (0.11–0.14) | 2.5 LD50 | 70.68 | 0.10 (0.09–0.12) | 0.06 | 1.33 | 5 LD50 | 21.37 | 0.67 (0.62–0.79) | 0.54 | 2.49 |
| F2b (Nk-T LNTX) | 0.09 (0.06–0.14) | 5 LD50 | 39.14 | 0.28 (0.18–0.43) | 0.22 | 4.89 | 5 LD50 | 16.05 | 0.67 (0.45–1.05) | 0.54 | 2.49 |
| F3a (Nk-T CTX-I) | 1.41 (1.08–1.85) | 1.5 LD50 | 53.16 | 0.875 (0.670–1.148) | 0.29 | 6.44 | 1.5 LD50 | 175.00 | 0.27 (0.20–0.35) | 0.09 | 0.41 |
| F4a (Nk-T CTX-II) | 1.75 (1.68–1.83) | 1.5 LD50 | 156.57 | 0.40 (0.39–0.42) | 0.13 | 2.89 | 1.5 LD50 | N.E. | N.E. | N.E. | N.E. |
| Hydrophis schistosus (Malaysia) | |||||||||||
| F1 (Hs-M SNTX) | 0.07 (0.05–0.09) * | 1.5 LD50 | 128.41 | 0.02 (0.01–0.02) | 0.01 | 0.22 | 5 LD50 | 17.67 | 0.44 (0.31–0.56) | 0.35 | 1.61 |
| F2 (Hs-M LNTX) | 0.18 (0.16–0.20) * | 5 LD50 | 81.25 | 0.22 (0.20–0.25) | 0.18 | 4.00 | 5 LD50 | 11.98 | 1.73 (1.54–1.92) | 1.38 | 6.35 |
| F3 (Hs-M basic PLA2) | 0.08 (0.06–0.10) * | 1.5 LD50 | 125.00 | 0.02 (0.01–0.02) | 0.01 | 0.22 | 5 LD50 | 5.62 | 1.57 (1.17–1.96) | 1.25 | 5.76 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms. Toxins 2016, 8, 86. https://doi.org/10.3390/toxins8040086
Tan KY, Tan CH, Fung SY, Tan NH. Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms. Toxins. 2016; 8(4):86. https://doi.org/10.3390/toxins8040086
Chicago/Turabian StyleTan, Kae Yi, Choo Hock Tan, Shin Yee Fung, and Nget Hong Tan. 2016. "Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms" Toxins 8, no. 4: 86. https://doi.org/10.3390/toxins8040086