Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding †
Abstract
:1. Introduction
2. Describing the Occurrence of Hemorrhage in Clinical and Experimental Viperid Snakebite Envenomings
3. Devising Methods to Quantify Venom-Induced Hemorrhage
4. Characterizing the Biochemical Properties of Hemorrhagic Toxins Present in Snake Venoms
5. Exploring the Pathological Effects Induced by Venoms and SVMPs on the Microvasculature
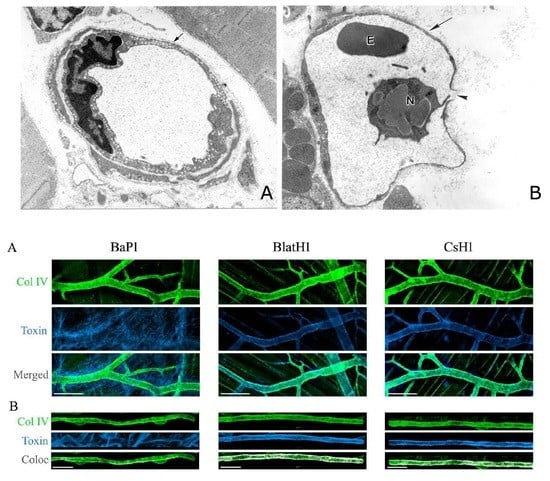
6. Are Endothelial Cells Directly Damaged by Hemorrhagic SVMPs? A Two-Step Hypothesis and the Role of Biophysical Factors Operating in Vivo
7. Exploring the Hydrolysis of Basement Membrane Components by SVMPs
8. Exploring the Hydrolysis of BM Components by SVMPs in Vivo
9. What Is the Basis for the Large Variation in the Hemorrhagic Potential of SVMPs?
10. SVMP-Induced Hemorrhage Viewed from a Holistic Perspective: Studying the Action of SVMP in the Context of the Whole Venom
11. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gutiérrez, J.M.; Theakston, R.D.G.; Warrell, D.A. Confronting the neglected problem of snake bite envenoming: The need for a global partnership. PLoS Med. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Gutiérrez, J.M.; Harrison, R.; Warrell, D.A.; White, J.; Winkel, K.D.; Gopalakrishnakone, P. The Global Snake Bite Initiative: An antidote for snake bite. Lancet 2010, 375, 89–91. [Google Scholar] [CrossRef]
- Calvete, J.J. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev. Proteom. 2011, 8, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.L.C.; França, F.O.S.; Wen, F.H.; Málaque, C.M.S.; Haddad, V., Jr. Animais Peçonhentos no Brasil. Biologia, Clínica e Terapêutica dos Acidentes, 2nd ed.; Sarvier: São Paulo, Brazil, 2009. (In Portuguese) [Google Scholar]
- Mitchel, S.W. Researchers upon the Venom of the Rattlesnake: With an Investigation of the Anatomy and Physiology of the Organs Concerned; Smithsonian Institution: Washington, DC, USA, 1860. [Google Scholar]
- Brazil, V. A Defesa Contra o Ophidismo; Pocai & Weiss: São Paulo, Brasil, 1911. (In Portuguese) [Google Scholar]
- Picado, C. Serpientes Venenosas de Costa Rica. Sus Venenos. In Seroterapia Antiofídica; Imprenta Alsina: San José, Costa Rica, 1931. (In Spanish) [Google Scholar]
- De Lacerda, J.B. Leçons sur le Venin des Serpents du Brésil; Lombaerts: Rio de Janeiro, Brasil, 1884. (In French) [Google Scholar]
- Mitchel, S.W.; Reichert, E.T. Researches upon the Venoms of Poisonous Serpents; Smithsonian Institution: Washington, DC, USA, 1886. [Google Scholar]
- Ohsaka, A. Hemorrhagic, necrotizing and edema-forming effects of snake venoms. In Handbook of Experimental Pharmacology; Springer-Verlag: Berlin, Germany, 1979; Volume 52, pp. 480–546. [Google Scholar]
- Kondo, H.; Kondo, S.; Ikezawa, H.; Murata, R.; Ohsaka, A. Studies on the quantitative method for the determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 1960, 13, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.G.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar] [PubMed]
- Tu, A.T.; Homma, M.; Hong, B.S. Hemorrhagic, myonecrotic, thrombotic and proteolytic activities of viper venoms. Toxicon 1969, 6, 175–178. [Google Scholar] [CrossRef]
- Bjarnason, J.B.; Tu, A.T. Hemorrhagic toxins from Western diamondback rattlesnake (Crotalus atrox) venom: Isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e. Biochemistry 1978, 17, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Gené, J.A.; Rojas, G.; Cerdas, L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 1985, 23, 887–893. [Google Scholar] [CrossRef]
- Rucavado, A.; Escalante, T.; Teixeira, C.F.P.; Fernándes, C.M.; Díaz, C.; Gutiérrez, J.M. Increments in cytokines and matrix metalloproteinases in skeletal muscle after injection of tissue-damaging toxins from the venom of the snake Bothrops asper. Mediat. Inflamm. 2002, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bonta, I.L.; Vargaftig, V.V.; Bhargava, N.; de Vos, C.J. Method for study of snake venom induced hemorrhage. Toxicon 1970, 8, 3–10. [Google Scholar] [CrossRef]
- Ownby, C.L.; Colberg, T.R.; Odell, G.V. A new method for quantitating hemorrhage induced by rattlesnake venoms: Ability of polyvalent antivenom to neutralize hemorrhagic activity. Toxicon 1984, 22, 227–233. [Google Scholar] [CrossRef]
- Escalante, T.; Núñez, J.; Moura-da-Silva, A.M.; Theakston, R.D.G.; Gutiérrez, J.M. Pulmonary hemorrhage induced by jararhagin, a metalloproteinase from Bothrops jararaca snake venom. Toxicol. Appl. Pharmacol. 2003, 193, 17–28. [Google Scholar] [CrossRef]
- Flexner, S.; Noguchi, H. The constitution of snake venom and snake sera. J. Path. Bact. 1903, 8, 379–410. [Google Scholar] [CrossRef]
- Houssay, B.A. Classification des actions des venins de serpents sur l’organisme animal. Comp. Rend. Soc. Biol. Paris 1930, 105, 308–310. (In French) [Google Scholar]
- Flowers, H.N.; Goucher, C.R. The effect of EDTA on the extent of tissue damage caused by the venoms of Bothrops atrox and Agkistrodon piscivorus. Toxicon 1965, 2, 221–224. [Google Scholar] [CrossRef]
- Ohsaka, A.; Ikezawa, H.; Kondo, H.; Kondo, S.; Uchida, N. Haemorrhagic activities of Habu snake venom, and their relations to lethal toxicity, proteolytic activities and other pathological activities. Br. J. Exp. Pathol. 1960, 41, 478–486. [Google Scholar] [PubMed]
- Grotto, L.; Moroz, C.; de Vries, A.; Goldblum, N. Isolation of Vipera palestinae hemorrhagin and distinction between its hemorrhagic and proteolytic activities. Biochim. Biophys. Acta 1967, 133, 356–362. [Google Scholar] [CrossRef]
- Takahashi, T.; Ohsaka, A. Purification and some properties of two hemorrhagic principles (HR2a and HR2b) in the venom of Trimeresurus flavoviridis; complete separation of the principles from proteolytic activities. Biochim. Biophys. Acta 1970, 207, 65–75. [Google Scholar] [CrossRef]
- Kunitz, M. Crystalline soybean trypsin inhibitor. II. General properties. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, J.B.; Fox, J.W. Hemorrhagic toxins from snake venoms. J. Toxicol. Toxin Rev. 1988–1989, 7, 121–209. [Google Scholar] [CrossRef]
- Mandelbaum, F.R.; Reichl, A.P.; Assakura, M. Some physical and biochemical characteristics of HF2, one of the hemorrhagic factors in the venom of Bothrops jararaca. In Animal, Plant, and Microbial Toxins; Plenum Press: New York, NY, USA, 1976; Volume 1, pp. 111–121. [Google Scholar]
- Bjarnason, J.B.; Fox, J.W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994, 62, 325–372. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Bode, W.; Gomis-Rüth, F.X.; Stöckler, W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met turn) and topologies and should be grouped into a common family, the “metzincins”. FEBS Lett. 1993, 331, 134–140. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Snake venom metalloproteinases. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010; pp. 95–113. [Google Scholar]
- Casewell, N.R.; Sunagar, K.; Takacs, Z.; Calvete, J.J.; Jackson, T.N.W.; Fry, B.G. Snake venom metalloprotese enzymes. In Venomous Reptiles and Their Toxins. Evolution, Pathophysiology and Discovery; Oxford University Press: Oxford, UK, 2015; pp. 347–363. [Google Scholar]
- Taube, H.N.; Essex, H.E. Pathologic changes in the tissue of the dog following injections of rattlesnake venom. Arch. Pathol. 1937, 24, 43–51. [Google Scholar]
- Homma, M.; Tu, A.T. Morphology of local tissue damage in experimental snake envenomation. Br. J. Exp. Pathol. 1971, 52, 538–542. [Google Scholar] [PubMed]
- Ohsaka, A.; Suzuki, K.; Ohashi, M. The spurting of erythrocytes through junctions of the vascular endothelium treated with snake venom. Microvasc. Res. 1975, 10, 208–213. [Google Scholar] [CrossRef]
- Ohsaka, A.; Ohashi, M.; Tsuchiya, M.; Kamisaka, Y.; Fujishiro, Y. Action of Trimeresurus flavoviridis venom on the microcirculatory system of rat; dynamic aspects as revealed by cinematographic recording. Jpn. J. Med. Sci. Biol. 1971, 24, 34–39. [Google Scholar]
- McKay, D.G.; Moroz, C.; de Vries, A.; Csavossy, I.; Cruse, V. The action of hemorrhagin and phospholipase derived from Vipera palestinae venom on the microcirculation. Lab. Investig. 1970, 22, 387–399. [Google Scholar] [PubMed]
- Ownby, C.L.; Kainer, R.A.; Tu, A.T. Pathogenesis of hemorrhage induced by rattlesnake venom. An electron microscopic study. Am. J. Pathol. 1974, 76, 401–414. [Google Scholar] [PubMed]
- Ownby, C.L.; Bjarnason, J.B.; Tu, A.T. Hemorrhagic toxins from rattlesnake (Crotalus atrox) venom. Pathogenesis of hemorrhage induced by three purified toxins. Am. J. Pathol. 1978, 93, 201–218. [Google Scholar] [PubMed]
- Ownby, C.L.; Geren, C.R. Pathogenesis of hemorrhage induced by hemorrhagic proteinase IV from timber rattlesnake (Crotalus horridus horridus) venom. Toxicon 1987, 25, 517–526. [Google Scholar] [PubMed]
- Moreira, L.; Borkow, G.; Ovadia, M.; Gutiérrez, J.M. Pathological changes induced by BaH1, a hemorrhagic proteinase isolated from Bothrops asper (Terciopelo) snake venom, on mouse capillary blood vessels. Toxicon 1994, 32, 976–987. [Google Scholar] [CrossRef]
- Anderson, S.G.; Ownby, C.L. Pathogenesis of hemorrhage induced by proteinase H from eastern diamondback rattlesnake (Crotalus adamanteus) venom. Toxicon 1997, 35, 1291–1300. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Núñez, J.; Escalante, T.; Rucavado, A. Blood flow is required for rapid endothelial cell damage induced by a snake venom hemorrhagic metalloproteinase. Microvasc. Res. 2006, 71, 55–63. [Google Scholar] [PubMed]
- Gonçalves, L.R.C.; Mariano, M. Local haemorrhage induced by Bothrops jararaca venom: Relationship to neurogenic inflammation. Mediat. Inflamm. 2000, 9, 101–107. [Google Scholar]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Gallin, J.I.; Snyderman, R. Inflammation: Basic Principles and Clinical Correlates; Lippincot: Philadelphia, PA, USA, 1999. [Google Scholar]
- Malucelli, B.E.; Mariano, M. The haemorrhagic exudate and its possible relationship to neurogenic inflammation. J. Pathol. 1980, 130, 193–200. [Google Scholar] [PubMed]
- Lomonte, B.; Lundgren, J.; Johansson, B.; Bagge, U. The dynamics of local tissue damage induced by Bothrops asper snake venom and myotoxin II on the mouse cremaster muscle. Toxicon 1994, 32, 41–55. [Google Scholar] [PubMed]
- Rucavado, A.; Lomonte, B.; Ovadia, M.; Gutiérrez, J.M. Local tissue damage induced by BaP1, a metalloproteinase isolated from Bothrops asper (Terciopelo) snake venom. Exp. Mol. Pathol. 1995, 63, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Gutiérrez, J.M.; Borkow, G.; Tarkowski, A.; Hanson, L.Å. Activity of hemorrhagic metalloproteinase BaH-1 and myotoxin II from Bothrops asper snake venom on capillary endothelial cells in vitro. Toxicon 1994, 32, 505–510. [Google Scholar] [CrossRef]
- Borkow, G.; Gutiérrez, J.M.; Ovadia, M. In vitro activity of BaH1, the main hemorrhagic toxin of Bothrops asper snake venom on bovine endothelial cells. Toxicon 1995, 33, 1387–1391. [Google Scholar] [CrossRef]
- Wu, W.B.; Chang, S.C.; Liau, M.Y.; Huang, T.F. Purification, molecular cloning and mechanism of action of graminelysin I, a snake-venom-derived metalloproteinase that induces apoptosis of human endothelial cells. Biochem. J. 2001, 357, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Ohta, T.; Kaji, K.; Fox, J.W.; Hayashi, H.; Araki, S. cDNA cloning and characterization of vascular apoptosis-inducing protein 1. Biochem. Biophys. Res. Commun. 2000, 278, 197–204. [Google Scholar] [CrossRef] [PubMed]
- You, W.K.; Seo, H.J.; Chung, K.H.; Kim, D.S. A novel metalloprotease from Gloydius halys venom induces endothelial cell apoptosis through its protease and disintegrin-like domains. J. Biochem. 2003, 134, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Valverde, L.; Brenes, O.; Rucavado, A.; Gutiérrez, J.M. Characterization of events associated with apoptosis/anoikis induced by snake venom metalloproteinase BaP1 on human endothelial cells. J. Cell. Biochem. 2005, 94, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Tanjoni, I.; Weinlich, R.; Della-Casa, M.S.; Clissa, P.B.; Saldanha-Gama, R.F.; de Freitas, M.S.; Barja-Fidalgo, C.; Amarante-Mendes, G.P.; Moura-da-Silva, A.M. Jararhagin, a snake venom metalloproreinase, induces a specialized form of apoptosis (anoikis) selective to endothelial cells. Apoptosis 2005, 10, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Brenes, O.; Muñoz, E.; Roldán-Rodríguez, R.; Díaz, C. Cell death induced by Bothrops asper snake venom metalloproteinase on endothelial and other cell lines. Exp. Mol. Pathol. 2010, 88, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.B.; Wang, T.F. Activation of MMP-2, cleavage of matrix proteins, and adherens junctions during a snake venom metalloproteinase-induced endothelial cell apoptosis. Exp. Cell Res. 2003, 288, 143–157. [Google Scholar] [CrossRef]
- Baldo, C.; Lopes, D.S.; Faquim-Mauro, E.L.; Jacysyn, J.F.; Niland, S.; Eble, J.A.; Clissa, P.B.; Moura-da-Silva, A.M. Jararhagin disruption of endothelial cell anchorage is enhanced in collagen enriched matrices. Toxicon 2015, 108, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Milnor, W.R. Principles of hemodynamics. In Medical Physiology; Mosby: St. Louis, MO, USA, 1980; pp. 1017–1032. [Google Scholar]
- Ballerman, B.J.; Dardik, A.; Eng, E.; Liu, A. Shear stress and the endothelium. Kidney Int. 1998, 67, S100–S108. [Google Scholar] [CrossRef]
- Murphy, M.E.; Johnson, P.C. Possible contribution of basement membrane to the structural rigidity of blood capillaries. Microvasc. Res. 1975, 9, 242–245. [Google Scholar] [CrossRef]
- Lee, J.; Schmid-Schönbein, G.W. Biomechanics of skeletal muscle capillaries: Hemodynamic resistance, endothelial distensibility, and pseudopod formation. Ann. Biomed. Eng. 1995, 23, 226–246. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rucavado, A.; Fox, J.W.; Gutiérrez, J.M. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J. Proteom. 2011, 74, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Ohsaka, A.; Just, M.; Habermann, E. Action of snake venom hemorrhagic principles on isolated glomerular basement membrane. Biochim. Biophys. Acta 1973, 323, 415–438. [Google Scholar] [CrossRef]
- Bjarnason, J.B.; Hamilton, D.; Fox, J.W. Studies on the mechanism of hemorrhage production by five proteolytic hemorrhagic toxins from Crotalus atrox venom. Biol. Chem. Hoppe Seyler 1988, 369, 121–129. [Google Scholar] [PubMed]
- Baramova, E.N.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Degradation of extracellular matrix proteins by hemorrhagic metalloproteinases. Arch. Biochem. Biophys. 1989, 275, 63–71. [Google Scholar] [CrossRef]
- Baramova, E.N.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Identification of the cleavage sites by a hemorrhagic metalloproteinase in type IV collagen. Matrix 1990, 10, 91–97. [Google Scholar] [CrossRef]
- Baramova, E.N.; Shannon, J.D.; Fox, J.W.; Bjarnason, J.B. Proteolytic digestion of non-collagenous basement membrane proteins by the hemorrhagic metalloproteinase Ht-e from Crotalus atrox venom. Biomed. Biochim. Acta 1991, 50, 763–768. [Google Scholar] [PubMed]
- Maruyama, M.; Sugiki, M.; Yoshida, E.; Shimaya, K.; Mihara, H. Broad substrate specificity of snake venom fibrinolytic enzymes: Possible role in haemorrhage. Toxicon 1992, 30, 1387–1397. [Google Scholar] [CrossRef]
- Escalante, T.; Shannon, J.; Moura-da-Silva, A.M.; Gutiérrez, J.M.; Fox, J.W. Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: A biochemical and immunohistochemical study. Arch. Biochem. Biophys. 2006, 455, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.K.; Paes-Leme, A.F.; Asega, A.F.; Camargo, A.C.; Fox, J.W.; Serrano, S.M.T. New insights into the structural elements involved in the skin haemorrhage induced by snake venom metalloproteinases. Thromb. Haemost. 2010, 104, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.P.; Menaldo, D.L.; Camacho, E.; Rosa, J.C.; Escalante, T.; Rucavado, A.; Lomonte, B.; Gutiérrez, J.M.; Sampaio, S.V. Proteomic analysis of Bothrops pirajai snake venom and characterization of BpirMP, a new P-I metalloproteinase. J. Proteom. 2013, 27, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Flores-Sanchez, E.; Franceschi, A.; Magalhaes, A.; Gutiérrez, J.M. Characterization of the local tissue damage induced by LHF-II, a metalloproteinase with weak hemorrhagic activity isolated from Lachesis muta muta snake venom. Toxicon 1999, 37, 1297–1312. [Google Scholar] [CrossRef]
- Rodrigues, V.M.; Soares, A.M.; Guerra-Sá, R.; Rodrigues, V.; Fontes, M.R.; Giglio, J.R. Structural and functional characterization of neuwiedase, a nonhemorrhagic fibrin(ogen)olytic metalloprotease from Bothrops neuwiedi snake venom. Arch. Biochem. Biophys. 2000, 381, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Ortiz, N.; Rucavado, A.; Sanchez, E.F.; Richardson, M.; Fox, J.W.; Gutiérrez, J.M. Role of collagens and perlecan in microvascular stability: Exploring the mechanism of capillary vessel damage by snake venom metalloproteinases. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumor angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Timpl, R.; Brown, J.C. The laminins. Matrix Biol. 1994, 14, 275–281. [Google Scholar] [CrossRef]
- Yurchenko, P.D.; Amenta, P.S.; Patton, B.L. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004, 22, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Baldo, C.; Jamora, C.; Yamanouye, N.; Zorn, T.M.; Moura-da-Silva, A.M. Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: Tissue distribution and in situ hydrolysis. PLoS Negl. Trop. Dis. 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Escalante, T.; Voisin, M.B.; Rucavado, A.; Morazán, D.; Macêdo, J.K.; Calvete, J.M.; Sanz, L.; Nourshargh, S.; Gutiérrez, J.M.; et al. Tissue localization and extracellular matrix degradation by PI, PII and PIII snake venom metalloproteinases: Clues on the mechanisms of venom-induced hemorrhage. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rucavado, A.; Pinto, A.F.; Terra, R.M.; Gutiérrez, J.M.; Fox, J.W. Wound exudate as a proteomic window to reveal different mechanisms of tissue damage by snake venom toxins. J. Proteome Res. 2009, 8, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Paes-Leme, A.F.; Sherman, N.E.; Smalley, D.M.; Sizukusa, M.O.; Oliveira, A.K.; Menezes, M.C.; Fox, J.W.; Serrano, S.M.T. Hemorrhagic activity of HF3, a snake venom metalloproteinase: Insights from the proteomic analysis of mouse skin and blood plasma. J. Proteome Res. 2012, 11, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.J.; Maslen, C.L.; Keene, D.R.; Glanville, R.W. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem. 1997, 272, 26522–26529. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V. Basement membrane proteoglycans: From cellar to ceiling. Nat. Rev. Mol. Cell. Biol. 2005, 6, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Nikai, T.; Taniguchi, K.; Komori, Y.; Masuda, K.; Fox, J.W.; Sugihara, H. Primary structure and functional characterization of bilitoxin-1, a novel dimeric P-II snake venom metalloproteinase from Agkistrodon bilineatus venom. Arch. Biochem. Biophys. 2000, 378, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.; Villalobos, E.; Sanz, L.; Pérez, A.; Escalante, T.; Lomonte, B.; Calvete, J.J.; Gutiérrez, J.M.; Rucavado, A. Understanding structural and functional aspects of PII snake venom metalloproteinases: Characterization of BlatH1, a hemorrhagic dimeric enzyme from the venom of Bothriechis lateralis. Biochimie 2014, 101, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Ramos, O.H.; Baldo, C.; Niland, S.; Hansen, U.; Ventura, J.S.; Furlan, S.; Butera, D.; Della-Casa, M.S.; Tanjoni, I.; et al. Collagen binding is a key factor for the hemorrhagic activity of snake venom metalloproteinases. Biochimie 2008, 90, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Tanjoni, I.; Evangelista, K.; Della-Casa, M.S.; Butera, D.; Magalhães, G.S.; Baldo, C.; Clissa, P.B.; Fernandes, I.; Eble, J.; Moura-da-Silva, A.M. Different regions of the class P-III snake venom metalloproteinase jararhagin are involved in binding to α2β1 integrin and collagen. Toxicon 2010, 55, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Jia, L.G.; Wang, D.; Shannon, J.D.; Fox, J.W. Function of the cysteine-rich domain of the hemorrhagic metalloproteinase atrolysin A: Targeting adhesion proteins collagen I and von Willebrand factor. Biochem. J. 2005, 391, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Kim, J.; Wang, D.; Dragulev, B.; Shannon, J.D.; Mann, H.H.; Veit, G.; Wagener, R.; Koch, M.; Fox, J.W. The cysteine-rich domain of snake venom metalloproteinases is a ligand for von Willebrand factor A domains: Role in substrate targeting. J. Biol. Chem. 2006, 281, 39746–39756. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Wang, D.; Shannon, J.D.; Pinto, A.F.; Polanowska-Grabowska, R.K.; Fox, J.W. Interaction of the cysteine-rich domain of snake venom metalloproteinases with the A1 domain of von Willebrand factor promotes site-specific proteolysis of von Willebrand factor and inhibition of von Willebrand factor-mediated platelet aggregation. FEBS J. 2007, 274, 3611–3621. [Google Scholar] [CrossRef] [PubMed]
- Baramova, E.; Shannon, J.D.; Bjarnason, J.B.; Gonias, S.L.; Fox, J.W. Interaction of hemorrhagic metalloproteinases with human α2-macroglobulin. Biochemistry 1990, 29, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Desmond, H.P.; Theakston, R.D.G.; Hay, C.R.; Zuzel, M. Ineffectiveness of the inhibition of the main haemorrhagic metalloproteinase from Bothrops jararaca venom by its only plasma inhibitor, α2-macrogloblin. Biochim. Biophys. Acta 1994, 1200, 307–314. [Google Scholar] [CrossRef]
- Estêvão-Costa, M.I.; Diniz, C.R.; Magalhães, A.; Markland, F.S.; Sanchez, E.F. Action of metalloproteinases mutalysin I and II on several components of the hemostatic and fibrinolytic systems. Thromb. Res. 2000, 99, 363–376. [Google Scholar] [CrossRef]
- Escalante, T.; Rucavado, A.; Kamiguti, A.S.; Theakston, R.D.G.; Gutiérrez, J.M. Bothrops asper metalloproteinase BaP1 is inhibited by α2-macroglobulin and mouse serum and does not induce systemic hemorrhage or coagulopathy. Toxicon 2004, 43, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S. Platelets as targets of snake venom metalloproteinases. Toxicon 2005, 45, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Hay, C.R.; Theakston, R.D.G.; Zuzel, M. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon 1996, 34, 627–642. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Lomonte, B.; Angulo, Y.; Fox, J.W. Tissue pathology induced by snake venoms: How to understand a complex pattern of alterations from a systems biology perspective? Toxicon 2010, 55, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- White, J. Snake venoms and coagulopathy. Toxicon 2005, 45, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Loría, G.D.; Rucavado, A.; Kamiguti, A.S.; Theakston, R.D.G.; Fox, J.W.; Alape, A.; Gutiérrez, J.M. Characterization of “basparin A”, a prothrombin-activating metalloproteinase, from the venom of the snake Bothrops asper that inhibits platelet aggregation and induces defibrination and thrombosis. Arch. Biochem. Biophys. 2003, 418, 13–24. [Google Scholar] [CrossRef]
- Malbranque, S.; Piercecchi-Marti, M.D.; Thomas, L.; Barbey, C.; Courcier, D.; Bucher, B.; Ridrch, A.; Smadja, D.; Warrell, D.A. Fatal diffuse thrombotic microangiopahy after a bite by the “Fer-de-Lance” pit viper (Bothrops lanceolatus) of Martinique. Am. J. Trop. Med. Hyg. 2008, 78, 856–861. [Google Scholar] [PubMed]
- Santoro, M.L.; Sano-Martins, I.S. Platelet dysfunction during Bothrops jararaca snake envenomation in rabbits. Thromb. Haemost. 2004, 92, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Soto, M.; Kamiguti, A.S.; Theakston, R.D.G.; Fox, J.W.; Escalante, T.; Gutiérrez, J.M. Characterization of aspercetin, a platelet aggregating component from the venom of the snake Bothrops asper which induces thrombocytopenia and potentiates metalloproteinase-induced hemorrhage. Thromb. Haemost. 2001, 85, 710–715. [Google Scholar] [PubMed]
- Calvete, J.J.; Marcinkiewicz, C.; Monleón, D.; Esteve, V.; Celda, B.; Juárez, P.; Sanz, L. Snake venom disintegrins: Evolution of structure and function. Toxicon 2005, 45, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Navdaev, A.; Clemetson, J.M.; Clemetson, K.J. Snake venom C-type lectins interacting with platelet receptors. Structure-function relationships and effects on hemostasis. Toxicon 2005, 45, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Sano-Martins, I.S.; Santoro, M.L.; Castro, S.C.; Fan, H.W.; Cardoso, J.L.C.; Theakston, R.D.G. Platelet aggregation in patients bitten by the Brazilian snake Bothrops jararaca. Thromb. Res. 1997, 87, 183–195. [Google Scholar] [CrossRef]
- Rucavado, A.; Soto, M.; Escalante, T.; Loría, G.D.; Arni, R.; Gutiérrez, J.M. Thrombocytopenia and platelet hypoaggregation induced by Bothrops asper snake venom. Toxins involved and their contribution to metalloproteinase-induced pulmonary hemorrhage. Thromb. Haemost. 2005, 94, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.T.; Hendon, R.R. Characterization of lizard venom hyaluronidase and evidence for its action as a spreading factor. Comp. Biochem. Physiol. B 1983, 76, 377–383. [Google Scholar] [CrossRef]
- Kemparaju, K.; Girish, K.S.; Nagaraju, S. Hyaluronidases, a neglected class of glycosidases from snake venom. Beyond a spreading factor. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010; pp. 237–258. [Google Scholar]
- Bustillo, S.; García-Denegri, M.E.; Gay, C.; van de Velde, A.C.; Acosta, O.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Leiva, L. Phospholipase A2 enhances the endothelial cell detachment effect of a snake venom metalloproteinase in the absence of catalysis. Chem. Biol. Interact. 2015, 240, 30–36. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. https://doi.org/10.3390/toxins8040093
Gutiérrez JM, Escalante T, Rucavado A, Herrera C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins. 2016; 8(4):93. https://doi.org/10.3390/toxins8040093
Chicago/Turabian StyleGutiérrez, José María, Teresa Escalante, Alexandra Rucavado, and Cristina Herrera. 2016. "Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding" Toxins 8, no. 4: 93. https://doi.org/10.3390/toxins8040093