Cyanobacterial Neurotoxin BMAA and Mercury in Sharks
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Sample Collection
3.2. HPLC Sample Preparation
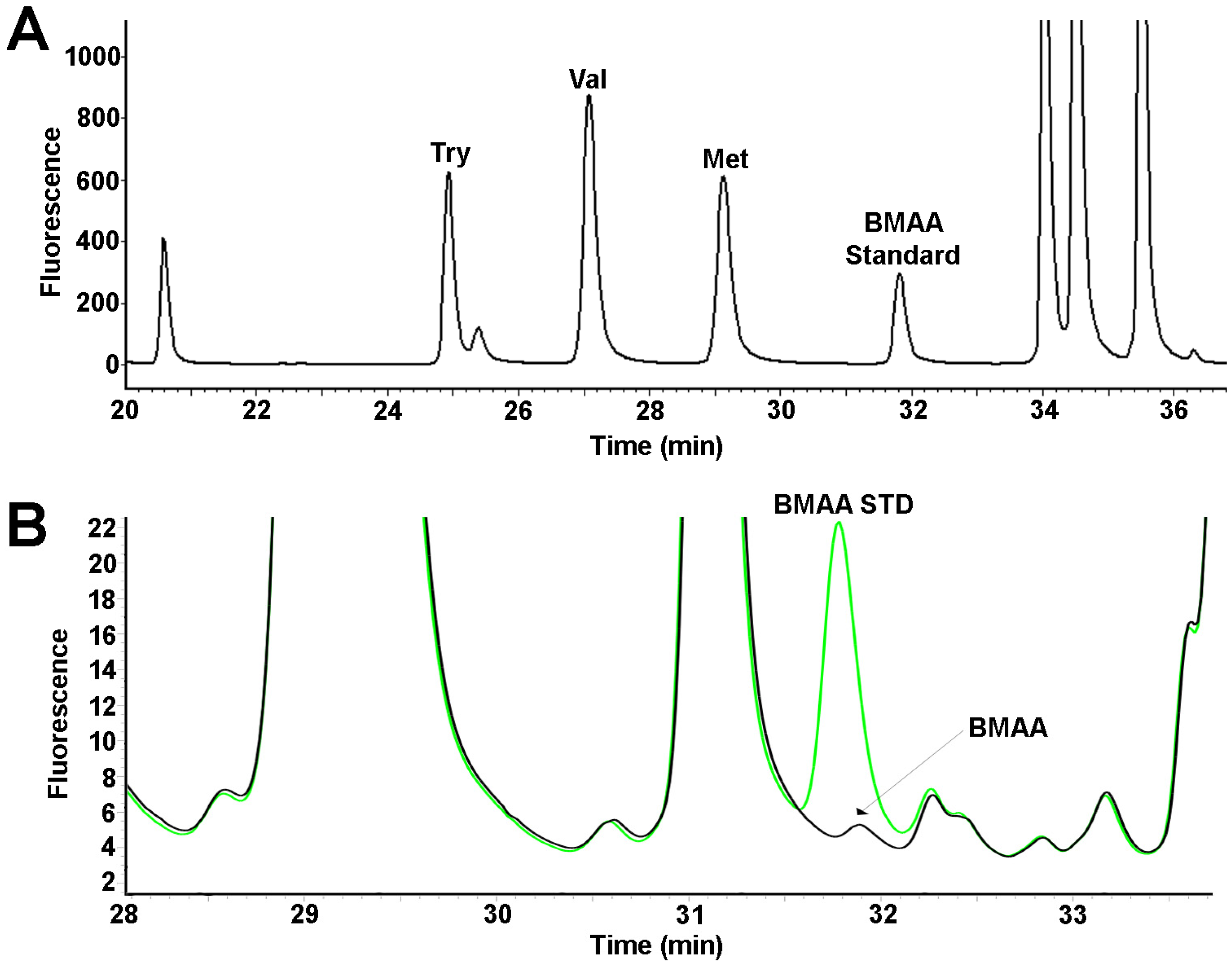
3.3. Fluorescence HPLC Methods for Analysis of BMAA
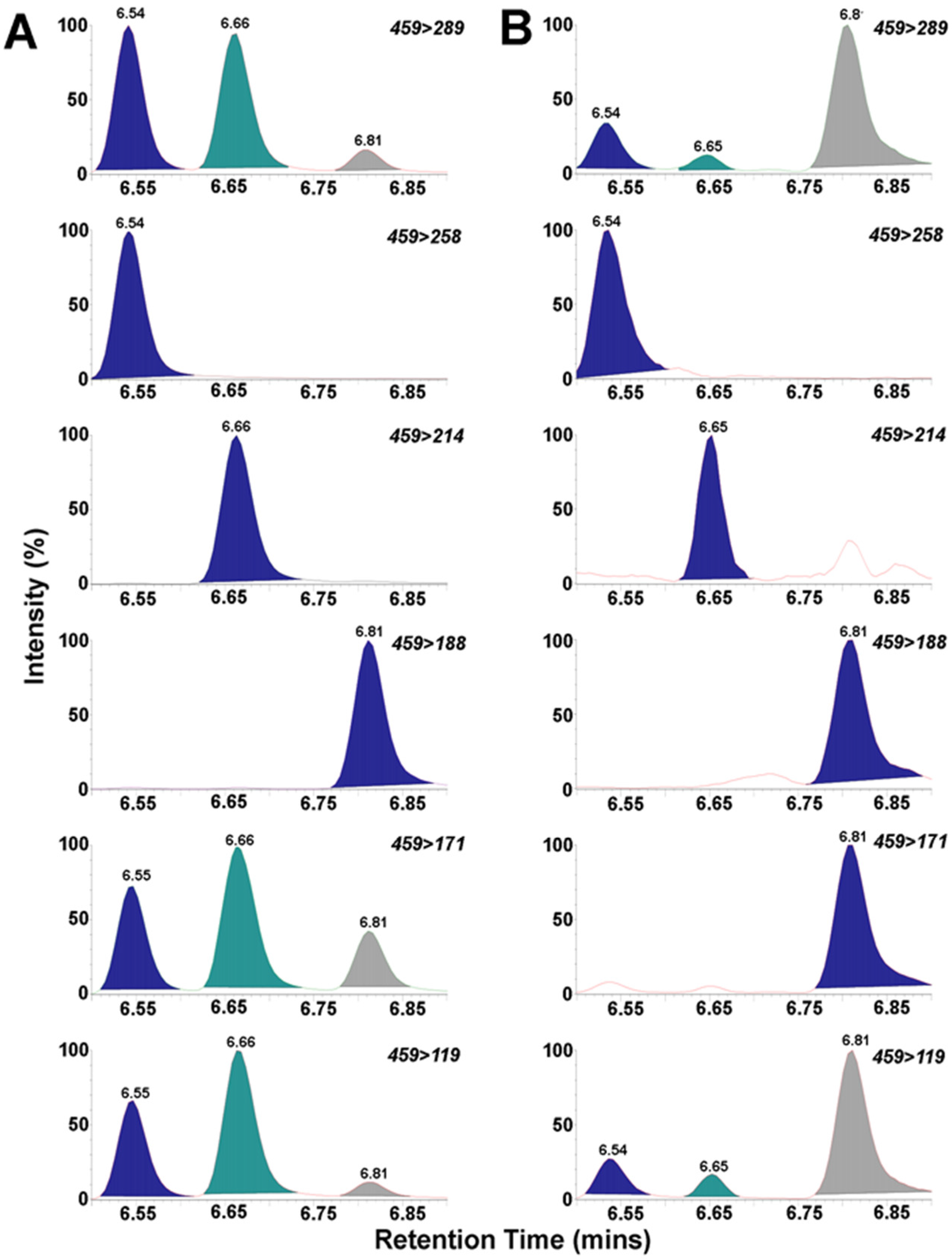
3.4. UPLC/MS/MS of BMAA
3.5. Determination of Hg in Fins (CVAFS Method)
3.6. Determination of Total Mercury in Muscles (Thermal Decomposition Method)
Supplementary Materials
Acknowledgments
Author Contributions
Abbreviations
| ALS/PDC | Amyotrophic lateral sclerosis/parkinsonism dementia complex |
| AEG | N-(2-aminoethyl) glycine |
| AQC | 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate |
| BMAA | β-N-methylamino-l-alanine |
| CVAFS | Cold vapor atomic fluorescence spectrometry |
| CCS | Calibration Check Samples |
| CFm | Calibration factor |
| CFx | Calibration factor |
| DAB | 2,4-diaminobutyric acid |
| FDOH | Florida Department of Health |
| Hg | Mercury |
| HPLC-FD | High performance liquid chromatography with fluorescence detection |
| LOD | Limits of detection |
| LOQ | Limits of quantification |
| MDLs | Method detection limits |
| MeHg | Methyl mercury |
| QCS | Quality Control Sample |
| THg | Total mercury |
| UPLC-MS/MS | Ultra-performance liquid chromatography/mass spectrometry/mass spectrometry |
References
- Baum, J.K.; Myers, R.A.; Kehler, D.G.; Worm, B.; Harley, S.J.; Doherty, P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science 2003, 299, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Baum, J.; Clarke, S.; Compagno, L.J.; Cortes, E.; Domingo, A.; Fordham, S.; Fowler, S.; Francis, M.P.; Gibson, C.; et al. You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. 2008, 18, 459–482. [Google Scholar] [CrossRef]
- Camhi, M.D.; Valenti, S.V.; Fordham, S.V.; Fowler, S.L.; Gibson, C. The Conservation Status of Pelagic Sharks and Rays: Report of the IUCN Shark Specialist Group Pelagic Shark Red List Workshop; IUCN Species Survival Commission Shark Specialist Group: Newbury, UK, 2009. [Google Scholar]
- Molina, J.M.; Cooke, S.J. Trends in shark bycatch research: Current status and research needs. Rev. Fish Biol. Fish. 2012, 22, 719–737. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Kyne, P.M.; Hammerschlag, N. Ecological risk assessment and its application to elasmobranch conservation and management. J. Fish Biol. 2012, 80, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Braccini, M.; Newman, S.J.; Harvey, E.S. Global patterns in the bycatch of sharks and rays. Mar. Policy 2015, 54, 86–97. [Google Scholar] [CrossRef]
- Worm, B.; Davis, B.; Kettemer, L.; Ward-Paige, C.A.; Chapman, D.; Heithaus, M.R.; Kessel, S.T.; Gruber, S.H. Global catches, exploitation rates, and rebuilding options for sharks. Mar. Pol. 2013, 40, 194–204. [Google Scholar] [CrossRef]
- The state of world fisheries and aquaculture. Rome: Food and agriculture organization of the United States. Available online: http://www.fao.org/3/a-i3720e.pdf. (accessed on 15 January 2016).
- Shiffman, D.; Hammerschlag, N. Shark conservation and management policy: A review and primer for non-specialists. Anim. Consv. 2016. [Google Scholar] [CrossRef]
- Cunningham-Day, R. Sharks in Danger: Global Shark Conservation Status with Reference to Management Plans and Legislation; Universal Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Spiegel, J. Even Jaws Deserves to Keep His Fins: Outlawing Shark Finning Throughout Global Waters. Boston Coll. Law Rev. 2000, 24, 409–437. [Google Scholar]
- Clarke, S.C.; Milner-Gulland, E.J.; Bjorndal, T. Social, economic, and regulatory drivers of the shark fin trade. Mar. Resour. Econ. 2007, 22, 305–327. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. Elife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Templer, R. Food for Thought: Chicken Soup for the Ostentatious Soul—Tonic or Tragedy? East, West Divided Over Shark’s Fin. Asian Wall St J. 1999. The Wall Street Journel. Availble online: http://www.wsj.com/articles/SB934473109149930399 (accessed on 31 January 2011).
- Mahr, K. Shark-Fin Soup and the Conservation Challenge. Time, 2010 9 August. [Google Scholar]Mahr, K. Shark-Fin Soup and the Conservation Challenge. Available online: http://content.time.com/time/magazine/article/0,9171,2021071,00.html (accessed on 25 June 2016).
- Mondo, K.; Broc Glover, W.; Murch, S.J.; Liu, G.; Cai, Y.; Davis, D.A.; Mash, D.C. Environmental neurotoxins beta-N-methylamino-l-alanine (BMAA) and mercury in shark cartilage dietary supplements. Food Chem. Toxicol. 2014, 70, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Merly, L.; Smith, S.L. Pro-inflammatory properties of shark cartilage supplements. Immunopharmacol. Immunotoxicol. 2015, 37, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Delshad, S.T.; Mousavi, S.A.; Islami, H.R.; Pazira, A. Mercury concentration of the whitecheek shark, Carcharhinus dussumieri (Elasmobranchii, Chondrichthyes), and its relation with length and sex. Pan-Am. J. Aquat. Sci. 2012, 7, 135–142. [Google Scholar]
- Escobar-Sanchez, O.; Galvan-Magana, F.; Rosiles-Martinez, R. Mercury and selenium bioaccumulation in the smooth hammerhead shark, Sphyrna zygaena Linnaeus, from the Mexican Pacific Ocean. Bull. Environ. Contam Toxicol. 2010, 84, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Pethybridge, H.; Cossa, D.; Butler, E.C. Mercury in 16 demersal sharks from southeast Australia: Biotic and abiotic sources of variation and consumer health implications. Mar. Environ. Res. 2010, 69, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, D.; Wasno, R.; Hammerschlag, N.; Volety, A. Mercury accumulation in sharks from the coastal waters of southwest Florida. Arch. Environ. Contam. Toxicol. 2014, 67, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H., Jr. Mercury levels in four species of sharks from the Atlantic coast of Florida. Fish. Bull. 1999, 97, 372–329. [Google Scholar]
- Health FDo. Seafood Consumption, Get Fresh with Florida Fish. Available online: http://www.doh.state.fl.us/floridafishadvice/ (accessed on 25 June 2016).
- Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. Avaible online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2012.2985/abstract (accessed on 28 June 2016).
- Mondo, K.; Hammerschlag, N.; Basile, M.; Pablo, J.; Banack, S.A.; Mash, D.C. Cyanobacterial neurotoxin beta-N-methylamino-l-alanine (BMAA) in shark fins. Mar. Drugs 2012, 10, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Field, N.C.; Metcalf, J.S.; Caller, T.A.; Banack, S.A.; Cox, P.A.; Stommel, E.W. Linking beta-methylamino-l-alanine exposure to sporadic amyotrophic lateral sclerosis in Annapolis, MD. Toxicon 2013, 70, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.B.; McDowell, K.A.; Siebert, A.A.; Clark, S.M.; Dugger, N.V.; Valentino, K.M.; Jinnah, H.A.; Sztalryd, C.; Fishman, P.S.; Shaw, C.A.; et al. Environmental neurotoxin-induced progressive model of parkinsonism in rats. Ann. Neurol. 2010, 68, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.S.; Nunn, P.B.; Hugon, J.; Ludolph, A.C.; Ross, S.M.; Roy, D.N.; Robertson, R.C. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science 1987, 237, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Rush, T.; Liu, X.; Lobner, D. Synergistic toxicity of the environmental neurotoxins methylmercury and beta-N-methylamino-l-alanine. Neuroreport 2012, 23, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. R. Soc. B Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.; Ceci, E.; Storelli, A.; Marcotrigiano, G. Polychlorinated biphenyl, heavy metal and methylmercury residues in hammerhead sharks: Contaminant status and assessment. Mar. Pollut. Bull. 2003, 46, 1035–1039. [Google Scholar] [CrossRef]
- Nam, D.; Adams, D.; Reyier, E.; Basu, N. Mercury and selenium levels in lemon sharks (Negaprion brevirostris) in relation to a harmful red tide event. Environ. Monit. Assess. 2010, 176, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, D.; Baumann, Z.; Abercrombie, D.L.; Chapman, D.D.; Hammerschmidt, C.R.; Fisher, N.S. Methylmercury in dried shark fins and shark fin soup from American restaurants. Sci. Total Environ. 2014, 496, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Nature IUfCo. THE IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org (accessed on 28 February 2016).
- Al-sammak, M.A.; Hoagland, K.D.; Snow, D.D.; Cassada, D. Toxicon Methods for simultaneous detection of the cyanotoxins BMAA, DABA, and anatoxin-a in environmental samples. Toxicon 2013, 76, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Gillissen, F.; Lürling, M. A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria. PLoS ONE 2012, 7, e36667. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.; Suzuki, M. Distribution of Benthic Chlorophyll in Florida Bay Sediments. In Proceedings of the 1999 Florida Bay and Adjacent Marine Systems Science Conference, Key Largo, FL, USA, 1–5 November 1999; p. 129.
- Bethea, D.M.; Hale, L.; Carlson, J.K.; Cortés, E.; Manire, C.A.; Gelsleichter, J. Geographic and ontogenetic variation in the diet and daily ration of the bonnethead shark, Sphyrna tiburo, from the eastern Gulf of Mexico. Mar. Biol. 2007, 152, 1009–1020. [Google Scholar] [CrossRef]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial Blooms and the Occurrence of the neurotoxin beta-N-methylamino-l-alanine (BMAA) in South Florida Aquatic Food Webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Adminstration USFaD. 2016. Available online: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/ucm077969.htm-merc (accessed on 15 June 2016).
- Agency USEP. Available online: http://www.epa.gov/mercury (accessed on 20 April 2015).
- Hueter, R.; Fong, W.; Henderson, G.; French, M.; Manire, C. Methylmercury concentration in shark muscle by species, size and distribution of sharks in Florida coastal waters. Water Air Soil Pollut. 1995, 80, 893–899. [Google Scholar] [CrossRef]
- Adams, D.H., Jr.; Henderson, G.E. Mercury Levels in Marine and Estuarine Fishes of Florida 1989–2001; Florida Marine Research Institute: Port Charlotte, FL, USA, 2003. [Google Scholar]
- Holtcamp, W. The emerging science of BMAA: Do cyanobacteria contribute to neurodegenerative disease? Environ. Health Perspect. 2012, 120, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Basile, M.; Mash, D.C. Cerebral uptake and protein incorporation of cyanobacterial toxin beta-N-methylamino-l-alanine. Neuroreport 2013, 24, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.A.; Cox, P.A.; Banack, S.A.; Rodgers, K.J. The non-protein amino acid BMAA is misincorporated into human proteins in place of l-serine causing protein misfolding and aggregation. PLoS ONE 2013, 8, e75376. [Google Scholar] [CrossRef] [PubMed]
- Glover, W.B.; Mash, D.C.; Murch, S.J. The natural non-protein amino acid N-beta-methylamino-l-alanine (BMAA) is incorporated into protein during synthesis. Amino Acids 2014, 46, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Weiyue, F.; Meng, W.; Ming, G.; Yuan, H.; Junwen, S.; Bing, W.; Motao, Z.; Hong, O.; Yuliang, Z.; Zhifang, C. Mercury speciation and mercury-binding protein study by HPLC-ICP-MS on the estimation of mercury toxicity between maternal and infant rats. J. Anal. At. Spectrom. 2011, 26, 156–164. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Sundseth, K.; Munthe, J.; Kindbom, K.; Wilson, S.; Maxson, S.P. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 2010, 44, 2487–2499. [Google Scholar] [CrossRef]
- Compeau, G.C.; Bartha, R. Principal Methylators. Microbiology 1985, 50, 498–502. [Google Scholar]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wang, W.; Wu, J.J.; Liu, L.; Theodoratou, E.; Car, J.; Middleton, L.; Russ, T.C.; Deary, I.J.; Campbell, H.; et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet 2013, 381, 2016–2023. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Midlife obesity and dementia: Meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity 2013, 21, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Dickman, M.D.; Leung, C.K.; Leong, M.K. Hong Kong male subfertility links to mercury in human hair and fish. Sci. Total Environ. 1998, 214, 165–174. [Google Scholar] [CrossRef]
- 2016 Alzhemer's Disease Facts And Figures. Availble online: https://www.alz.org/documents_custom/2016-facts-and-figures.pdf (accessed on 25 July 2016).
- Schindler, D.W. Detecting ecosystem responses to anthropogenic stress. J. Fish. Aquat. Sci. 1987, 44, S6–S25. [Google Scholar] [CrossRef]
- Torres, P.; da Cunha, R.T.; Maia, R.; Dos Santos Rodrigues, A. Trophic ecology and bioindicator potential of the North Atlantic tope shark. Sci. Total Environ. 2014, 481, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Glover, W.B.; Baker, T.C.; Murch, S.J.; Brown, P.N. Determination of beta-N-methylamino-l-alanine, N-(2-aminoethyl)glycine, and 2,4-diaminobutyric acid in Food Products Containing Cyanobacteria by Ultra-Performance Liquid Chromatography and Tandem Mass Spectrometry: Single-Laboratory Validation. J. AOAC Int. 2015, 98, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Agency USEP. Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. Available online: http://www.epa.gov/sites/production/files/2015-08/documents/method_1631e_2002.pdf (accessed on 17 September 2002).
- EPA Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. Available online: https://www.epa.gov/homeland-security-research/epa-method-7473-sw-846-mercury-solids-and-solutions-thermal-decomposition (accessed on 15 September 2015).
| Scientific Name | International Union for Conservation of Nature Red List Category | Common Name | Location | Month |
|---|---|---|---|---|
| Carharhinus acronotus | Near Threatened | Blacknose b | 25.09417oN 81.04234oW | March |
| - | - | Blacknose b | 25.00858oN 81.00089oW | April |
| - | - | Blacknose a | 25.62099oN 80.15602oW | October |
| - | - | Blacknose a | Biscayne Bay | June |
| - | - | Blacknose b | 25.09417oN 81.04234oW | December |
| - | - | Blacknose b | 25.01089oN 81.00419oW | April |
| Carcharhinus limbatus | Near Threatened | Blacktip b | 25.00644oN 80.99969oW | March |
| - | - | Blacktip b | 25.00644oN 80.99969oW | September |
| - | - | Blacktip a | 25.59968oN 80.15205oW | July |
| - | - | Blacktip b | 25.01109oN 80.99832oW | September |
| - | - | Blacktip b | 25.00644oN 80.99969oW | March |
| - | - | Blacktip a | 25.62592oN 80.15442oW | October |
| - | - | Blacktip a | 25.61905oN 80.1714oW | October |
| - | - | Blacktip a | 25.64757oN 80.1881oW | April |
| - | - | Blacktip a | 25.67199oN 80.18144oW | September |
| - | - | Blacktip b | 25.01089oN 81.00419oW | September |
| - | - | Blacktip b | 25.00976oN 81.00079oW | September |
| - | - | Blacktip b | 25.00644oN 80.99969oW | September |
| - | - | Blacktip b | 25.01715oN 81.01056oW | October |
| - | - | Blacktip b | 25.01715oN 81.01056oW | February |
| - | - | Blacktip b | 25.01089oN 81.00419oW | April |
| - | - | Blacktip b | 25.00858oN 81.00089oW | December |
| - | - | Blacktip b | 25.00623oN 80.99723oW | March |
| Sphyrna tiburo | Least concerned | Bonnethead a | 25.36711oN 80.14806oW | March |
| - | - | Bonnethead a | 25.36711oN 80.14806oW | March |
| - | - | Bonnethead a | 25.40807oN 80.21806oW | October |
| - | - | Bonnethead b | 25.36711oN 80.14806oW | March |
| Carcharhinus leucas | Near threatened | Bull b | 25.01715oN 81.01056oW | September |
| - | - | Bull b | 25.01309oN 80.00129oW | September |
| - | - | Bull b | 25.00623oN 80.99723oW | March |
| Sphyrna mokarran | Endangered | Great Hammerhead a | 25.62138oN 80.15656oW | July |
| - | - | Great Hammerhead b | 25.01715oN 81.01056oW | September |
| - | - | Great Hammerhead a | 25.740092oN 79.967258oW | May |
| - | - | Great Hammerhead b | 26.61587oN 79.96725oW | February |
| - | - | Great Hammerhead b | 26.457892oN 80.053938 oW | April |
| Negaprion brevirostris | Near threatened | Lemon b | 25.00644oN 80.99969oW | June |
| - | - | Lemon b | 25.00644oN 80.99969oW | March |
| Ginglymostoma cirraum | Data deficient | Nurse a | 25.61942oN 80.1835 oW | September |
| - | - | Nurse b | 24.88335oN 80.84475 oW | April |
| - | - | Nurse b | 25.00644oN 80.99969 oW | March |
| - | - | Nurse a | 25.62311oN 80.15626oW | August |
| - | - | Nurse a | 25.60062oN 80.15214 oW | August |
| - | - | Nurse a | 25.60569oN 80.1534 oW | August |
| - | - | Nurse a | 25.62311oN 80.15626 oW | August |
| - | - | Nurse b | 25.00858oN 80.00089 oW | September |
| - | - | Nurse b | Florida Bay | January |
| - | - | Nurse b | 25.00983oN 80.99305oW | March |
| Rhizoprionodon terraenovae | Least Concerned | Atlantic Sharpnose b | 25.00858oN 81.00089oW | April |
| - | - | Atlantic Sharpnose b | 25.10566oN 81.04757oW | April |
| - | - | Atlantic Sharpnose b | Florida Bay | April |
| Sphyrna zygaena | Vulnerable | Smooth Hammerhead a | 26.117727oN 80.09734oW | February |
| Galeocerdo cuvier | Near threatened | Tiger c | −21.12055oN 149.22416oE | January |
| - | - | Tiger c | −32.78278oN 152.41171oE | January |
| - | - | Tiger c | −24.81665oN 152.47257 oE | September |
| - | - | Tiger c | −24.81665oN 152.47257 oE | March |
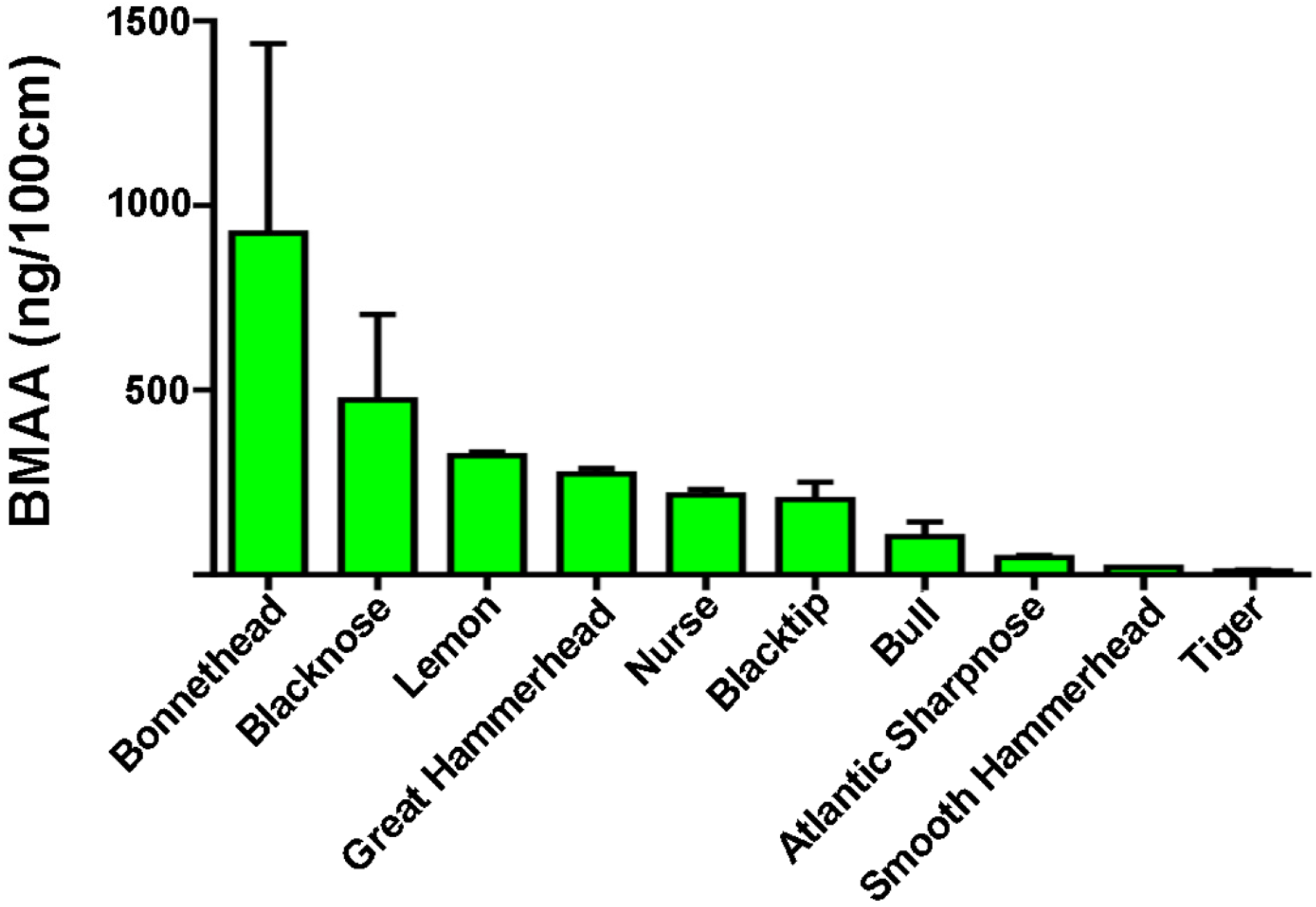
| Species | Range (ng/mg) | Detected Mean ± SE (ng/mg) | BMAA/Length (ng/100 cm) |
|---|---|---|---|
| Blacknose (n = 6) a | ND–1663 | 573 ± 322 * | 473 |
| Blacktip (n = 17) a | ND–811 | 282 ± 72 * | 203 |
| Bonnethead (n = 4) a | 40–1836 | 707 ± 395 | 925 |
| Bull (n = 3) a | 43–264 | 180 ± 69 | 103 |
| Great Hammerhead (n = 5) a | 42–1528 | 576 ± 272 | 273 |
| Lemon (n = 2) a | 556–628 | 592 ± 36 | 322 |
| Nurse (n = 10) a | ND–2011 | 442 ± 315 * | 216 |
| Sharpnose (n = 3) a | 40–115 | 68 ± 24 | 47 |
| Smooth Hammerhead (n = 1) a | - | 43 | 21 |
| Tiger (n = 4) b | 34–44 | 39 ± 2 | 11 |
| Species | HPLC-FD * (ng/mg) | UPLC-MS/MS * (ng/mg) |
|---|---|---|
| Galeocerdo cuvier | - | - |
| Tiger a | 35.60 ± 1.90 | 19.20 ± 7.10 |
| Tiger a | 31.50 ± 2.60 | 20.68 ± 3.50 |
| Tiger a | 39.60 ± 4.70 | 33.15 ± 5.60 |
| Tiger a | 38.90 ± 5.10 | 20.17 ± 2.40 |
| Species | Range Hg (ng/mg) | THg (ng/mg) * | MeHg (ng/mg) * | BMAA:THg |
|---|---|---|---|---|
| Blacknose a | 0.05–5.65 | 1.93 ± 2.27 (n = 3) | 0.71 ± 0.02 (n = 2) | 429:1 |
| Blacktip a | 0.22–7.73 | 3.70 ± 0.69 (n = 16) | 1.40 ± 0.75 (n = 7) | 368:1 |
| Bonnethead a | 0.41–1.77 | 0.96 ± 0.32 (n = 4) | 0.56 ± 0.44 (n = 4) | 668:1 |
| Bull a | 3.24–13.23 | 7.26 ± 3.04 (n = 3) | 2.32 (n = 1) | 27:1 |
| Great Hammerhead a | - | 3.29 (n = 1) | N/A | 465:1 |
| Lemon a | 0.27–1.34 | 0.81 ± 0.54 (n = 2) | 0.26 ± 0.08 (n = 2) | 1390:1 |
| Nurse a | 0.06–0.48 | 0.24 ± 0.04 (n = 10) | N/A | 1509:1 |
| Sharpnose a | 0.44–2.41 | 1.42 ± 0.98 (n = 2) | 0.25 (n = 1) | 70:1 |
| Smooth Hammerhead a | - | 2.85 (n = 1) | N/A | 15:1 |
| Tiger b | 0.12–1.61 | 0.74 ± 0.36 (n = 4) | N/A | 23:1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammerschlag, N.; Davis, D.A.; Mondo, K.; Seely, M.S.; Murch, S.J.; Glover, W.B.; Divoll, T.; Evers, D.C.; Mash, D.C. Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins 2016, 8, 238. https://doi.org/10.3390/toxins8080238
Hammerschlag N, Davis DA, Mondo K, Seely MS, Murch SJ, Glover WB, Divoll T, Evers DC, Mash DC. Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins. 2016; 8(8):238. https://doi.org/10.3390/toxins8080238
Chicago/Turabian StyleHammerschlag, Neil, David A. Davis, Kiyo Mondo, Matthew S. Seely, Susan J. Murch, William Broc Glover, Timothy Divoll, David C. Evers, and Deborah C. Mash. 2016. "Cyanobacterial Neurotoxin BMAA and Mercury in Sharks" Toxins 8, no. 8: 238. https://doi.org/10.3390/toxins8080238
APA StyleHammerschlag, N., Davis, D. A., Mondo, K., Seely, M. S., Murch, S. J., Glover, W. B., Divoll, T., Evers, D. C., & Mash, D. C. (2016). Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins, 8(8), 238. https://doi.org/10.3390/toxins8080238