Chronic Exposure to the Fusarium Mycotoxin Deoxynivalenol: Impact on Performance, Immune Organ, and Intestinal Integrity of Slow-Growing Chickens
Abstract
:1. Introduction
2. Results
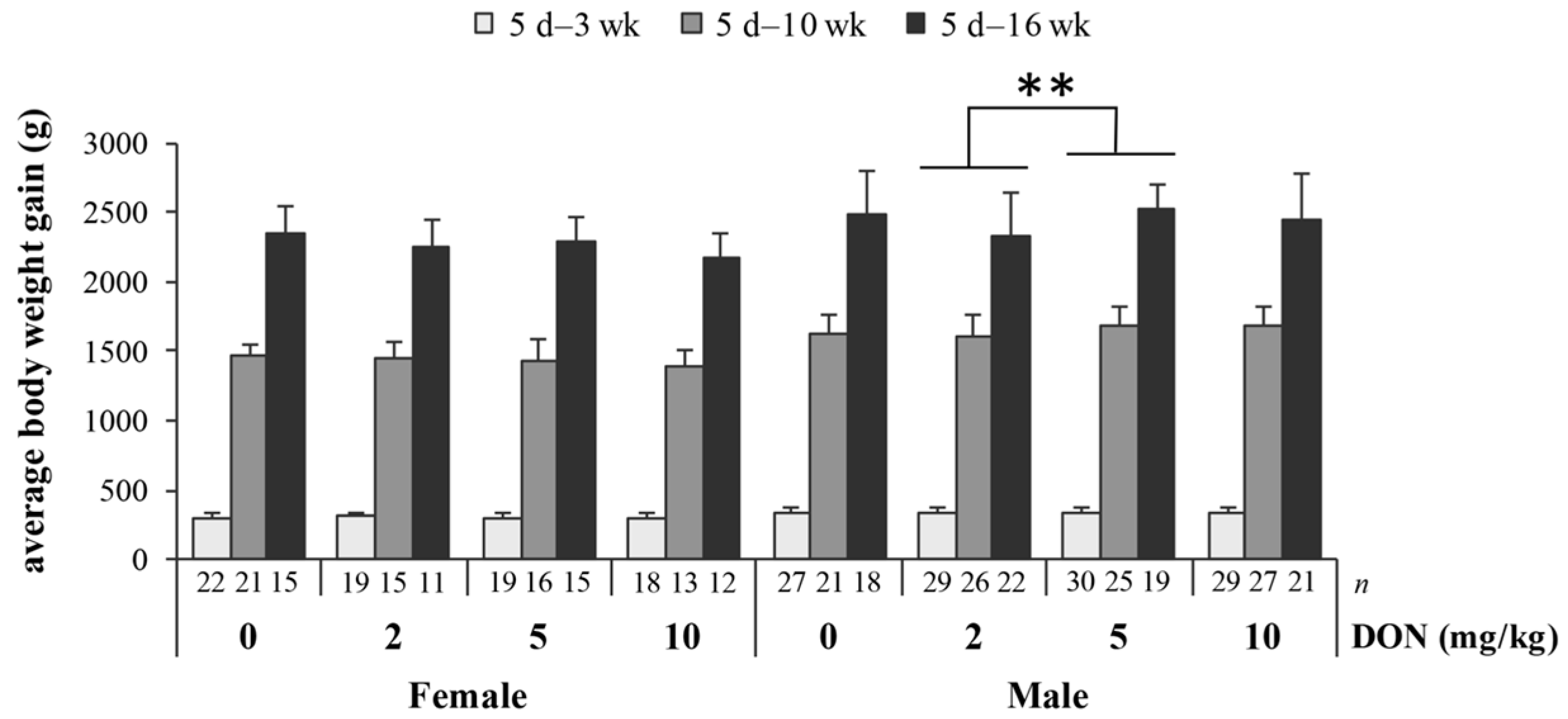
2.1. Dietary Composition, Deoxynivalenol Concentrations, Chicken Performance, and Carryover
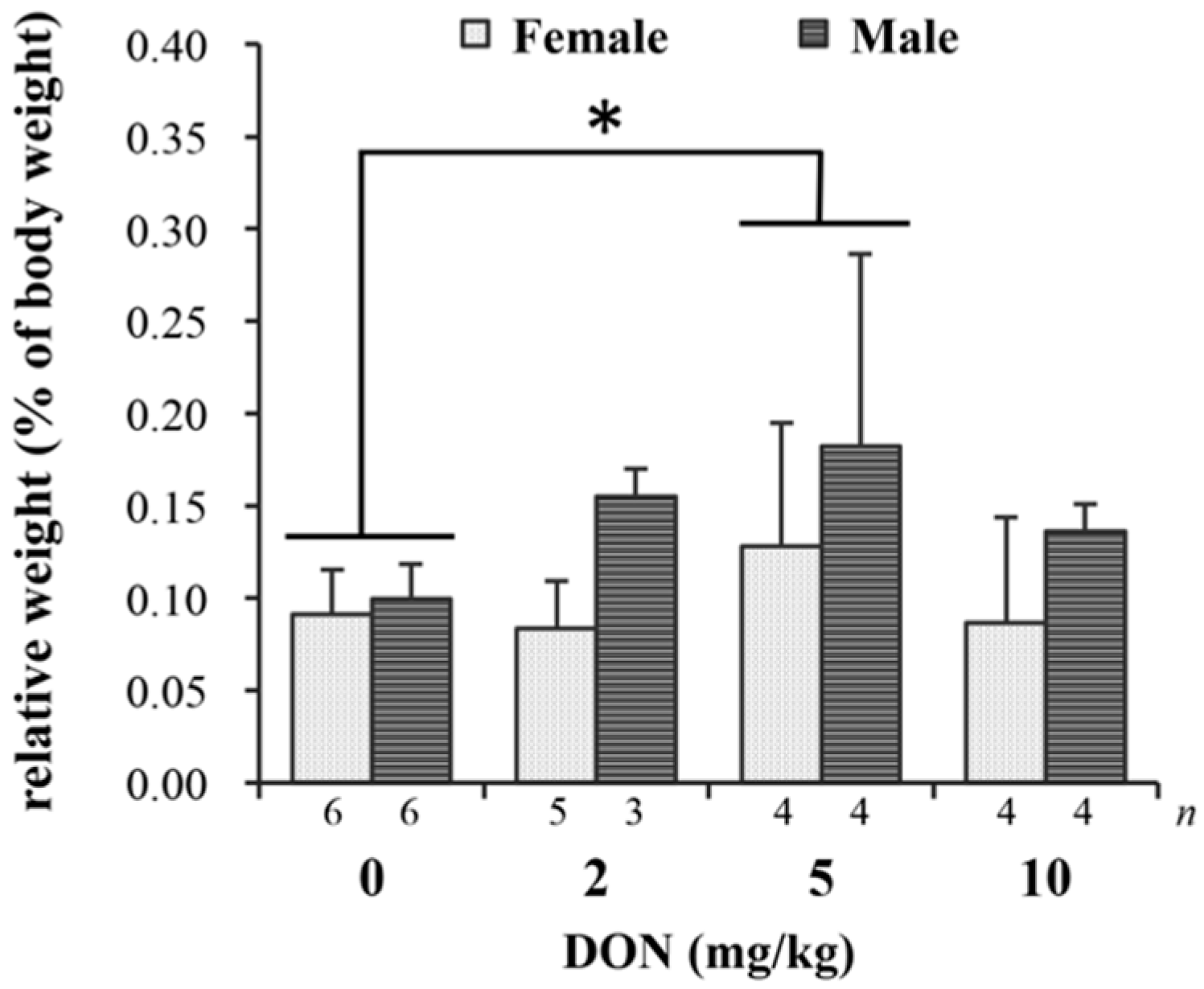
2.2. Blood Biochemistry and Relative Organ Weights
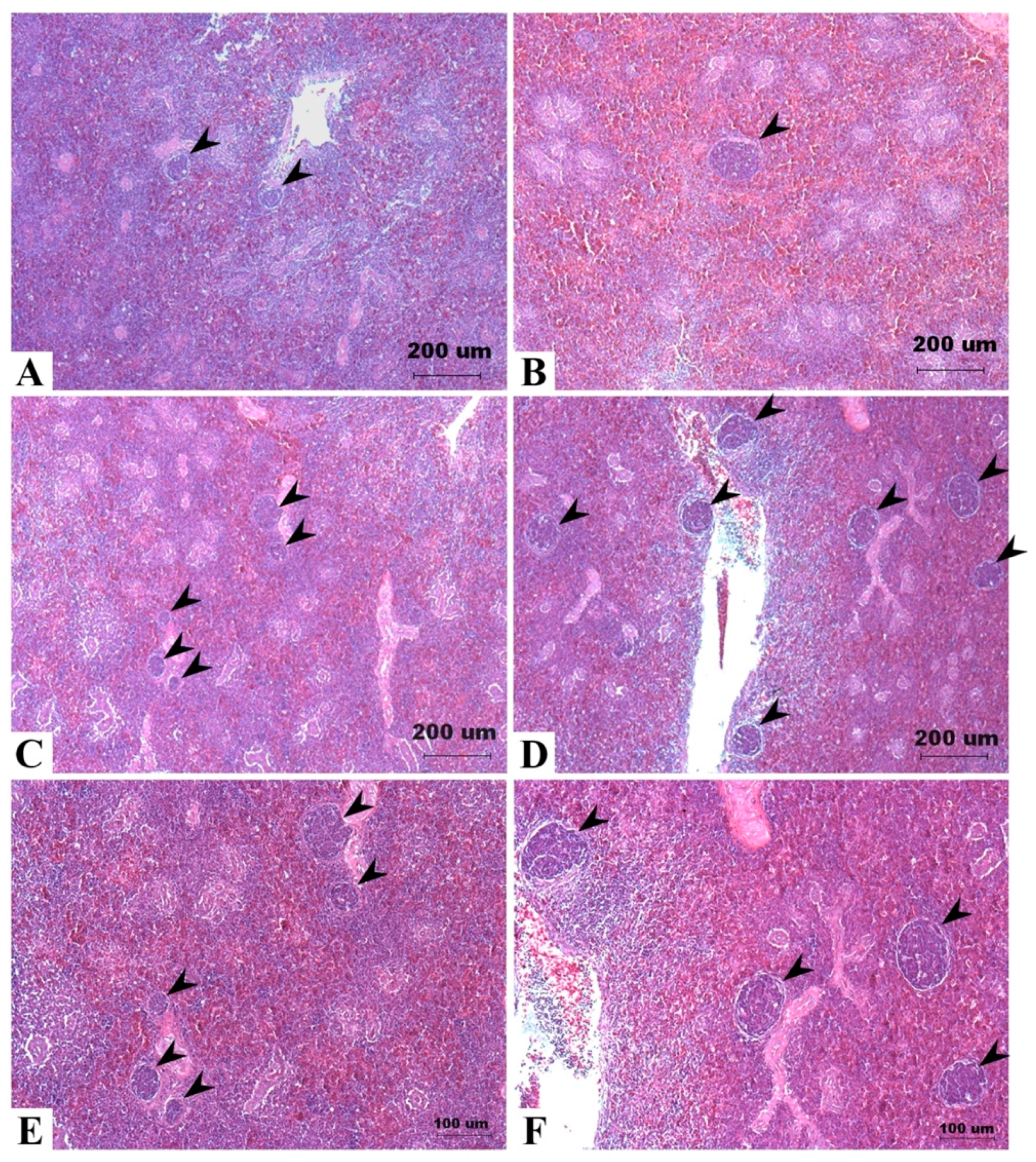
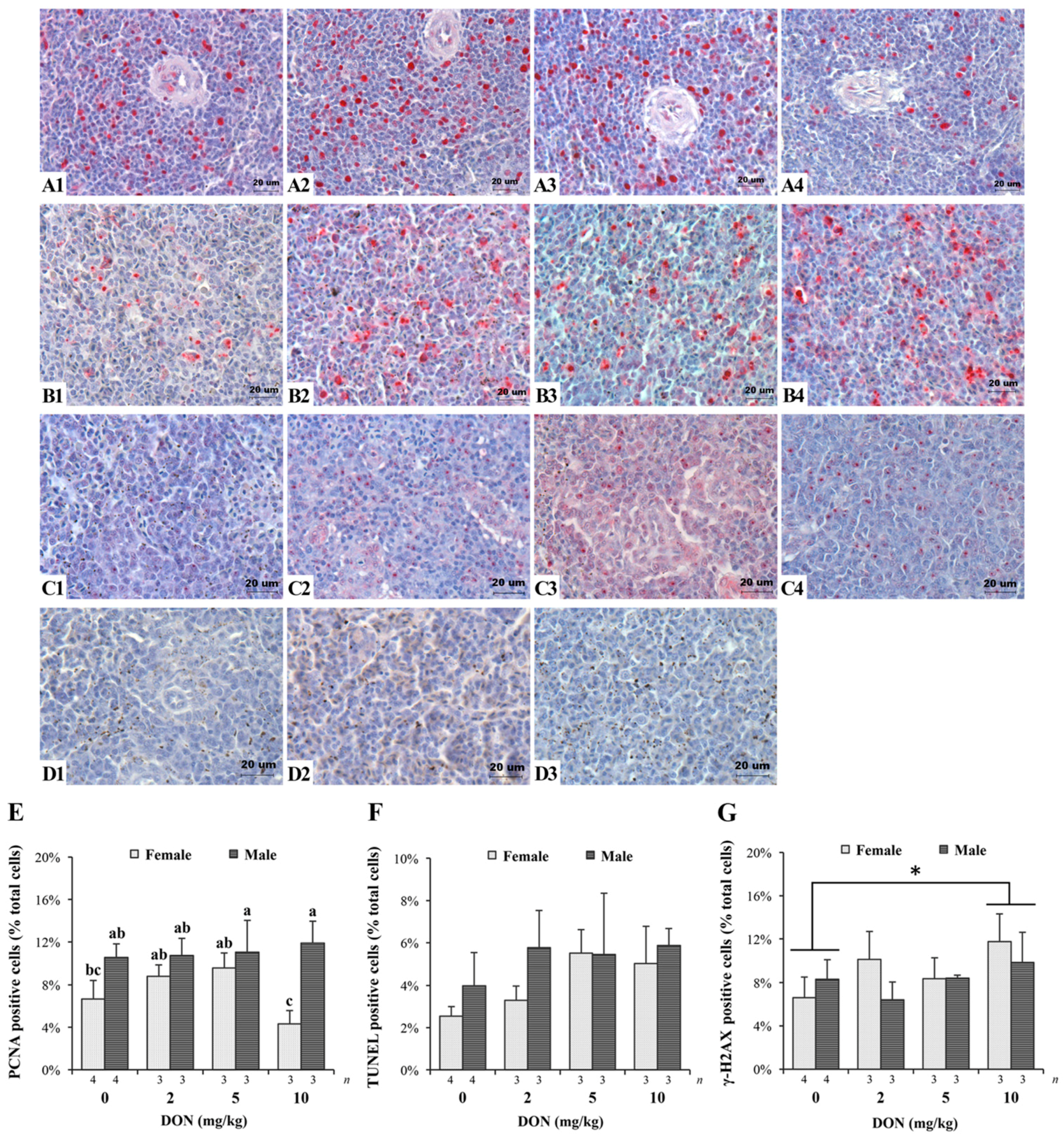
2.3. Histopathology, Immunohistochemistry, and TUNEL Assay of the Spleen
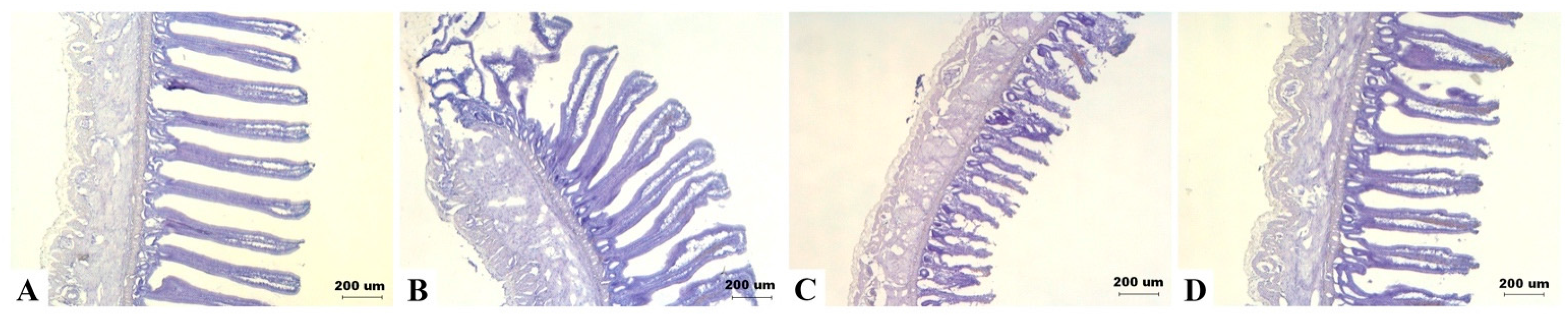
2.4. Intestinal Morphology
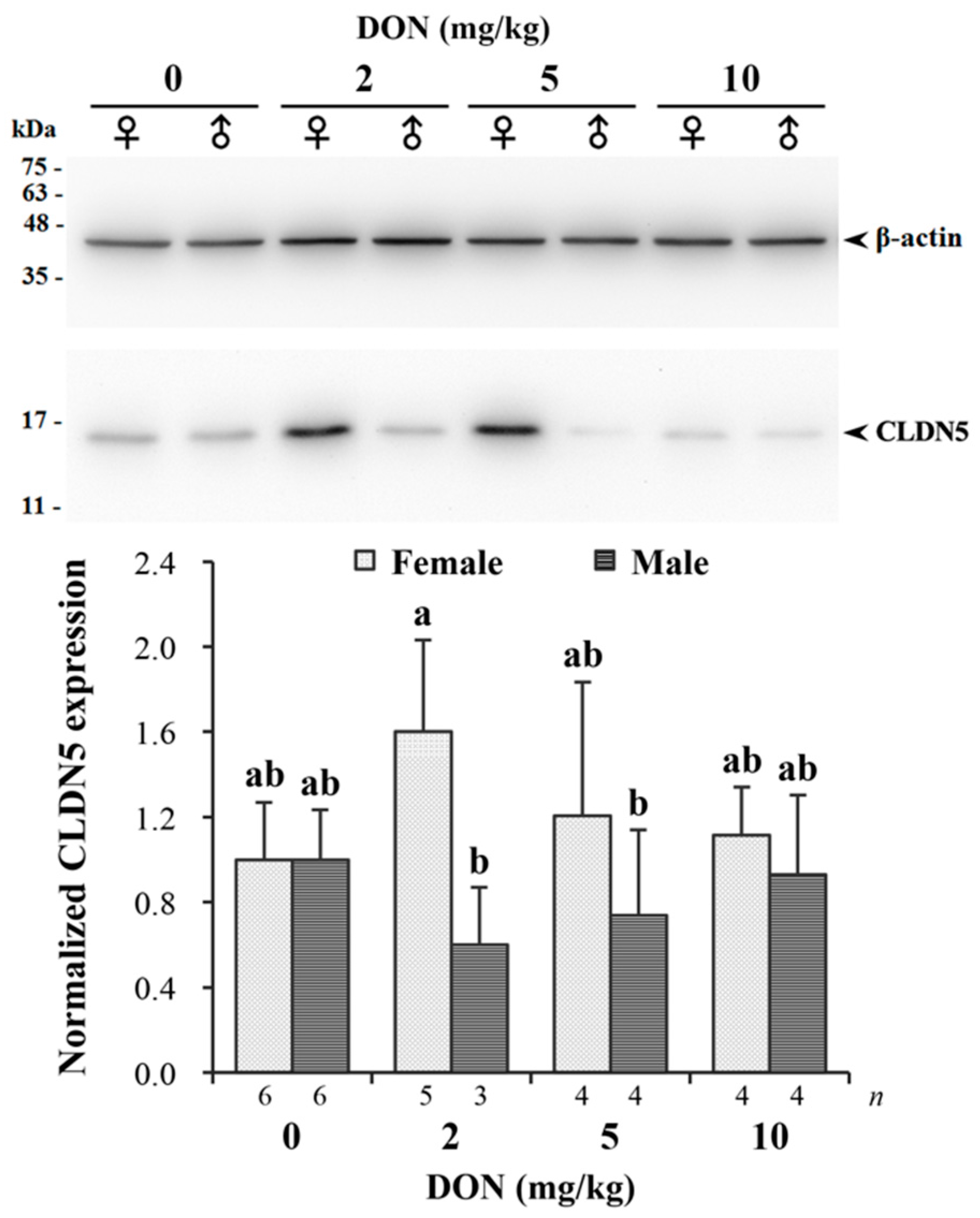
2.5. Relative Expression of Tight Junction Protein
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Design, Birds, and Diets
5.2. Sampling and Tissue Collection
5.3. Spleen Sectioning and Staining
5.3.1. Immunohistochemical Evaluation
5.3.2. Detection of Apoptosis
5.3.3. Image Analysis
5.4. Intestinal Morphology
5.5. Tissue Protein Extraction, Separation, and Western Blotting
5.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Canady, R.; Coker, R.; Egan, S.; Krska, R.; Kuiper-Goodman, T.; Olsen, M.; Pestka, J.; Resnik, S.; Schlatter, J. Deoxynivalenol. Safety evaluation of certain mycotoxins in food. Joint Expert Committee on Food Additives (JECFA). In WHO Food Additives Series; World Health Organization (WHO): Geneva, Switzerland, 2001; pp. 419–555. [Google Scholar]
- BIOMIN Holding GmbH. 2016 BIOMIN Mycotoxin Survey Report. Available online: http://www.biomin.net/en/press-releases/latest-biomin-mycotoxin-survey-reveals-high-risk-to-livestock-globally (accessed on 29 September 2017).
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhou, H.R.; Pestka, J.J. Mechanisms for ribotoxin-induced ribosomal RNA cleavage. Toxicol. Appl. Pharm. 2012, 265, 10–18. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhou, H.R.; Pestka, J.J. Targets and intracellular signaling mechanisms for deoxynivalenol-induced ribosomal RNA cleavage. Toxicol. Sci. 2012, 127, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol-induced proinflammatory gene expression: Mechanisms and pathological sequelae. Toxins 2010, 2, 1300–1317. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Wang, X.; Yang, W.; Nussler, A.; Xiong, L.Y.; Kuca, K.; Dohnal, V.; Zhang, X.J.; Yuan, Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Gerdes, R.G.; Underhill, K.L.; Rotter, B.A.; Jui, P.Y.; Trenholm, H.L. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat. Toxins 1994, 2, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rotter, B.A. Invited review: Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health A 1996, 48, 1–34. [Google Scholar] [CrossRef]
- European Commission. Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004, 114, 205–239. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Böhm, J. The toxicity of Fusarium mycotoxin deoxynivalenol in poultry feeding. World’s Poult. Sci. J. 2012, 68, 651–667. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.A.; Zebeli, Q.; Böhm, J. Deoxynivalenol in chicken feed alters the vaccinal immune response and clinical biochemical serum parameters but not the intestinal and carcass characteristics. J. Anim. Physiol. Anim. Nutr. 2016, 100, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Osselaere, A.; Devreese, M.; Goossens, J.; Vandenbroucke, V.; De Baere, S.; De Backer, P.; Croubels, S. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem. Toxicol. 2013, 51, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Goyarts, T.; Dänicke, S. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from naturally contaminated wheat for the pig. Toxicol. Lett. 2006, 163, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Zimmermann, H.E.; Fruhmann, P.; Dänicke, S.; Wiesenberger, G.; Caha, S.; Weber, J.; Berthiller, F. Metabolism of deoxynivalenol and deepoxy-deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins 2015, 7, 4706–4729. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Huang, L.L.; Pan, Y.H.; Wu, Q.H.; Chen, D.M.; Tao, Y.F.; Wang, X.; Liu, Z.L.; Li, J.; Wang, L.Y.; et al. Metabolism, distribution, and excretion of deoxynivalenol with combined techniques of radiotracing, high-performance liquid chromatography ion trap time-of-flight mass spectrometry, and online radiometric detection. J. Agric. Food Chem. 2014, 62, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, M.; Twarużek, M.; Grajewski, J.; Kosicki, R.; Böhm, J.; Zentek, J. The impact of the Fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int. J. Mol. Sci. 2011, 12, 7996–8012. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Vahjen, W.; Aschenbach, J.R.; Zentek, J. A diet naturally contaminated with the Fusarium mycotoxin deoxynivalenol (DON) downregulates gene expression of glucose transporters in the intestine of broiler chickens. Livest. Sci. 2011, 140, 72–79. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrede, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osselaere, A.; Santos, R.; Hautekiet, V.; De Backer, P.; Chiers, K.; Ducatelle, R.; Croubels, S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE 2013, 8, e69014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dänicke, S.; Matthes, S.; Halle, I.; Ueberschär, K.H.; Döll, S.; Valenta, H. Effects of graded levels of Fusarium toxin-contaminated wheat and of a detoxifying agent in broiler diets on performance, nutrient digestibility and blood chemical parameters. Br. Poult. Sci. 2003, 44, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Jiang, S.Z.; Wu, Y.B. Effects of feed-borne Fusarium mycotoxins with or without yeast cell wall adsorbent on organ weight, serum biochemistry, and immunological parameters of broiler chickens. Poult. Sci. 2012, 91, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Ghareeb, K.; Twaruzek, M.; Grajewski, J.; Böhm, J. Deoxynivalenol as a contaminant of broiler feed: Effects on bird performance and response to common vaccines. Poult. Sci. 2012, 91, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Chaytor, A.C.; See, M.T.; Hansen, J.A.; de Souza, A.L.P.; Middleton, T.F.; Kim, S.W. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 2011, 89, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Mikami, O.; Yamaguchi, H.; Murata, H.; Nakajima, Y.; Miyazaki, S. Induction of apoptotic lesions in liver and lymphoid tissues and modulation of cytokine mRNA expression by acute exposure to deoxynivalenol in piglets. J. Vet. Sci. 2010, 11, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, Y.L.; Xue, C.Y.; Ma, J.Y.; Xie, Q.M.; Bi, Y.Z.; Cao, Y.C. The combination of deoxynivalenol and zearalenone at permitted feed concentrations causes serious physiological effects in young pigs. J. Vet. Sci. 2008, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Böhm, J.; Zentek, J. The toxicological impacts of the Fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins 2013, 5, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Smith, T.K.; Boermans, H.J.; Sefton, A.E.; Downey, R.; Woodward, B. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on performance, metabolism, hematology, and immunocompetence of ducklings. Poult. Sci. 2005, 84, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Dadak, A.; Hess, M.; Böhm, J. Single and combined effects of deoxynivalenol mycotoxin and a microbial feed additive on lymphocyte DNA damage and oxidative stress in broiler chickens. PLoS ONE 2014, 9, e88028. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, Y.; Deng, H.; Deng, Y.; Deng, J.; Zuo, Z.; Wang, Y.; Peng, X.; Cui, H.; Shen, L. Deoxynivalenol induces apoptosis in chicken splenic lymphocytes via the reactive oxygen species-mediated mitochondrial pathway. Environ. Toxicol. Pharmacol. 2015, 39, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-R.; Yan, D.; Pestka, J.J. Differential cytokine mRNA expression in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): Dose response and time course. Toxicol. Appl. Pharmacol. 1997, 144, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Kaspers, B.; Kaiser, P. Avian Immunology, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Bravo, R.; Frank, R.; Blundell, P.A.; Macdonald-Bravo, H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature 1987, 326, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Zhao, H. Detection of DNA strand breaks in apoptotic cells by flow-and image-cytometry. In DNA Damage Detection In Situ, Ex Vivo, and In Vivo: Methods and Protocols; Didenko, V.V., Ed.; Humana Press: New York, NY, USA, 2011; pp. 91–101. [Google Scholar]
- Redon, C.E.; Nakamura, A.J.; Sordet, O.; Dickey, J.S.; Gouliaeva, K.; Tabb, B.; Lawrence, S.; Kinders, R.J.; Bonner, W.M.; Sedelnikova, O.A. Gamma-H2AX detection in peripheral blood lymphocytes, splenocytes, bone marrow, xenografts, and skin. In DNA Damage Detection In Situ, Ex Vivo, and In Vivo: Methods and Protocols; Didenko, V.V., Ed.; Humana Press: New York, NY, USA, 2011; pp. 249–270. [Google Scholar]
- Rothkamm, K.; Horn, S. Gamma-H2AX as protein biomarker for radiation exposure. Ann. Ist. Super. Sanita 2009, 45, 265–271. [Google Scholar] [PubMed]
- Hu, C.-N. The Production Performance of Native Chicken in Taiwan. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 1 January 2007. [Google Scholar]
- Poultry Association Republic of China. Ji de Zhong Lei: Hei Yu Tu Ji [The Variety of Chickens: Black-Feathered Country Chickens]. Available online: http://www.poultry.org.tw/variety.php?cate_index=3 (accessed on 6 April 2017).
- Ozden, O.; Black, B.L.; Ashwell, C.M.; Tipsmark, C.K.; Borski, R.J.; Grubb, B.J. Developmental profile of claudin-3, -5, and -16 proteins in the epithelium of chick intestine. Anat. Rec. 2010, 293, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Gambacorta, L.; Visconti, A. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins 2014, 6, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Van Le Thanh, B.; Lemay, M.; Bastien, A.; Lapointe, J.; Lessard, M.; Chorfi, Y.; Guay, F. The potential effects of antioxidant feed additives in mitigating the adverse effects of corn naturally contaminated with Fusarium mycotoxins on antioxidant systems in the intestinal mucosa, plasma, and liver in weaned pigs. Mycotoxin Res. 2016, 32, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Swamy, H.V.; Smith, T.K.; Cotter, P.F.; Boermans, H.J.; Sefton, A.E. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on production and metabolism in broilers. Poult. Sci. 2002, 81, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Chowdhury, S.R.; Oinas, N.; MacDonald, E.J.; Smith, T.K. Effects of feeding grains naturally contaminated with Fusarium mycotoxins on brain regional neurochemistry of laying hens, turkey poults, and broiler breeder hens. Poult. Sci. 2006, 85, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Klapáčová, K.; Faixová, Z.; Faix, Š.; Miklósová, L.; Leng, L. Effects of feeding wheat naturally contaminated with Fusarium mycotoxins on blood biochemistry and the effectiveness of dietary lignin treatment to alleviate mycotoxin adverse effects in broiler chickens. Acta Vet. 2011, 61, 227–237. [Google Scholar] [CrossRef]
- Hamilton, R.M.G.; Thompson, B.K.; Trenholm, H.L.; Fiser, P.S.; Greenhalgh, R. Effects of feeding White Leghorn hens diets that contain deoxynivalenol (vomitoxin)-contaminated wheat. Poult. Sci. 1985, 64, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Reevaluation of the fundamental dose–Response relationship: A new database suggests that the U-shaped, rather than the sigmoidal, curve predominates. BioScience 1999, 49, 725–732. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Smith, T.K. Effects of feeding grains naturally contaminated with Fusarium mycotoxins on hepatic fractional protein synthesis rates of laying hens and the efficacy of a polymeric glucomannan mycotoxin adsorbent. Poult. Sci. 2005, 84, 1671–1674. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Ueberschär, K.H.; Valenta, H.; Matthes, S.; Matthäus, K.; Halle, I. Effects of graded levels of Fusarium toxin-contaminated wheat in Pekin duck diets on performance, health and metabolism of deoxynivalenol and zearalenone. Br. Poult. Sci. 2004, 45, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Matthes, S. On the interactions between Fusarium toxin-contaminated wheat and nonstarch polysaccharide hydrolyzing enzymes in diets of broilers on performance, intestinal viscosity, and carryover of deoxynivalenol. Poult. Sci. 2007, 86, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Ueberschär, K.H.; Matthes, S. On the interactions between Fusarium toxin-contaminated wheat and nonstarch polysaccharide hydrolyzing enzymes in turkey diets on performance, health and carry-over of deoxynivalenol and zearalenone. Br. Poult. Sci. 2007, 48, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Brezina, U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.K.; Smith, T.K.; Boermans, H.J.; Kumar, P.A.; Girgis, G.N. Effects of dietary Fusarium mycotoxins on intestinal lymphocyte subset populations, cell proliferation and histological changes in avian lymphoid organs. Food Chem. Toxicol. 2010, 48, 3000–3007. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Goyarts, T.; Döll, S.; Grove, N.; Spolders, M.; Flachowsky, G. Effects of the Fusarium toxin deoxynivalenol on tissue protein synthesis in pigs. Toxicol. Lett. 2006, 165, 297–311. [Google Scholar]
- Malovrh, T.; Jakovac-Strajn, B. Feed contaminated with Fusarium toxins alter lymphocyte proliferation and apoptosis in primiparous sows during the perinatal period. Food Chem. Toxicol. 2010, 48, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Øvernes, G.; Matre, T.; Sivertsen, T.; Larsen, H.J.S.; Langseth, W.; Reitan, L.J.; Jansen, J.H. Effects of diets with graded levels of naturally deoxynivalenol-contaminated oats on immune response in growing pigs. J. Vet. Med. A 1997, 44, 539–550. [Google Scholar] [CrossRef]
- Döll, S.; Goyarts, T.; Tiemann, U.; Dänicke, S. Practically relevant concentrations of deoxynivalenol in diets for growing-finishing pigs offered as mash or pellets. Arch. Anim. Nutr. 2007, 61, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brüssow, K.-P.; Jonas, L.; Pöhland, R.; Schneider, F.; Dänicke, S. Effects of diets with cereal grains contaminated by graded levels of two toxins on selected immunological and histological measurements in the spleen of gilts. J. Anim. Sci. 2006, 84, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Goyarts, T.; Dänicke, S.; Tiemann, U.; Rothkötter, H.-J. Effect of the Fusarium toxin deoxynivalenol (DON) on IgA, IgM and IgG concentrations and proliferation of porcine blood lymphocytes. Toxicol. In Vitro 2006, 20, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Accensi, F.; Beauchamp, E.; Cossalter, A.M.; Callu, P.; Grosjean, F.; Oswald, I.P. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. 2008, 177, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Yahi, N.; Younès-Sakr, L.; Boyron, M.; Caporiccio, B.; Fantini, J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: Stimulation of interleukin-8 secretion, potentiation of interleukin-1β effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol. 2008, 228, 84–92. [Google Scholar] [PubMed]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Swamy, H.V.; Smith, T.K.; Karrow, N.A.; Boermans, H.J. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on growth and immunological parameters of broiler chickens. Poult. Sci. 2004, 83, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Blajet-Kosicka, A.; Kosicki, R.; Khan, M.Z.; Rehman, H.; Böhm, J. Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult. Sci. 2012, 91, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhou, T.; Gong, J.; Young, C.; Su, X.; Li, X.Z.; Zhu, H.; Tsao, R.; Yang, R. Isolation of deoxynivalenol-transforming bacteria from the chicken intestines using the approach of PCR-DGGE guided microbial selection. BMC Microbiol. 2010, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Pitman, R.S.; Blumberg, R.S. First line of defense: The role of the intestinal epithelium as an active component of the mucosal immune system. J. Gastroenterol. 2000, 35, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Osselaere, A.; Devreese, M.; Watteyn, A.; Vandenbroucke, V.; Goossens, J.; Hautekiet, V.; Eeckhout, M.; De Saeger, S.; De Baere, S.; De Backer, P. Efficacy and safety testing of mycotoxin-detoxifying agents in broilers following the European Food Safety Authority guidelines. Poult. Sci. 2012, 91, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Li, M.L.; Lin, C.Y.; Shi, B.L.; Xu, A.L.; Pan, J.M. Jia qin ying yang fen xu yao liang shou ce—Tu ji, ya, e [Handbook of nutrient requirements for poultry—Country chicken, duck, and goose]. In Xing Zheng Yuan Nong Ye Wei Yuan Hui Xu Chan Shi Yan Suo Zhuan Ji; Livestock Research Institute, Council of Agriculture, Executive Yuan: Tainan, Taiwan, 1999. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2004. [Google Scholar]
- Lillie, R.D. Histopathologic Technic and Practical Histochemistry; Blakiston Division, McGraw-Hill: New York, NY, USA, 1954. [Google Scholar]
- Shi, S.R.; Cote, R.J.; Taylor, C.R. Antigen retrieval immunohistochemistry: Past, present, and future. J. Histochem. Cytochem. 1997, 45, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, T. Antigen retrieval on formaldehyde-fixed paraffin sections: Its potential drawbacks and optimization for double immunostaining. Micron 2000, 31, 639–649. [Google Scholar] [CrossRef]
- Huang, J.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wu, B. The association between splenocyte apoptosis and alterations of Bax, Bcl-2 and caspase-3 mRNA expression, and oxidative stress induced by dietary nickel chloride in broilers. Int. J. Environ. Res. Public Health 2013, 10, 7310–7326. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cui, Y.; Cui, W.; Deng, J.; Cui, H. The decrease of relative weight, lesions, and apoptosis of bursa of Fabricius induced by excess dietary selenium in chickens. Biol. Trace Elem. Res. 2009, 131, 33–42. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, L.; Garay, E.; Quirós, M. Identification of claudins by Western blot and immunofluorescence in different cell lines and tissues. In Claudins; Turksen, K., Ed.; Humana Press: New York, NY, USA, 2011; pp. 213–231. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Egbeyale, L.T.; Abiola, S.S.; Sogunle, O.M.; Ozoje, M.O. Effect of egg size and strain on growth performance of cockerel. Agric. Biol. J. N. Am. 2011, 2, 1445–1453. [Google Scholar] [CrossRef]
- Pinchasov, Y. Relationship between the weight of hatching eggs and subsequent early performance of broiler chicks. Br. Poult. Sci. 1991, 32, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sklan, D.; Heifetz, S.; Halevy, O. Heavier chicks at hatch improves marketing body weight by enhancing skeletal muscle growth. Poult. Sci. 2003, 82, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
| Ingredient (g/kg as Fed Basis) | Diet | ||
|---|---|---|---|
| I | II | III | |
| Corn | 510 | 570 | 600 |
| Soybean meal | 360 | 320 | 310 |
| Fish meal | 25 | 15 | - |
| Soybean oil | 55 | 45 | 39 |
| Brown rice 1 | 15 | 15 | 15 |
| Monocalcium phosphate | 15 | 11 | 11 |
| Limestone | 11 | 15 | 15 |
| Iodized salt | 3 | 4 | 4 |
| DL-Methionine | 2 | 2 | 2 |
| Vitamin Premix 2 | 1 | 1 | 1 |
| Mineral Premix 3 | 1 | 1 | 1 |
| Choline chloride, 50% | 1 | 1 | 1 |
| Total | 1000 | 1000 | 1000 |
| Calculated value | |||
| Metabolizable Energy (kcal/kg) | 3159 | 3150 | 3146 |
| Crude Protein (g/kg) | 220.1 | 200.1 | 186.9 |
| Analyzed value | |||
| Dry Matter (g/kg) | 887.1 | 884.7 | 886.1 |
| Crude Protein (g/kg) | 224.6 | 207.7 | 191.2 |
| Ether Extract (g/kg of DM) | 90.6 | 81.2 | 70.1 |
| Crude Fiber (g/kg of DM) | 34.9 | 30.2 | 33.1 |
| Treatment | Diet | ||
|---|---|---|---|
| I | II | III | |
| Basal diet | ND | ND | ND |
| 2 mg/kg | 2.39 | 2.21 | 2.07 |
| 5 mg/kg | 5.17 | 5.27 | 5.31 |
| 10 mg/kg | 10.88 | 10.01 | 10.92 |
| Age | Treatment (DON, mg/kg) | p-Values 1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | ||||||||||||
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | D | S | L | D × S | D × L | S × L | D × S × L | |
| Body weight (g) | |||||||||||||||
| Initial (5 d) | 49.3 ± 5.2 (n = 24) | 49.4 ± 4.8 (n = 28) | 48.8 ± 5.0 (n = 20) | 49.5 ± 4.8 (n = 30) | 50.9 ± 3.9 (n = 20) | 48.1 ± 5.0 (n = 32) | 48.9 ± 5.2 (n = 19) | 49.4 ± 4.3 (n = 31) | 0.999 | 0.629 | - | 0.247 | - | - | - |
| 3 wk | 348 ± 41 (n = 22) | 383 ± 33 (n = 27) | 356 ± 37 (n = 19) | 387 ± 43 (n = 29) | 354 ± 32 (n = 18) | 384 ± 40 (n = 30) | 350 ± 31 (n = 17) | 388 ± 38 (n = 29) | 0.120 | <0.0001 | <0.0001 | 0.002 | 0.135 | <0.0001 | 0.350 |
| 10 wk | 1509 ± 96 (n = 21) | 1681 ± 130 (n = 21) | 1505 ± 112 (n = 15) | 1654 ± 162 (n = 26) | 1480 ± 151 (n = 16) | 1724 ± 148 (n = 25) | 1435 ± 114 (n = 13) | 1739 ± 141 (n = 27) | |||||||
| 16 wk | 2399 ± 203 (n = 15) | 2526 ± 311 (n = 18) | 2301 ± 183 (n = 11) | 2386 ± 302 (n = 22) | 2332 ± 178 (n = 15) | 2569 ± 181 (n = 19) | 2218 ± 174 (n = 12) | 2498 ± 329 (n = 21) | |||||||
| Feed intake (g/bird) | |||||||||||||||
| 5 d–3 wk | 519 ± 18 (n = 4) | 526 ± 19 (n = 4) | 520 ± 18 (n = 4) | 538 ± 27 (n = 4) | 0.288 | - | <0.0001 | - | 0.405 | - | - | ||||
| 5 d–10 wk | 3727 ± 179 (n = 4) | 3676 ± 198 (n = 4) | 3719 ± 191 (n = 4) | 3727 ± 61 (n = 4) | |||||||||||
| 5 d–16 wk | 8507 ± 474 (n = 4) | 7992 ± 406 (n = 4) | 8391 ± 356 (n = 4) | 8225 ± 281 (n = 4) | |||||||||||
| Feed efficiency | |||||||||||||||
| 5 d–3 wk | 0.61 ± 0.03 (n = 4) | 0.61 ± 0.02 (n = 4) | 0.60 ± 0.02 (n = 4) | 0.59 ± 0.02 (n = 4) | 0.871 | - | <0.0001 | - | 0.654 | - | - | ||||
| 5 d–10 wk | 0.42 ± 0.00 (n = 4) | 0.42 ± 0.02 (n = 4) | 0.43 ± 0.01 (n = 4) | 0.43 ± 0.01 (n = 4) | |||||||||||
| 5 d–16 wk | 0.28 ± 0.01 (n = 4) | 0.29 ± 0.02 (n = 4) | 0.29 ± 0.01 (n = 4) | 0.29 ± 0.01 (n = 4) | |||||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.S.; Li, Y.-H.; Lin, M.-F. Chronic Exposure to the Fusarium Mycotoxin Deoxynivalenol: Impact on Performance, Immune Organ, and Intestinal Integrity of Slow-Growing Chickens. Toxins 2017, 9, 334. https://doi.org/10.3390/toxins9100334
Chen SS, Li Y-H, Lin M-F. Chronic Exposure to the Fusarium Mycotoxin Deoxynivalenol: Impact on Performance, Immune Organ, and Intestinal Integrity of Slow-Growing Chickens. Toxins. 2017; 9(10):334. https://doi.org/10.3390/toxins9100334
Chicago/Turabian StyleChen, Stephanie S., Yi-Hung Li, and Mei-Fong Lin. 2017. "Chronic Exposure to the Fusarium Mycotoxin Deoxynivalenol: Impact on Performance, Immune Organ, and Intestinal Integrity of Slow-Growing Chickens" Toxins 9, no. 10: 334. https://doi.org/10.3390/toxins9100334





