Efficacy of Bee Venom Acupuncture for Chronic Low Back Pain: A Randomized, Double-Blinded, Sham-Controlled Trial
Abstract
:1. Introduction
2. Results
2.1. Study Participants
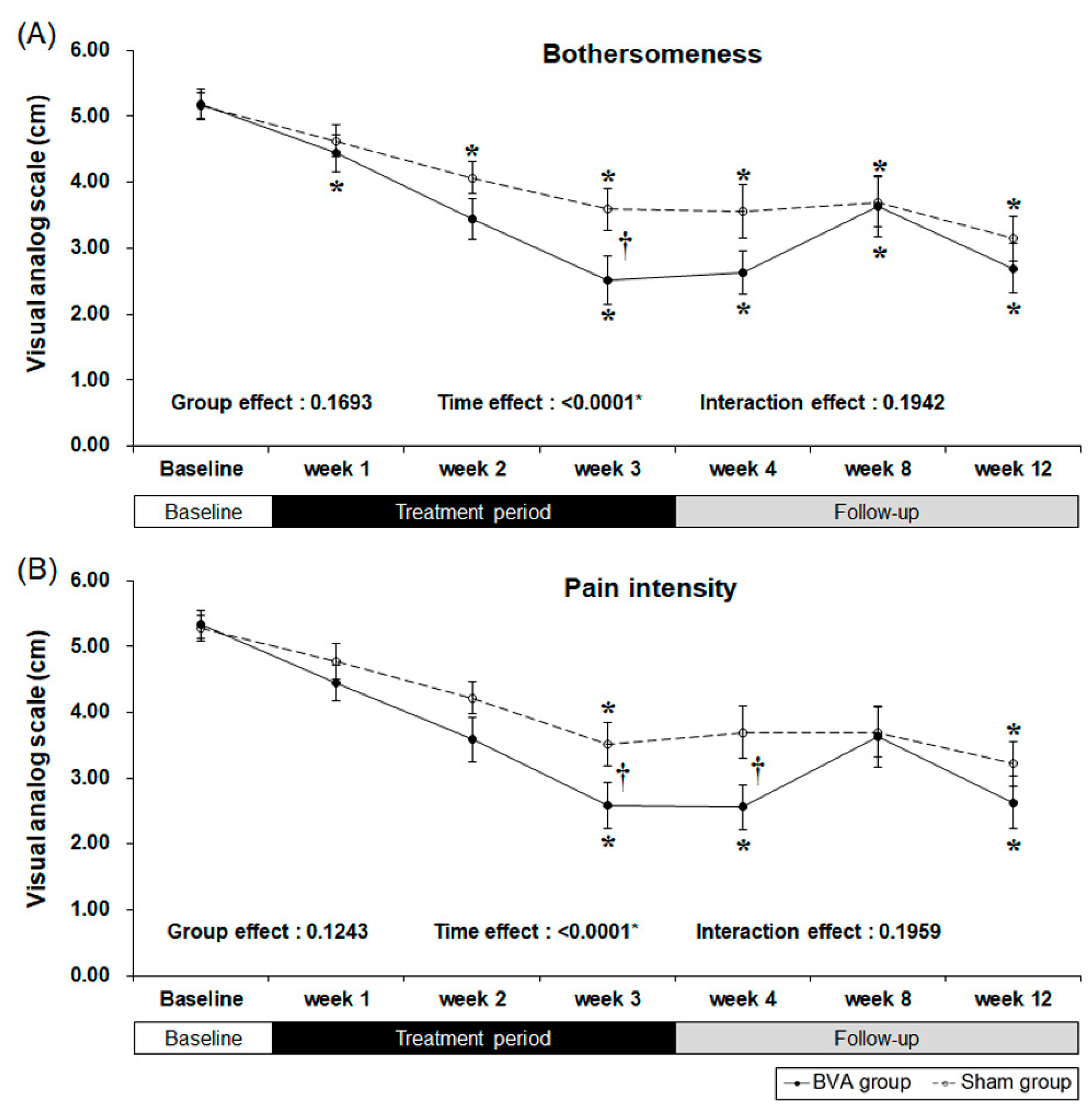
2.2. Primary Outcome
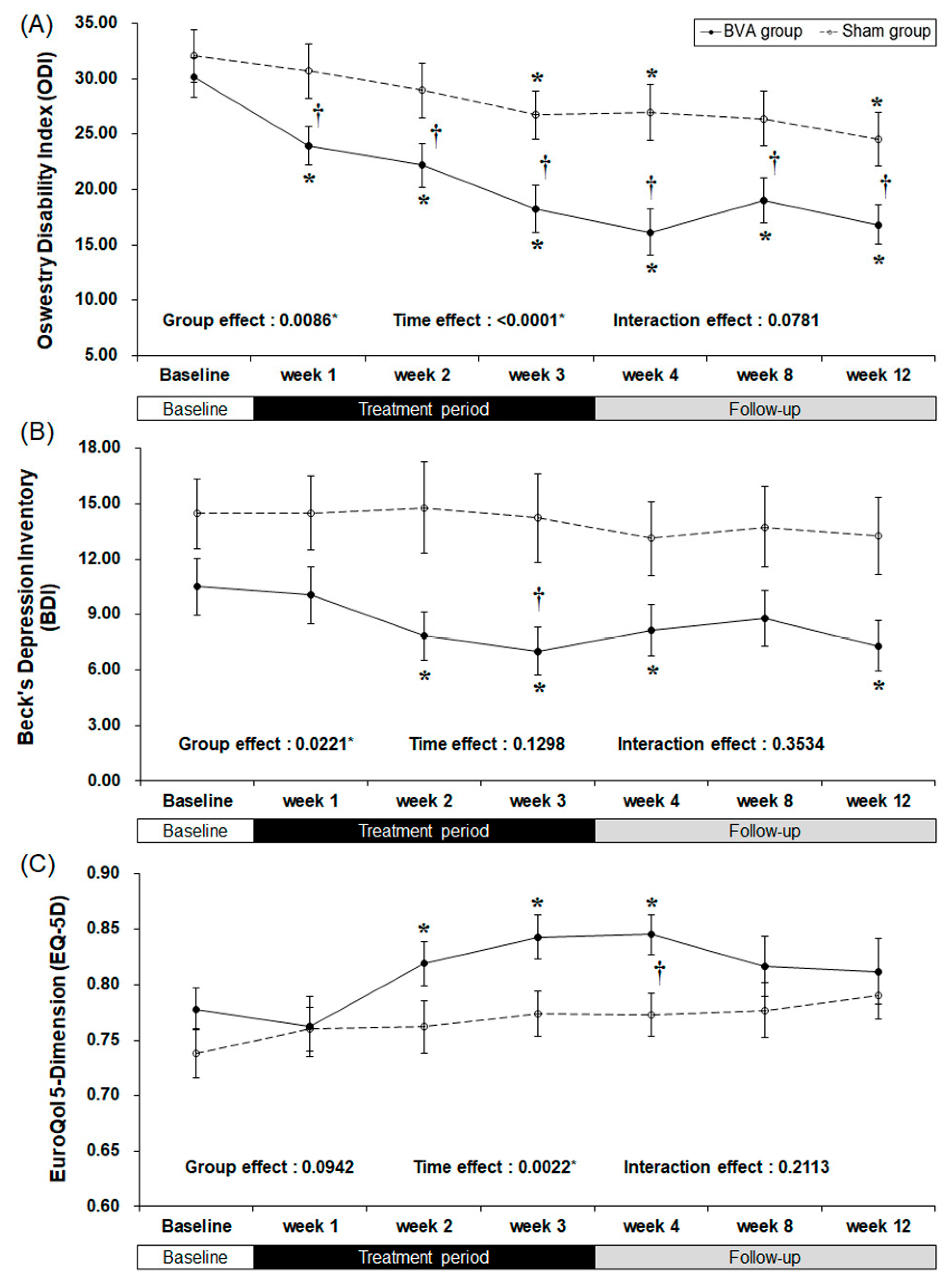
2.3. Secondary Outcomes
2.4. Credibility Analysis
2.5. Adverse Events
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Size Calculation
4.3. Eligibility Criteria
4.4. Randomization and Blinding
4.5. Interventions
4.6. Outcomes Measures
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Valat, J.P.; Goupille, P.; Vedere, V. Low back pain: Risk factors for chronicity. Revue Rhum. 1997, 64, 189–194. [Google Scholar]
- Gore, M.; Sadosky, A.; Stacey, B.R.; Tai, K.S.; Leslie, D. The burden of chronic low back pain: Clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012, 37, E668–E677. [Google Scholar] [CrossRef] [PubMed]
- Chenot, J.F.; Becker, A.; Leonhardt, C.; Keller, S.; Donner-Banzhoff, N.; Baum, E.; Pfingsten, M.; Hildebrandt, J.; Basler, H.D.; Kochen, M.M. Use of complementary alternative medicine for low back pain consulting in general practice: A cohort study. BMC Complement. Altern. Med. 2007, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Tai, K.S.; Sadosky, A.; Leslie, D.; Stacey, B.R. Use and costs of prescription medications and alternative treatments in patients with osteoarthritis and chronic low back pain in community-based settings. Pain Pract. 2012, 12, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.M.; Langevin, H.M.; Witt, C.M.; Dubner, R. Acupuncture for chronic low back pain. N. Engl. J. Med. 2010, 363, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Galvin, R.; Curry, P. Effectiveness of acupuncture for nonspecific chronic low back pain: A systematic review and meta-analysis. Spine 2013, 38, 2124–2138. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Kang, M.S.; Kim, H.W.; Ham, T.W.; Yim, Y.K.; Jeong, S.H.; Park, D.S.; Choi, D.Y.; Han, H.J.; Beitz, A.J.; et al. Antinociceptive effects of bee venom acupuncture (apipuncture) in rodent animal models: A comparative study of acupoint versus non-acupoint stimulation. Acupunct. Electrother. Res. 2001, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Pittler, M.H.; Shin, B.C.; Kong, J.C.; Ernst, E. Bee venom acupuncture for musculoskeletal pain: A review. J. Pain 2008, 9, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, J.S.; Lee, J.; Kim, M.R.; Park, K.B.; Lee, H.D.; Lee, Y.; Hong, J.; Ha, I.H. Usage report of pharmacopuncture in musculoskeletal patients visiting Korean medicine hospitals and clinics in Korea. BMC Complement. Altern. Med. 2016, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Park, S.; Sun, S.; Lee, K. Research on Korean pharmacopuncture in South Korea since 2007. J. Pharm. 2014, 17, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi do, Y.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by alpha2-adrenoceptors. Brain Res. 2006, 1073–1074, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Qu, F.; He, X.; Liao, D.; Kang, S.M.; Lu, S.J. The anti-nociceptive effect and the possible mechanism of acupoint stimulation caused by chemical irritants in the bee venom pain model. Brain Res. 2010, 1355, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Park, H.J.; Chae, Y.; Lim, S. An overview of bee venom acupuncture in the treatment of arthritis. Evid.-Based Complement. Altern. Med. 2005, 2, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, D.I. A descriptive statistical approach to the Korean pharmacopuncture therapy. J. Acupunct. Meridian Stud. 2010, 3, 141–149. [Google Scholar] [CrossRef]
- Shin, B.-C.; Kong, J.C.; Park, T.-Y.; Yang, C.-Y.; Kang, K.-W.; Choi, S.-M. Bee venom acupuncture for chronic low back pain: A randomised, sham-controlled, triple-blind clinical trial. Eur. J. Integr. Med. 2012, 4, e271–e280. [Google Scholar] [CrossRef]
- Dunn, K.M.; Croft, P.R. Classification of low back pain in primary care: Using “bothersomeness” to identify the most severe cases. Spine 2005, 30, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Lee, J.; Kim, M.R.; Jung, J.; Shin, B.C.; Lee, M.S.; Ha, I.H. The short-term effect of integrated complementary and alternative medicine treatment in inpatients diagnosed with lumbar intervertebral disc herniation: A prospective observational study. J. Altern. Complement. Med. 2016, 22, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Song, H.S. The effectiveness of bee venom acupuncture therapy on the treatment of sprain of l-spine (a randomized controlled trial: Double blinding). Acupuncture 2005, 22, 185–195. [Google Scholar]
- Kwon, Y.B.; Kang, M.S.; Han, H.J.; Beitz, A.J.; Lee, J.H. Visceral antinociception produced by bee venom stimulation of the zhongwan acupuncture point in mice: Role of alpha(2) adrenoceptors. Neurosci. Lett. 2001, 308, 133–137. [Google Scholar] [CrossRef]
- Kim, H.W.; Kwon, Y.B.; Ham, T.W.; Roh, D.H.; Yoon, S.Y.; Lee, H.J.; Han, H.J.; Yang, I.S.; Beitz, A.J.; Lee, J.H. Acupoint stimulation using bee venom attenuates formalin-induced pain behavior and spinal cord fos expression in rats. J. Vet. Ned. Sci. 2003, 65, 349–355. [Google Scholar] [CrossRef]
- Nam, K.W.; Je, K.H.; Lee, J.H.; Han, H.J.; Lee, H.J.; Kang, S.K.; Mar, W. Inhibition of cox-2 activity and proinflammatory cytokines (tnf-alpha and il-1beta) production by water-soluble sub-fractionated parts from bee (apis mellifera) venom. Arch. Pharm. Res. 2003, 26, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Rim, G.S.; Yang, H.I.; Yin, C.S.; Koh, H.G.; Jang, M.H.; Kim, C.J.; Choe, B.K.; Chung, J.H. Bee venom induces apoptosis through caspase-3 activation in synovial fibroblasts of patients with rheumatoid arthritis. Toxicon 2005, 46, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Steen, N.; Hutchinson, A.; McColl, E.; Eccles, M.P.; Hewison, J.; Meadows, K.A.; Blades, S.M.; Fowler, P. Development of a symptom based outcome measure for asthma. BMJ 1994, 309, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Cherkin, D.C.; Deyo, R.A.; Street, J.H.; Barlow, W. Predicting poor outcomes for back pain seen in primary care using patients’ own criteria. Spine 1996, 21, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Cherkin, D.C.; Wheeler, K.J.; Barlow, W.; Deyo, R.A. Medication use for low back pain in primary care. Spine 1998, 23, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Grovle, L.; Haugen, A.J.; Keller, A.; Natvig, B.; Brox, J.I.; Grotle, M. The bothersomeness of sciatica: Patients’ self-report of paresthesia, weakness and leg pain. Eur. Spine J. 2010, 19, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.L.; Deyo, R.A.; Atlas, S.J.; Singer, D.E.; Chapin, A.; Keller, R.B. Assessing health-related quality of life in patients with sciatica. Spine 1995, 20, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Baras, M.; Zeev, A.; Epstein, L. Low back pain: Reliability of a set of pain measurement tools. Arch. Phys. Med. Rehabil. 2001, 82, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yim, B.K.; Lee, J.H.; Lee, S.; Kim, T.H. Risk associated with bee venom therapy: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126971. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.; Lee, H.G.; Choi, Y.M.; Song, B.Y.; Yook, T.H.; Kim, J.U. The clinical study on 130 cases with sweet bee venom treatment. Acupuncture 2013, 30, 211–217. [Google Scholar] [CrossRef]
- Seo, B.K.; Lee, J.H.; Sung, W.S.; Song, E.M.; Jo, D.J. Bee venom acupuncture for the treatment of chronic low back pain: Study protocol for a randomized, double-blinded, sham-controlled trial. Trials 2013, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Zaringhalam, J.; Manaheji, H.; Rastqar, A.; Zaringhalam, M. Reduction of chronic non-specific low back pain: A randomised controlled clinical trial on acupuncture and baclofen. Chin. Med. 2010, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Revill, S.I.; Robinson, J.O.; Rosen, M.; Hogg, M.I. The reliability of a linear analogue for evaluating pain. Anaesthesia 1976, 31, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.M. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983, 16, 87–101. [Google Scholar] [CrossRef]
- Roland, M.; Morris, R. A study of the natural history of back pain. Part i: Development of a reliable and sensitive measure of disability in low-back pain. Spine 1983, 8, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.H.; Kim, D.J.; Kim, S.K.; Kim, D.J.; Lee, H.M.; Park, H.J. Validation in the cross-cultural adaptation of the Korean version of the oswestry disability index. J. Korean Med. Sci. 2006, 21, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R.; de Charro, F. EQ-SD: A measure of health status from the euroqol group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Cho, Y.S.; Uhm, W.S.; Kim, S.; Bae, S.C. Cross-cultural adaptation and validation of the Korean version of the eq-5d in patients with rheumatic diseases. Qual. Life Res. 2005, 14, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, J.Y. A study of the reliability and the validity of the BDI, SDS, and MMPI-D scales. Korean J. Clin. Psychol. 1991, 10, 98–113. [Google Scholar]
- Vincent, C.; Lewith, G. Placebo controls for acupuncture studies. J. R. Soc. Med. 1995, 88, 199–202. [Google Scholar] [PubMed]
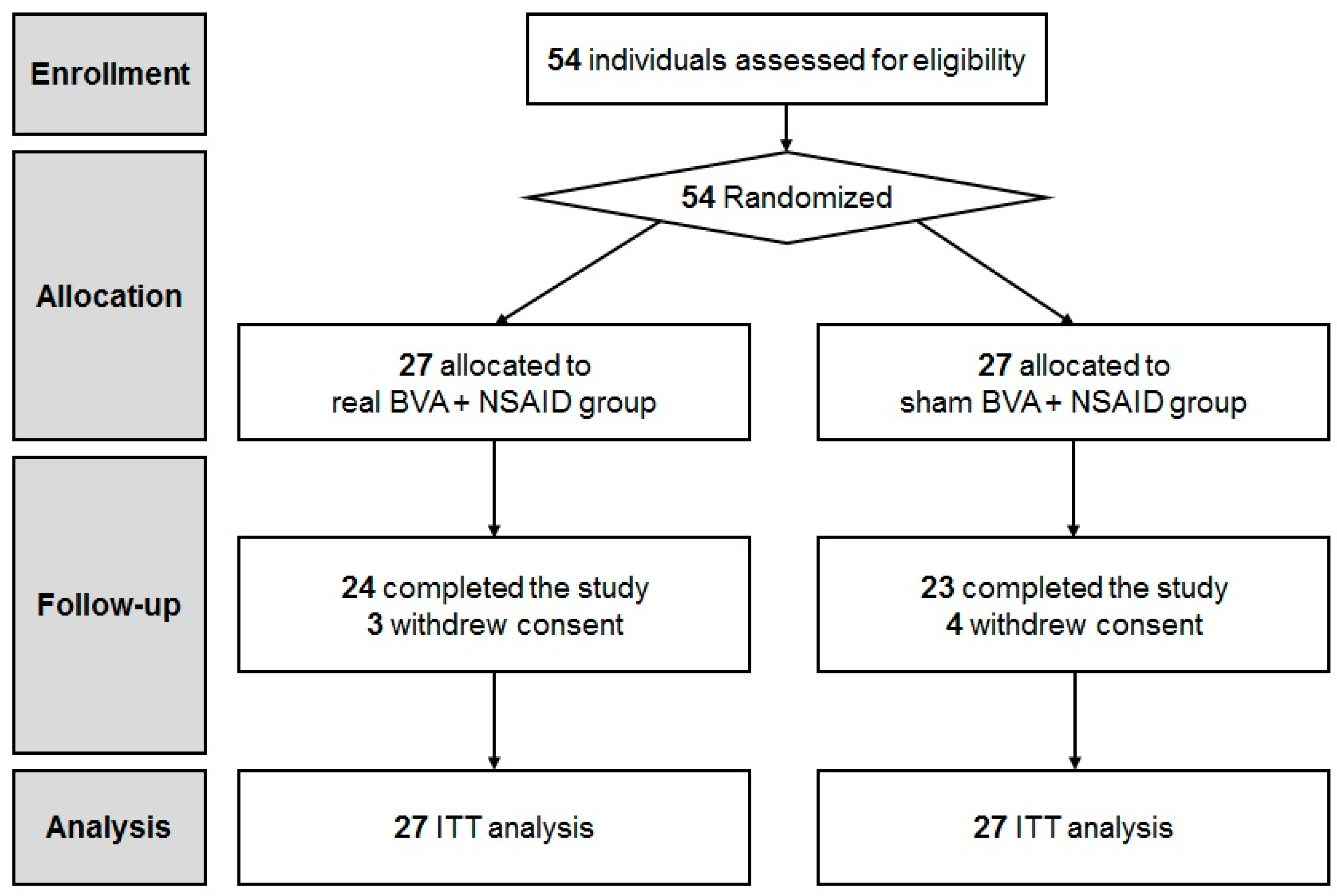
| Characteristics | BVA Group (n = 27) | Sham Group (n = 27) | p-Value |
|---|---|---|---|
| Gender (Male/Female) † | 9 (33.33%)/18 (66.67%) | 4 (14.81%)/23 (85.19%) | 0.1115 |
| Age (years) ‡ | 49.85 (14.44) | 50.07 (11.06) | 0.7175 |
| Height (cm) § | 162.03 (7.65) | 160.35 (7.15) | 0.4099 |
| Weight (kg) § | 64.09 (11.02) | 61.60 (10.26) | 0.3928 |
| Vital sign | |||
| SBP (mmHg) § | 122.78 (12.63) | 120.26 (15.34) | 0.5131 |
| DBP (mmHg) § | 75.56 (8.10) | 72.93 (10.48) | 0.3071 |
| Pulse (times/minute) ‡ | 75.81 (9.85) | 78.44 (10.30) | 0.3763 |
| Temp (°C) ‡ | 36.15 (0.28) | 36.10 (0.26) | 0.4681 |
| Smoke (Yes/No) †† | 3 (12.00%)/22 (88.00%) | 0 (0.00%)/27 (100.00%) | 0.1041 |
| Drink (Yes/No) † | 10 (40.00%)/15 (60.00%) | 6 (23.08%)/20 (76.92%) | 0.1929 |
| VAS for bothersomeness (mm) ‡ | 5.19 (1.14) | 5.16 (1.07) | 0.9073 |
| VAS for pain (mm) ‡ | 5.33 (1.11) | 5.28 (1.02) | 0.8745 |
| Outcome | Time | BVA Group (n = 27) | Sham Group (n = 27) | Within (BG) † | Within (PG) † | p-Value ‡ | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| VAS score | ||||||||
| Bothersomeness | ||||||||
| Baseline | 5.19 | 1.14 | 5.16 | 1.07 | ||||
| week 3 | 2.52 | 1.89 | 3.59 | 1.67 | <0.0001 * | <0.0001 * | 0.0164 * | |
| week 4 | 2.63 | 1.74 | 3.56 | 2.08 | <0.0001 * | <0.0001 * | 0.0548 | |
| week 8 | 3.63 | 2.42 | 3.70 | 1.96 | 0.0061 * | 0.0002 * | 0.8823 | |
| week 12 | 2.70 | 2.00 | 3.15 | 1.77 | <0.0001 * | <0.0001 * | 0.3502 | |
| Pain intensity | ||||||||
| Baseline | 5.33 | 1.11 | 5.28 | 1.02 | ||||
| week 3 | 2.59 | 1.82 | 3.52 | 1.70 | <0.0001 * | <0.0001 * | 0.0486 * | |
| week 4 | 2.56 | 1.80 | 3.70 | 2.03 | <0.0001 * | 0.0005 * | 0.0273 | |
| week 8 | 3.63 | 2.42 | 3.70 | 1.96 | 0.0083 * | 0.0002 * | 0.9069 | |
| week 12 | 2.63 | 2.06 | 3.22 | 1.76 | <0.0001 * | <0.0001 * | 0.2478 | |
| ODI score | ||||||||
| Baseline | 30.14 | 9.17 | 32.07 | 12.48 | ||||
| week 3 | 18.25 | 11.16 | 26.76 | 11.28 | <0.0001 * | 0.0100 * | 0.0085 * | |
| week 4 | 16.15 | 10.71 | 26.96 | 13.01 | <0.0001 * | 0.0259 * | 0.0018 * | |
| week 8 | 19.06 | 10.60 | 26.40 | 12.84 | <0.0001 * | 0.0955 | 0.0349 * | |
| week 12 | 16.81 | 9.34 | 24.54 | 12.51 | <0.0001 * | 0.0407 * | 0.0171 * | |
| BDI score | ||||||||
| Baseline | 10.52 | 7.99 | 14.44 | 9.66 | ||||
| week 3 | 7.00 | 6.72 | 14.22 | 12.59 | 0.0026 * | 0.0719 | 0.0429 * | |
| week 4 | 8.15 | 7.23 | 13.11 | 10.32 | 0.0002 * | 0.0677 | 0.1670 | |
| week 8 | 8.78 | 7.76 | 13.74 | 11.37 | 0.1963 | 0.1337 | 0.2525 | |
| week 12 | 7.30 | 7.12 | 13.26 | 10.78 | 0.0064 * | 0.2018 | 0.0765 | |
| EQ-5D score | ||||||||
| Baseline | 0.778 | 0.097 | 0.738 | 0.115 | ||||
| week 3 | 0.843 | 0.102 | 0.774 | 0.106 | 0.0009* | 0.1567 | 0.0511 | |
| week 4 | 0.845 | 0.095 | 0.773 | 0.100 | 0.0005* | 0.1793 | 0.0278 * | |
| week 8 | 0.816 | 0.141 | 0.777 | 0.128 | 0.0997 | 0.1978 | 0.5776 | |
| week 12 | 0.812 | 0.155 | 0.790 | 0.110 | 0.1317 | 0.0961 | 0.9381 | |
| Outcome | Time | BVA Group (n = 27) | Sham Group (n = 27) | Within (BG) † | Within (PG) † | p-Value ‡ | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Credibility test | ||||||||
| Improvement expected | ||||||||
| Baseline | 5.15 | 0.60 | 4.89 | 0.58 | ||||
| week 3 | 5.15 | 0.66 | 4.85 | 0.66 | 0.9999 | 0.9999 | 0.3211 | |
| Recommendation to others | ||||||||
| Baseline | 4.67 | 0.78 | 4.56 | 1.01 | ||||
| week 3 | 4.74 | 0.66 | 4.67 | 0.83 | 0.9999 | 0.8359 | 0.8491 | |
| Treatment logical | ||||||||
| Baseline | 4.89 | 0.80 | 4.41 | 0.84 | ||||
| week 3 | 4.89 | 0.58 | 4.67 | 0.78 | 0.9999 | 0.1826 | 0.9938 | |
| Effective also for other diseases | ||||||||
| Baseline | 4.48 | 1.09 | 4.56 | 0.93 | ||||
| week 3 | 4.70 | 0.82 | 4.52 | 1.16 | 0.2500 | 0.9648 | 0.2349 | |
| Inclusion Criteria | |
| Age between 18 to 65 years old | |
| Experienced low back pain for the previous three months or more. | |
| Scoring more than 4 points on a 10 cm Visual Analog Scale (VAS) for bothersomeness of low back pain | |
| Exhibiting no abnormalities on neurological examination (for example, lumbosacral nerve function, deep tendon reflexes, plantar response, voluntary muscle activation, and sensory function) | |
| Having non-specific, uncomplicated low back pain that qualifies as the following International Classification of Diseases 10 codes: | |
| M513 | Other specified intervertebral disc degeneration |
| M545 | Low back pain |
| M548 | Other dorsalgia |
| M549 | Dorsalgia, unspecified |
| S335 | Sprain and strain of lumbar spine |
| S336 | Sprain and strain of sacroiliac joint |
| S337 | Sprain and strain of other and unspecified parts of the lumbar spine and pelvis |
| Participants who agreed and signed the informed consent | |
| Exclusion criteria | |
| Back pain with radicular pain | |
| Serious spinal disorders, including malignancy, vertebral fracture, spinal infection, and inflammatory spondylitis | |
| Other chronic diseases that could affect or interfere with the therapeutic outcomes, including cardiovascular disease, diabetic neuropathy, active hepatitis, fibromyalgia, rheumatoid arthritis, dementia, and epilepsy | |
| History of spinal surgery or subjects scheduled for spinal surgery during the study | |
| Pain induced by traffic accidents | |
| Musculoskeletal pain other than back pain | |
| Conditions that can be aggravated by bee venom treatment, including: clotting disorders, administration of anticoagulant agents, pregnancy, and seizure disorders | |
| Hypersensitive reactions to previous bee venom treatments, bee strings or insect bites | |
| Severe psychiatric or psychological disorders | |
| Current use of corticosteroids, muscle relaxants, narcotics, or herbal medicines. Use of any medication considered inappropriate by the investigator | |
| Pending lawsuit or receipt of compensation because of low back pain | |
| Subjects who refused to participate in the trial or provide informed consent | |
| Subjects unable to read and write in Korean language | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, B.-K.; Han, K.; Kwon, O.; Jo, D.-J.; Lee, J.-H. Efficacy of Bee Venom Acupuncture for Chronic Low Back Pain: A Randomized, Double-Blinded, Sham-Controlled Trial. Toxins 2017, 9, 361. https://doi.org/10.3390/toxins9110361
Seo B-K, Han K, Kwon O, Jo D-J, Lee J-H. Efficacy of Bee Venom Acupuncture for Chronic Low Back Pain: A Randomized, Double-Blinded, Sham-Controlled Trial. Toxins. 2017; 9(11):361. https://doi.org/10.3390/toxins9110361
Chicago/Turabian StyleSeo, Byung-Kwan, Kyungsun Han, Ojin Kwon, Dae-Jean Jo, and Jun-Hwan Lee. 2017. "Efficacy of Bee Venom Acupuncture for Chronic Low Back Pain: A Randomized, Double-Blinded, Sham-Controlled Trial" Toxins 9, no. 11: 361. https://doi.org/10.3390/toxins9110361
APA StyleSeo, B.-K., Han, K., Kwon, O., Jo, D.-J., & Lee, J.-H. (2017). Efficacy of Bee Venom Acupuncture for Chronic Low Back Pain: A Randomized, Double-Blinded, Sham-Controlled Trial. Toxins, 9(11), 361. https://doi.org/10.3390/toxins9110361






