In Vivo Crystallization of Three-Domain Cry Toxins
Abstract
:1. Introduction
- Separate crystallization domain open reading frames (ORFs)
- Other known crystallization factors
- Toxins with putative crystallization factors
- No known crystallization factors
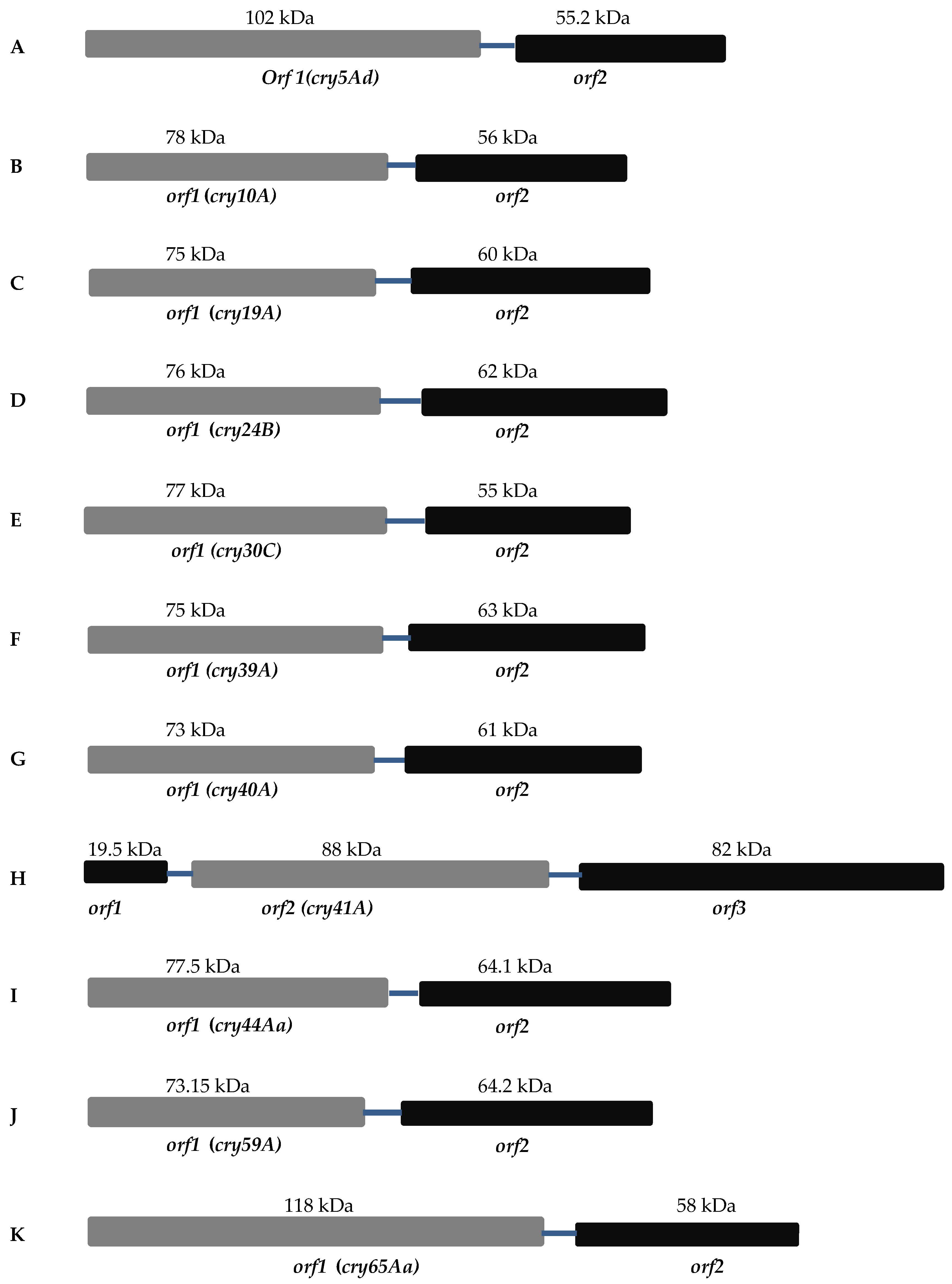
2. Separate Crystallization Domain ORF
2.1. Mosquitocidal Bt Toxins
2.2. Nematocidal Bt Toxins
2.3. Parasporin Toxins
2.3.1. Cry41A
2.3.2. Cry65Aa1
3. Other Known Crystallization Factors
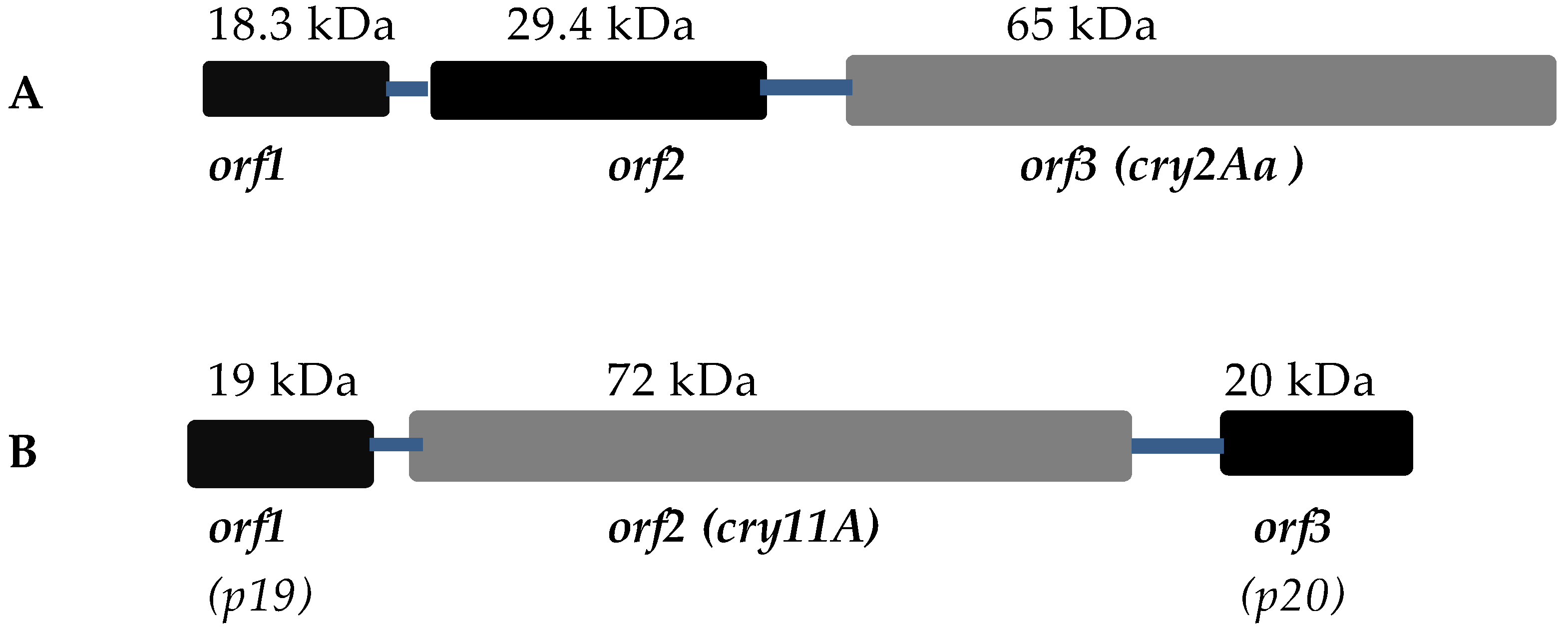
3.1. Cry2A Toxins and the ORF2 Repeat Proteins
3.2. Cry11A and p20
4. Toxins with Putative Crystallization Factors
4.1. Cry6A
4.2. Cry9Ca
4.3. Cry9Ec
4.4. Cry8Ea
4.5. Cry18Aa
5. No Known Crystallization Factors
5.1. Cry3A
5.1.1. Role of Cry3A Domains in Crystallization
5.1.2. STAB-SD Sequence
5.2. Cry1I
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [PubMed]
- Pardo-Lopez, L.; Soberon, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Rosas-García, N.M. Biopesticide production from Bacillus thuringiensis: An environmentally friendly alternative. Recent Pat. Biotechnol. 2009, 3, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Lereclus, D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J. Bacteriol. 1995, 177, 6027–6032. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Lereclus, D. STAB-SD: A Shine-Dalgarno sequence in the 5′ untranslated region is a determinant of mRNA stability. Mol. Microbiol. 1996, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Malvar, T. Regulation of insecticidal crystal protein production in Bacillus thuringiensis. Mol. Microbiol. 1995, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Grant, R.; Aronson, A. Regulation of the packaging of Bacillus thuringiensis δ-endotoxins into inclusions. Appl. Environ. Microbiol. 2001, 67, 5032–5036. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, M.; Walter, T.; Aronson, A. Regulation by overlapping promoters of the rate of synthesis and deposition into crystalline inclusions of Bacillus thuringiensis δ-endotoxins. J. Bacteriol. 2000, 182, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Peng, Q.; Song, F.; Lereclus, D. Regulation of cry gene expression in Bacillus thuringiensis. Toxins 2014, 6, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, A.G.; Moshiri, F.; Sturman, E.J.; Rydel, T.J.; Zheng, M.; Seale, J.W.; Franklin, S. Structure of the full-length insecticidal protein Cry1Ac reveals intriguing details of toxin packaging into in vivo formed crystals. Protein Sci. 2014, 23, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A.I. The two faces of Bacillus thuringiensis: Insecticidal proteins and post—Exponential survival. Mol. Microbiol. 1993, 7, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bietlot, H.P.; Vishnubhatla, I.; Carey, P.R.; Pozsgay, M.; Kaplan, H. Characterization of the cysteine residues and disulphide linkages in the protein crystal of Bacillus thuringiensis. Biochem. J. 1990, 267, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Couche, G.A.; Pfannenstiel, M.A.; Nickerson, K. Structural disulfide bonds in the Bacillus thuringiensis subsp. israelensis protein crystal. J. Bacteriol. 1987, 169, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Sato, S.; Iwamoto, S.; Sudo, S.; Sakamoto, Y.; Yamashita, T.; Uchida, M.; Matsushima, K.; Kashino, Y.; Sakai, H. Novel strategy for protein production using a peptide tag derived from Bacillus thuringiensis Cry4Aa. FEBS J. 2010, 277, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Koni, P.; Ellar, D. Cloning and characterization of a novel Bacillus thuringiensis cytolytic delta-endotoxin. J. Mol. Biol. 1993, 229, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Soto, A.; Del Rincón-Castro, M.C.; Espinoza, A.M.; Ibarra, J.E. Parasporal body formation via overexpression of the Cry10Aa toxin of Bacillus thuringiensis subsp. israelensis, and Cry10Aa-Cyt1Aa synergism. Appl. Environ. Microbiol. 2009, 75, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Barboza-Corona, J.E.; Park, H.W.; Bideshi, D.K.; Federici, B.A. The 60-kilodalton protein encoded by orf2 in the cry19A operon of Bacillus thuringiensis subsp. jegathesan functions like a C-terminal crystallization domain. Appl. Environ. Microbiol. 2012, 78, 2005–2012. [Google Scholar]
- Rosso, M.-L.; Delecluse, A. Contribution of the 65-kilodalton protein encoded by the cloned gene cry19A to the mosquitocidal activity of Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 1997, 63, 4449–4455. [Google Scholar] [PubMed]
- Ohgushi, A.; Saitoh, H.; Wasano, N.; Uemori, A.; Ohba, M. Cloning and characterization of two novel genes, cry24B and s1orf2, from a mosquitocidal strain of Bacillus thuringiensis serovar sotto. Curr. Microbiol. 2005, 51, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, Q.; Xia, L.; Ding, X.; Hu, Q.; Federici, B.A.; Park, H.-W. Identification and characterization of three previously undescribed crystal proteins from Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 2013, 79, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sahara, K.; Bando, H.; Asano, S. Cloning and expression of novel crystal protein genes cry39A and 39 orf2 from Bacillus thuringiensis subsp. aizawai Bun1-14 encoding mosquitocidal proteins. J. Insect Biotechnol. Sericol. 2002, 71, 123–128. [Google Scholar]
- Ito, T.; Ikeya, T.; Sahara, K.; Bando, H.; Asano, S.-I. Cloning and expression of two crystal protein genes, cry30Ba1 and cry44Aa1, obtained from a highly mosquitocidal strain, Bacillus thuringiensis subsp. entomocidus INA288. Appl. Environ. Microbiol. 2006, 72, 5673–5676. [Google Scholar] [CrossRef] [PubMed]
- Noguera, P.A.; Ibarra, J.E. Detection of new cry genes of Bacillus thuringiensis by use of a novel PCR primer system. Appl. Environ. Microbiol. 2010, 76, 6150–6155. [Google Scholar] [CrossRef] [PubMed]
- De Maagd, R.A.; Bravo, A.; Berry, C.; Crickmore, N.; Schnepf, H.E. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 2003, 37, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Lenane, I.J.; Bagnall, N.H.; Josh, P.F.; Pearson, R.D.; Akhurst, R.J.; Kotze, A.C. A pair of adjacent genes, cry5Ad and orf2-5Ad, encode the typical N- and C-terminal regions of a Cry5A delta-endotoxin as two separate proteins in Bacillus thuringiensis strain L366. FEMS Microbiol. Lett. 2008, 278, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Katayama, H.; Saitoh, H.; Akao, T.; Park, Y.S.; Mizuki, E.; Ohba, M.; Ito, A. Typical three-domain Cry proteins of Bacillus thuringiensis strain A1462 exhibit cytocidal activity on limited human cancer cells. J. Biochem. 2005, 138, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hazes, B. The (QxW) 3 domain: A flexible lectin scaffold. Protein Sci. 1996, 5, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, K.; Kimura, K.; Fujii, N.; Yokosawa, N.; Indoh, T.; Murakami, T.; Oguma, K. Cloning and complete nucleotide sequence of the gene for the main component of hemagglutinin produced by Clostridium botulinum type C. Infect. Immun. 1990, 58, 3173–3177. [Google Scholar] [PubMed]
- Krishnan, V. Investigation of parasporins, the cytotoxic proteins from the bacterium Bacillus thuringiensis. Ph.D. Thesis, University of Sussex, Falmer, UK, 2013. [Google Scholar]
- Peng, D.-H.; Pang, C.-Y.; Wu, H.; Huang, Q.; Zheng, J.-S.; Sun, M. The expression and crystallization of Cry65Aa require two C-termini, revealing a novel evolutionary strategy of Bacillus thuringiensis Cry proteins. Sci. Rep. 2015, 5, 8291. [Google Scholar] [CrossRef] [PubMed]
- Widner, W.R.; Whiteley, H.R. Two highly related insecticidal crystal proteins of Bacillus thuringiensis subsp. kurstaki possess different host range specificities. J. Bacteriol. 1989, 171, 965–974. [Google Scholar] [PubMed]
- Wu, D.; Cao, X.; Bai, Y.; Aronson, A. Sequence of an operon containing a novel δ-endotoxin gene from Bacillus thuringiensis. FEMS Microbiol. Lett. 1991, 81, 31–35. [Google Scholar] [CrossRef]
- Brown, K.L. Transcriptional regulation of the Bacillus thuringiensis subsp. thompsoni crystal protein gene operon. J. Bacteriol. 1993, 175, 7951–7957. [Google Scholar] [PubMed]
- Ge, B.; Bideshi, D.; Moar, W.J.; Federici, B.A. Differential effects of helper proteins encoded by the cry2A and cry11A operons on the formation of Cry2A inclusions in Bacillus thuringiensis. FEMS Microbiol. Lett. 1998, 165, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Ellar, D.J. Involvement of a possible chaperonin in the efficient expression of a cloned CryllA δ-endotoxin gene in Bacillus thuringiensis. Mol. Microbiol. 1992, 6, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Staples, N.; Ellar, D.; Crickmore, N. Cellular localization and characterization of the Bacillus thuringiensis Orf2 crystallization factor. Curr. Microbiol. 2001, 42, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Wheeler, V.C.; Ellar, D.J. Use of an operon fusion to induce expression and crystallisation of a Bacillus thuringiensis δ-endotoxin encoded by a cryptic gene. Mol. Gen. Genet. 1994, 242, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Bietlot, H.P.; Schernthaner, J.P.; Milne, R.; Clairmont, F.R.; Bhella, R.; Kaplan, H. Evidence that the CryIA crystal protein from Bacillus thuringiensis is associated with DNA. J. Biol. Chem. 1993, 268, 8240–8245. [Google Scholar] [PubMed]
- Clairmont, F.R.; Milne, R.E.; Carrière, M.B.; Kaplan, H. Role of DNA in the activation of the Cry1A insecticidal crystal protein from Bacillus thuringiensis. J. Biol. Chem. 1998, 273, 9292–9296. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner, J.P.; Milne, R.E.; Kaplan, H. Characterization of a novel insect digestive DNase with a highly alkaline pH optimum. Insect Biochem. Mol. Biol. 2002, 32, 255–263. [Google Scholar] [CrossRef]
- Guo, S.; Li, J.; Liu, Y.; Song, F.; Zhang, J. The role of DNA binding with the Cry8Ea1 toxin of Bacillus thuringiensis. FEMS Microbiol. Lett. 2011, 317, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Höfte, H.; Whiteley, H. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 53, 242–255. [Google Scholar] [PubMed]
- Chilcott, C.N.; Ellar, D.J. Comparative toxicity of Bacillus thuringiensis var. israelensis crystal proteins in vivo and in vitro. Microbiology 1988, 134, 2551–2558. [Google Scholar]
- Adams, L.F.; Visick, J.E.; Whiteley, H.R. A 20-kilodalton protein is required for efficient production of the Bacillus thuringiensis subsp. israelensis 27-kilodalton crystal protein in Escherichia coli. J. Bacteriol. 1989, 171, 521–530. [Google Scholar] [PubMed]
- Dervyn, E.; Poncet, S.; Klier, A.; Rapoport, G. Transcriptional regulation of the cryIVD gene operon from Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 1995, 177, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Federici, B. Improved production of the insecticidal CryIVD protein in Bacillus thuringiensis using cryIA (c) promoters to express the gene for an associated 20-kDa protein. Appl. Microbiol. Biotechnol. 1995, 42, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Federici, B.A.; Park, H.-W.; Sakano, Y. Insecticidal protein crystals of Bacillus thuringiensis. In Inclusions in Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2006; pp. 195–236. [Google Scholar]
- Visick, J.E.; Whiteley, H. Effect of a 20-kilodalton protein from Bacillus thuringiensis subsp. israelensis on production of the CytA protein by Escherichia coli. J. Bacteriol. 1991, 173, 1748–1756. [Google Scholar] [PubMed]
- Chang, C.; Yu, Y.-M.; Dai, S.-M.; Law, S.; Gill, S. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein, and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl. Environ. Microbiol. 1993, 59, 815–821. [Google Scholar] [PubMed]
- Wu, D.; Federici, B.A. A 20-kilodalton protein preserves cell viability and promotes CytA crystal formation during sporulation in Bacillus thuringiensis. J. Bacteriol. 1993, 175, 5276–5280. [Google Scholar] [CrossRef] [PubMed]
- Sazhenskiy, V.; Zaritsky, A.; Itsko, M. Expression in Escherichia coli of the Native cyt1Aa from Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2010, 76, 3409–3411. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Liu, Z.; Yu, Z. Effects of the 20-kilodalton helper protein on Cry1Ac production and spore formation in Bacillus thuringiensis. Appl. Environ. Microbiol. 2001, 67, 5362–5369. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Mendoza, M.; Bideshi, D.K.; Ortego, F.; Farinós, G.P.; Federici, B.A. The 20-kDa chaperone-like protein of Bacillus thuringiensis ssp. israelensis enhances yield, crystal size and solubility of Cry3A. Lett. Appl. Microbiol. 2012, 54, 88–95. [Google Scholar] [PubMed]
- Rang, C.; Bes, M.; Lullien-Pellerin, V.; Wu, D.; Federici, B.A.; Frutos, R. Influence of the 20-kDa protein from Bacillus thuringiensis ssp. israelensis on the rate of production of truncated Cry1C proteins. FEMS Microbiol. Lett. 1996, 141, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Sasser, J.N.; Fackman, D.W. A World Perspective on Neamtology: The Role of the Society. In Vistas on Nematology: A Commemorationn of the 25th Anniversary of the Society of Nematologists; Veech, J.A., Dickson, D.W., Eds.; Society of Nematologists: Lakeland, FL, USA, 1987; pp. 7–14. [Google Scholar]
- Luo, H.; Xiong, J.; Zhou, Q.; Xia, L.; Yu, Z. The effects of Bacillus thuringiensis Cry6A on the survival, growth, reproduction, locomotion, and behavioral response of Caenorhabditis elegans. Appl. Microbiol. Biotechnol. 2013, 97, 10135–10142. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Bai, P.; Ye, W.; Zhang, F.; Ruan, L.; Sun, M. A novel negative regulatory factor for nematicidal Cry protein gene expression in Bacillus thuringiensis. J. Microbiol. Biotechnol. 2008, 18, 1033–1039. [Google Scholar] [PubMed]
- Lambert, B.; Buysse, L.; Decock, C.; Jansens, S.; Piens, C.; Saey, B.; Seurinck, J.; Van Audenhove, K.; Van Rie, J.; Van Vliet, A. A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family Noctuidae. Appl. Environ. Microbiol. 1996, 62, 80–86. [Google Scholar] [PubMed]
- Wasano, N.; Saitoh, H.; Maeda, M.; Ohgushi, A.; Mizuki, E.; Ohba, M. Cloning and characterization of a novel gene cry9Ec1 encoding lepidopteran-specific parasporal inclusion protein from a Bacillus thuringiensis serovar galleriae strain. Can. J. Microbiol. 2005, 51, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Lereclus, D.; Agaisse, H. Toxin and virulence gene expression in Bacillus thuringiensis. In Entomopathogenic Bacteria: From Laboratory to Field Application; Springer: Berlin/Heidelberg, Germany, 2000; pp. 127–142. [Google Scholar]
- Rosso, M.-L.; Mahillon, J.; Delécluse, A. Genetic and Genomic Contexts of Toxin Genes; Springer: Berlin/Heidelberg, Germany, 2000; pp. 143–166. [Google Scholar]
- Shu, C.; Yu, H.; Wang, R.; Fen, S.; Su, X.; Huang, D.; Zhang, J.; Song, F. Characterization of two novel cry8 genes from Bacillus thuringiensis strain BT185. Curr. Microbiol. 2009, 58, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Huang, D.; Gao, J.; Song, F. Characterization of Bacillus thuringiensis strain Bt185 toxic to the Asian cockchafer: Holotrichia parallela. Curr. Microbiol. 2006, 53, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Schairer, H.U.; Schnetter, W.; Lereclus, D.; Agaisse, H. Bacillus popilliae cry18Aa operon is transcribed by σE and σK forms of RNA polymerase from a single initiation site. Nucleic Acids Res. 1998, 26, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Qiu, L.; Peng, Q.; Lereclus, D.; Zhang, J.; Song, F.; Huang, D. Identification of the promoter in the intergenic region between orf1 and cry8Ea1 controlled by sigma H factor. Appl. Environ. Microbiol. 2012, 78, 4164–4168. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Li, J.; Feng, D.; Li, F.; Guo, S. The elimination of DNA from the Cry toxin-DNA complex is a necessary step in the mode of action of the Cry8 toxin. PLoS ONE 2013, 8, e81335. [Google Scholar] [CrossRef] [PubMed]
- Herrnstadt, C.; Gilroy, T.E.; Sobieski, D.A.; Bennett, B.D.; Gaertner, F.H. Nucleotide sequence and deduced amino acid sequence of a coleopteran-active delta-endotoxin gene from Bacillus thuringiensis subsp. san diego. Gene 1987, 57, 37–46. [Google Scholar] [CrossRef]
- Herrnstadt, C.; Soares, G.G.; Wilcox, E.R.; Edwards, D.L. A new strain of Bacillus thuringiensis with activity against coleopteran insects. Nat. Biotechnol. 1986, 4, 305–308. [Google Scholar] [CrossRef]
- Krieg, A.V.; Huger, A.; Langenbruch, G.; Schnetter, W. Bacillus thuringiensis var. tenebrionis: Ein neuer, gegenüber Larven von Coleopteren wirksamer Pathotyp. Z. Angew. Entomol. 1983, 96, 500–508. [Google Scholar]
- Sick, A.; Gaertner, F.; Wong, A. Nucleotide sequence of a coleopteran-active toxin gene from a new isolate of Bacillus thuringiensis subsp. tolworthi. Nucleic Acids Res. 1990, 18, 1305. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Zeigler, D.R.; Feitelson, J.; Schnepf, E.; Van Rie, J.; Lereclus, D.; Baum, J.; Dean, D.H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 807–813. [Google Scholar] [PubMed]
- Park, H.-W.; Federici, B.A. Effect of specific mutations in helix α7 of domain I on the stability and crystallization of Cry3A in Bacillus thuringiensis. Mol. Biotechnol. 2004, 27, 89–100. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. Structural and functional analysis of the promoter region involved in full expression of the cryIIIA toxin gene of Bacillus thuringiensis. Mol. Microbiol. 1994, 13, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Mathy, N.; Bénard, L.; Pellegrini, O.; Daou, R.; Wen, T.; Condon, C. 5′-to-3′ exoribonuclease activity in bacteria: Role of RNase J1 in rRNA maturation and 5′ stability of mRNA. Cell 2007, 129, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Tailor, R.; Tippett, J.; Gibb, G.; Pells, S.; Jordan, L.; Ely, S. Identification and characterization of a novel Bacillus thuringiensis δ-endotoxin entomocidal to coleopteran and lepidopteran larvae. Mol. Microbiol. 1992, 6, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Gleave, A.P.; Williams, R.; Hedges, R.J. Screening by polymerase chain reaction of Bacillus thuringiensis serotypes for the presence of cryV-like insecticidal protein genes and characterization of a cryV gene cloned from B. thuringiensis subsp. kurstaki. Appl. Environ. Microbiol. 1993, 59, 1683–1687. [Google Scholar] [PubMed]
- Masson, L.; Erlandson, M.; Puzstai-Carey, M.; Brousseau, R.; Juárez-Pérez, V.; Frutos, R. A holistic approach for determining the entomopathogenic potential of Bacillus thuringiensis strains. Appl. Environ. Microbiol. 1998, 64, 4782–4788. [Google Scholar] [PubMed]
- Kostichka, K.; Warren, G.W.; Mullins, M.; Mullins, A.D.; Palekar, N.V.; Craig, J.A.; Koziel, M.G.; Estruch, J.J. Cloning of a cryV-type insecticidal protein gene from Bacillus thuringiensis: The cryV-encoded protein is expressed early in stationary phase. J. Bacteriol. 1996, 178, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Varani, A.M.; Lemos, M.V.; Fernandes, C.C.; Lemos, E.G.; Alves, E.C.; Desidério, J.A. Draft genome sequence of Bacillus thuringiensis var. thuringiensis strain T01–328, a Brazilian isolate that produces a soluble pesticide protein, Cry1Ia. Genome Announc. 2013, 1, e00817-13. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, S.; Jaoua, S. Identification of a promoter for the crystal protein-encoding gene cry1Ia from Bacillus thuringiensis subsp. kurstaki. FEMS Microbiol. Lett. 2002, 208, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhang, J.; Gu, A.; Wu, Y.; Han, L.; He, K.; Chen, Z.; Yao, J.; Hu, Y.; Li, G. Identification of cry1I-type genes from Bacillus thuringiensis strains and characterization of a novel cry1I-type gene. Appl. Environ. Microbiol. 2003, 69, 5207–5211. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.-S.; Park, S.-H.; Choi, S.-K.; Koo, B.-T.; Lee, S.-T.; Kim, J.-I. Distribution of cryV-type insecticidal protein genes in Bacillus thuringiensis and cloning of cryV-type genes from Bacillus thuringiensis subsp. kurstaki and Bacillus thuringiensis subsp. entomocidus. Appl. Environ. Microbiol. 1995, 61, 2402–2407. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adalat, R.; Saleem, F.; Crickmore, N.; Naz, S.; Shakoori, A.R. In Vivo Crystallization of Three-Domain Cry Toxins. Toxins 2017, 9, 80. https://doi.org/10.3390/toxins9030080
Adalat R, Saleem F, Crickmore N, Naz S, Shakoori AR. In Vivo Crystallization of Three-Domain Cry Toxins. Toxins. 2017; 9(3):80. https://doi.org/10.3390/toxins9030080
Chicago/Turabian StyleAdalat, Rooma, Faiza Saleem, Neil Crickmore, Shagufta Naz, and Abdul Rauf Shakoori. 2017. "In Vivo Crystallization of Three-Domain Cry Toxins" Toxins 9, no. 3: 80. https://doi.org/10.3390/toxins9030080




