Establishment of the Inducible Tet-On System for the Activation of the Silent Trichosetin Gene Cluster in Fusarium fujikuroi
Abstract
:1. Introduction
2. Results
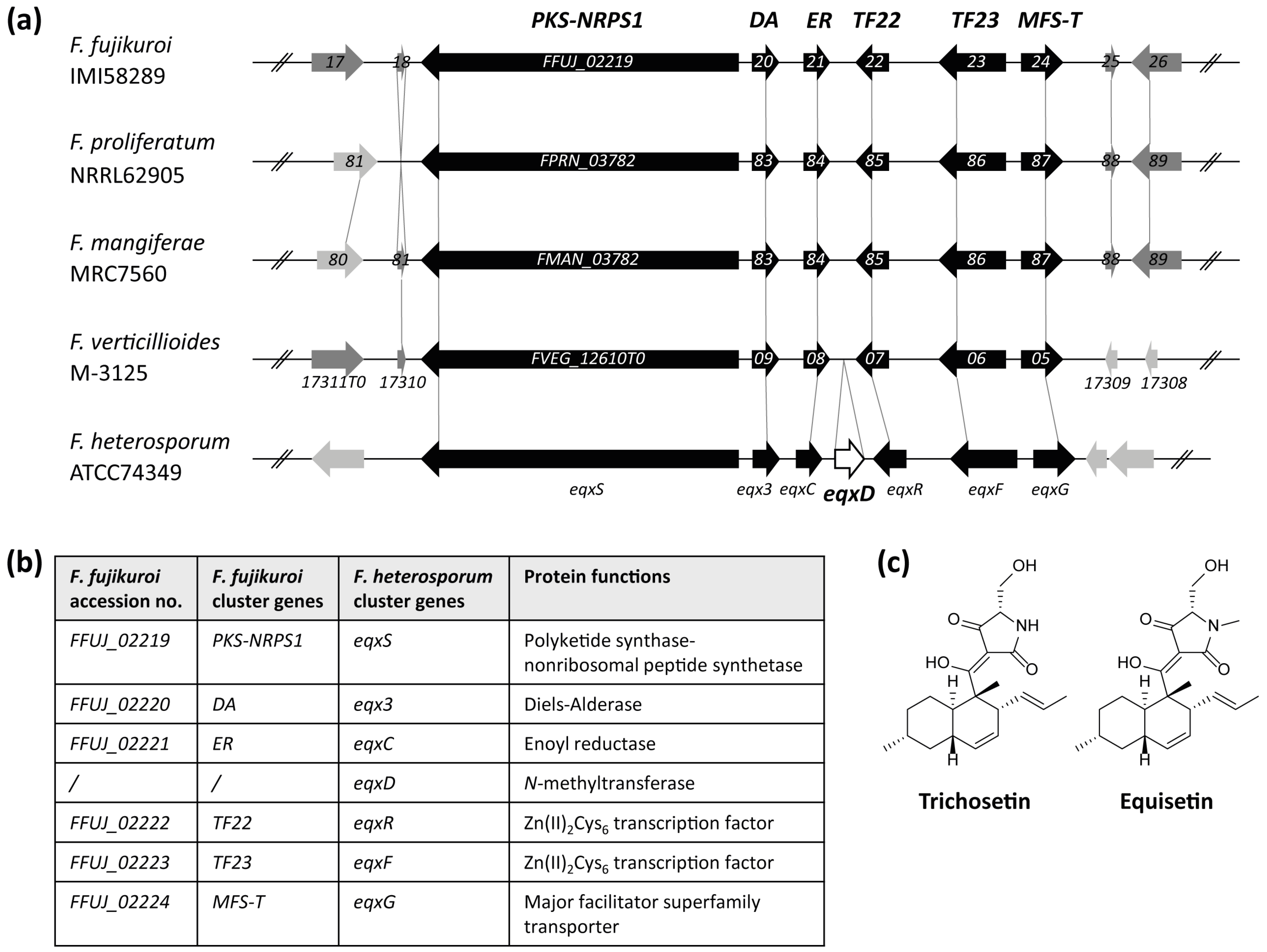
2.1. Identification of a Cluster Homologous to the F. heterosporum Equisetin Gene Cluster
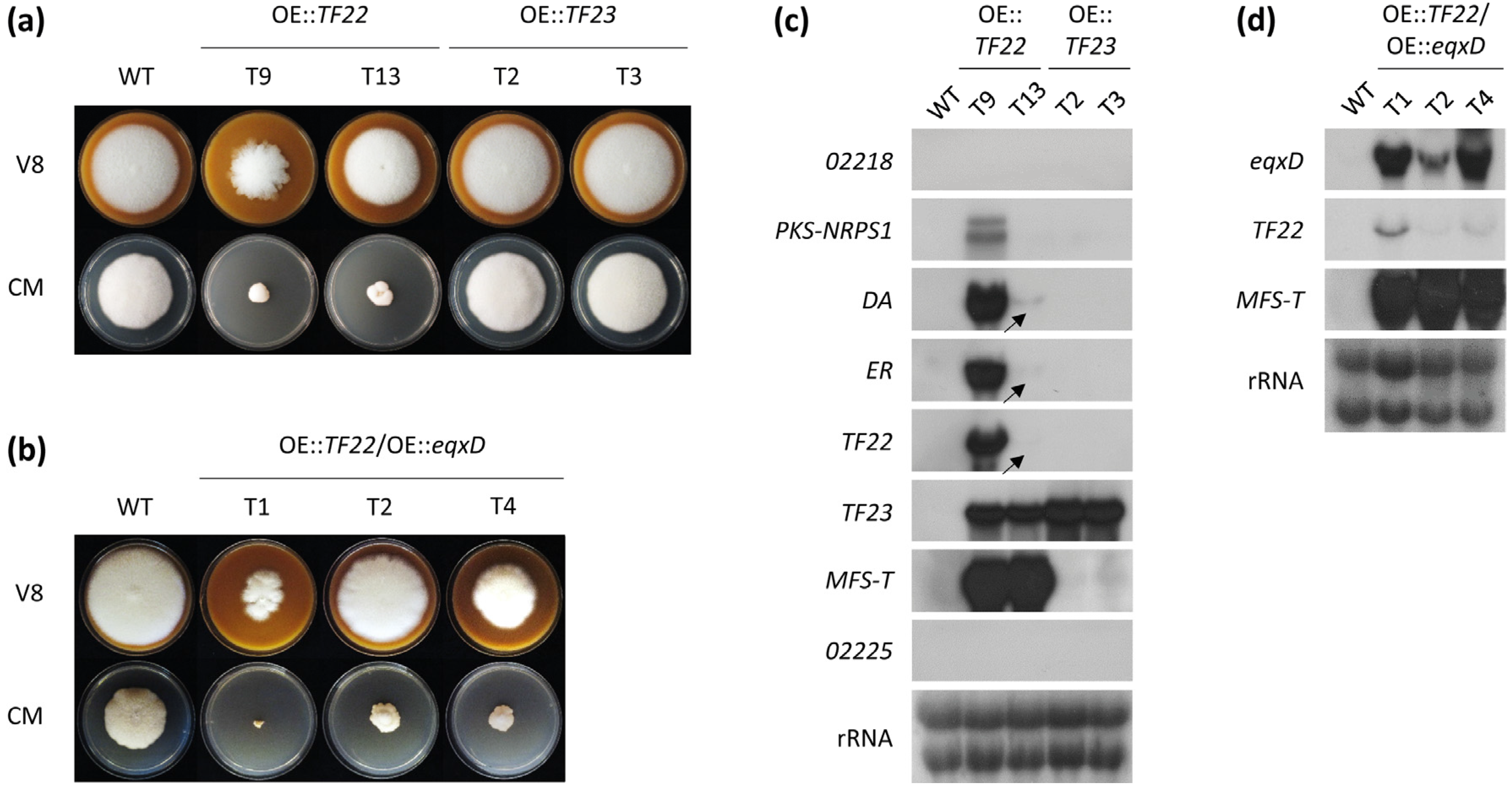
2.2. Activation of the F. fujikuroi Trichosetin Gene Cluster
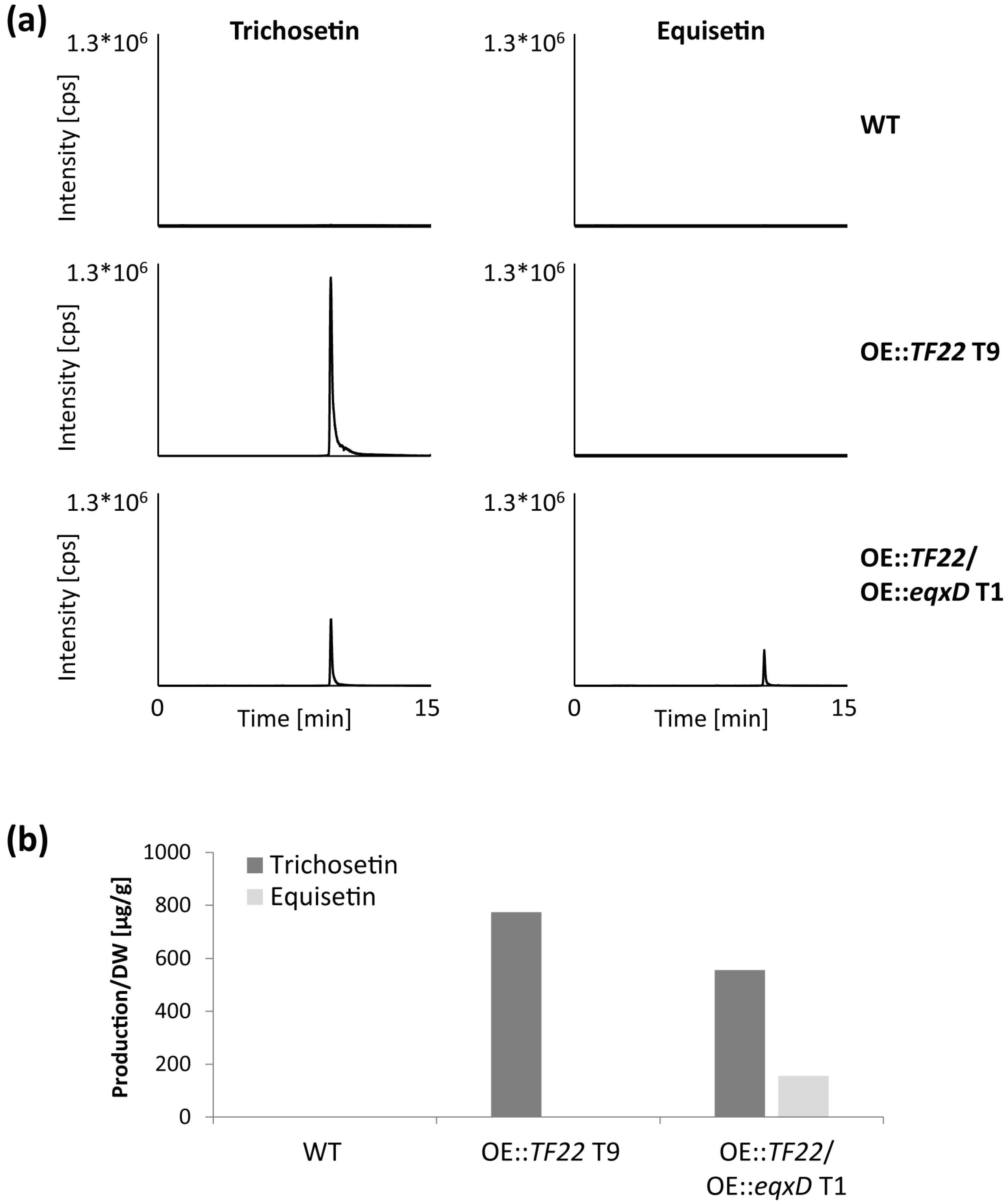
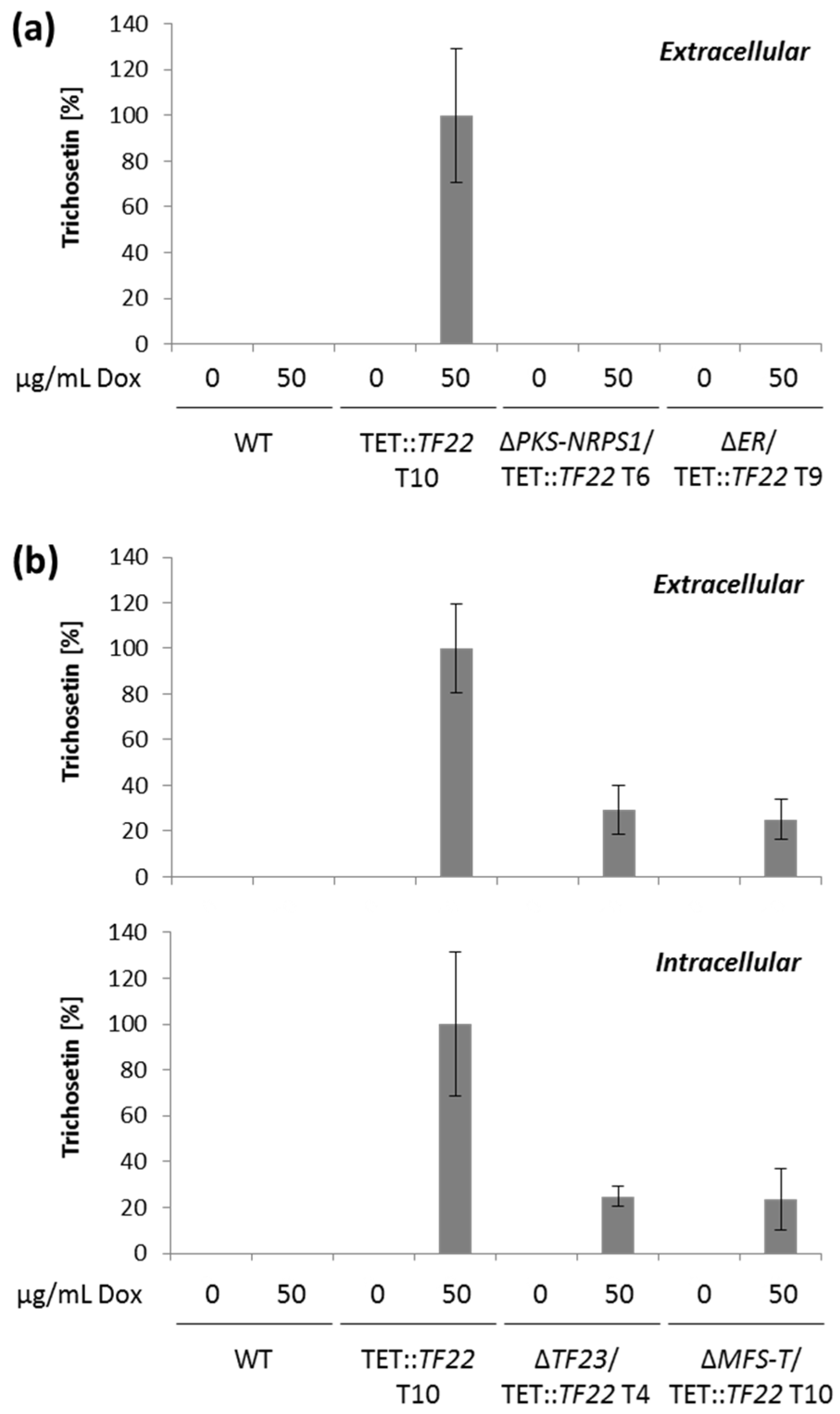
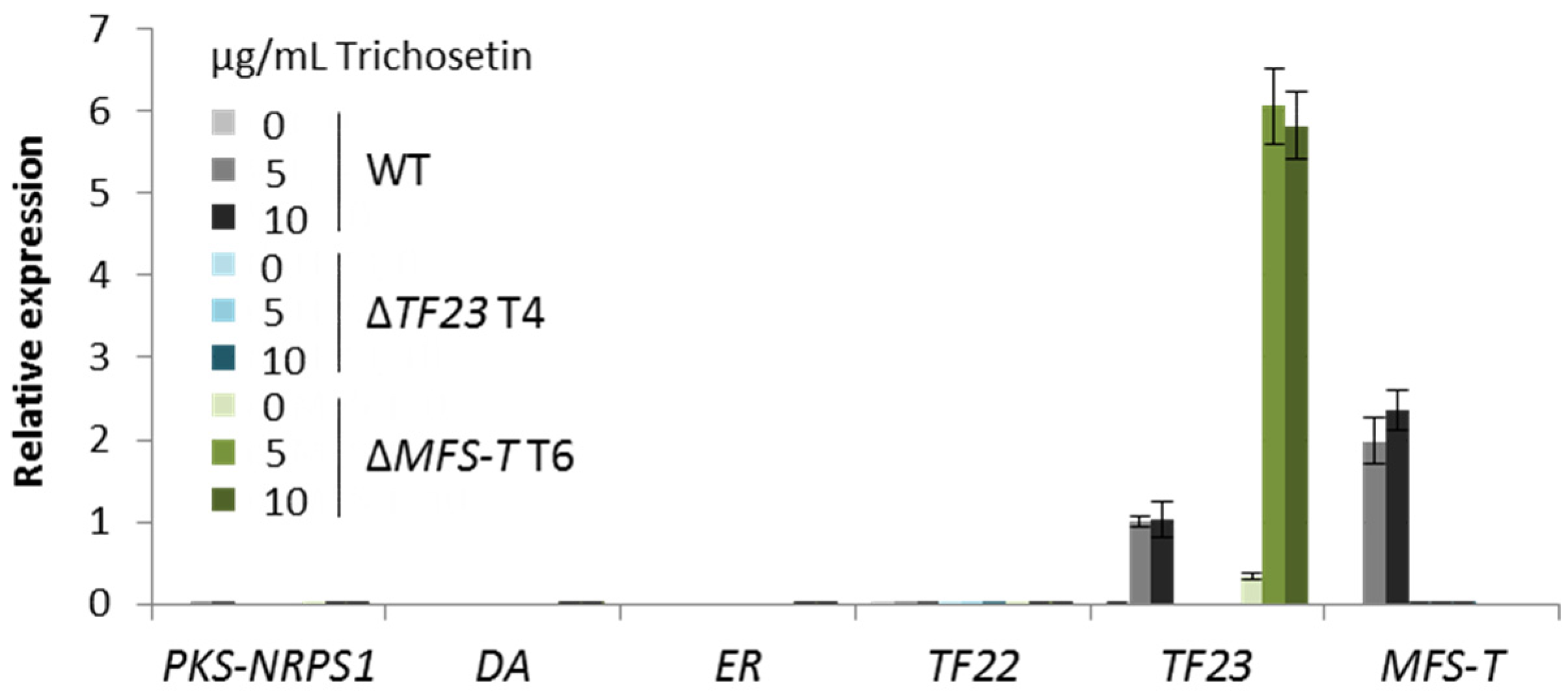
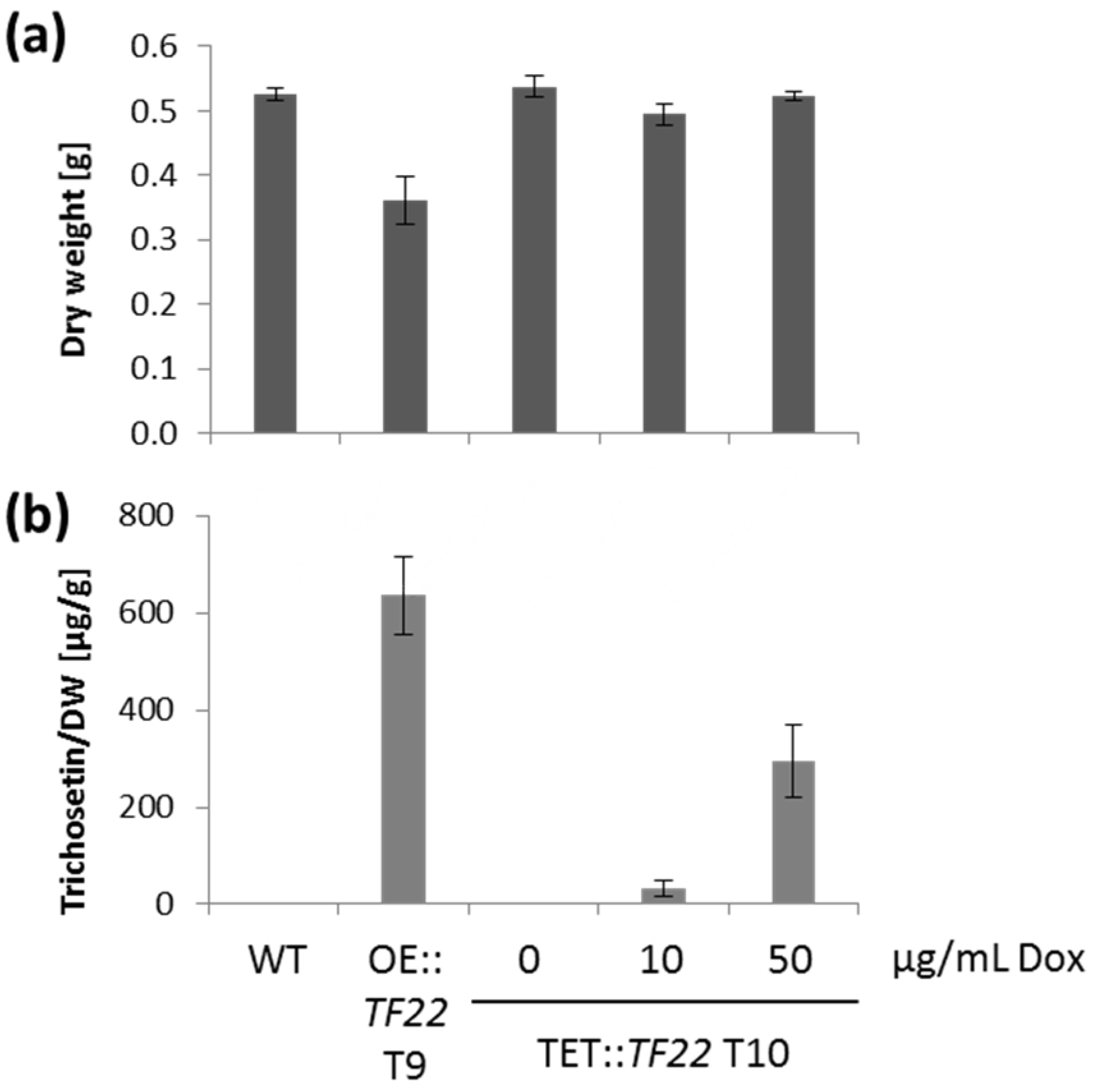
2.3. Inducible Overexpression of TF22
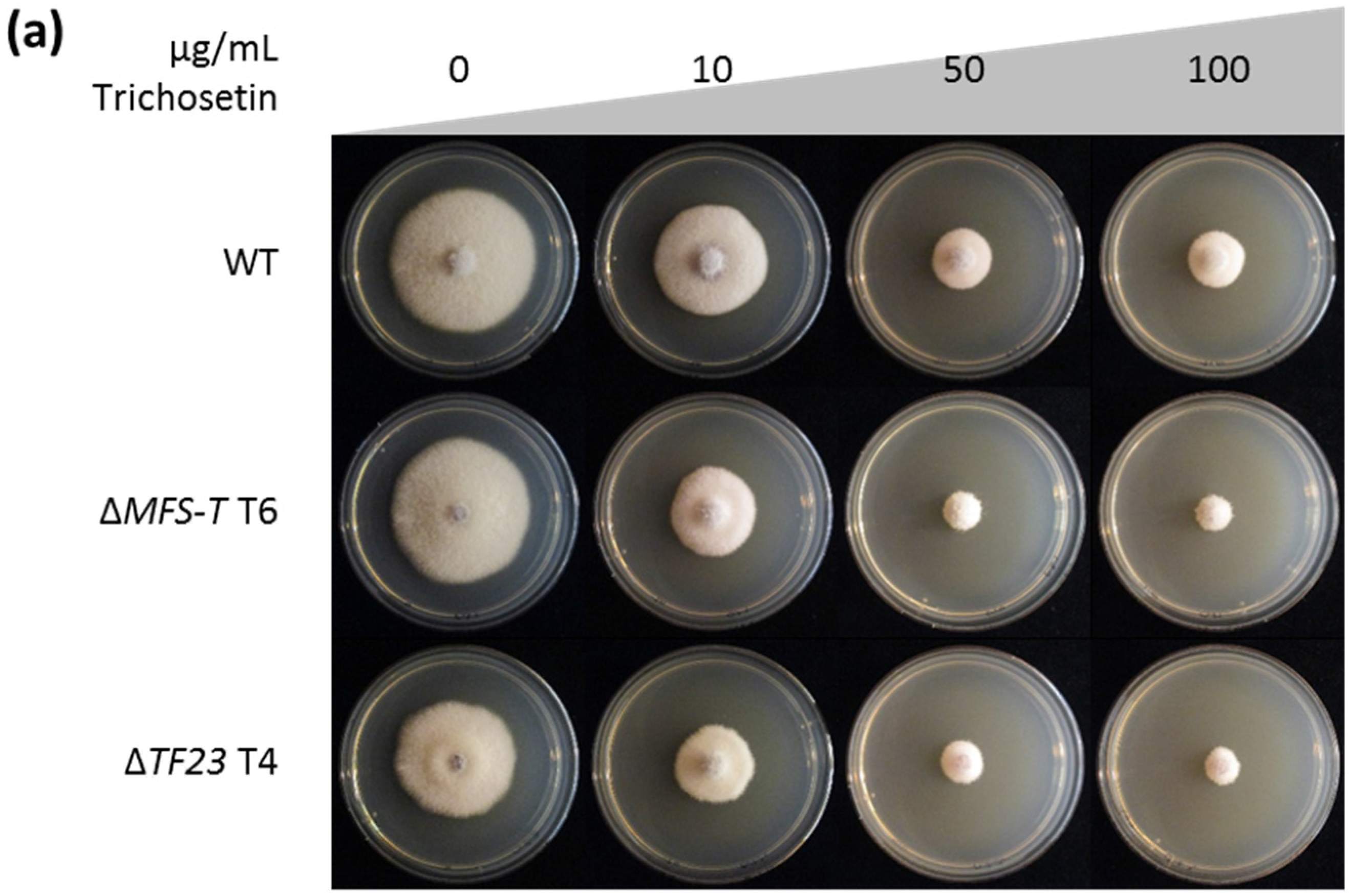
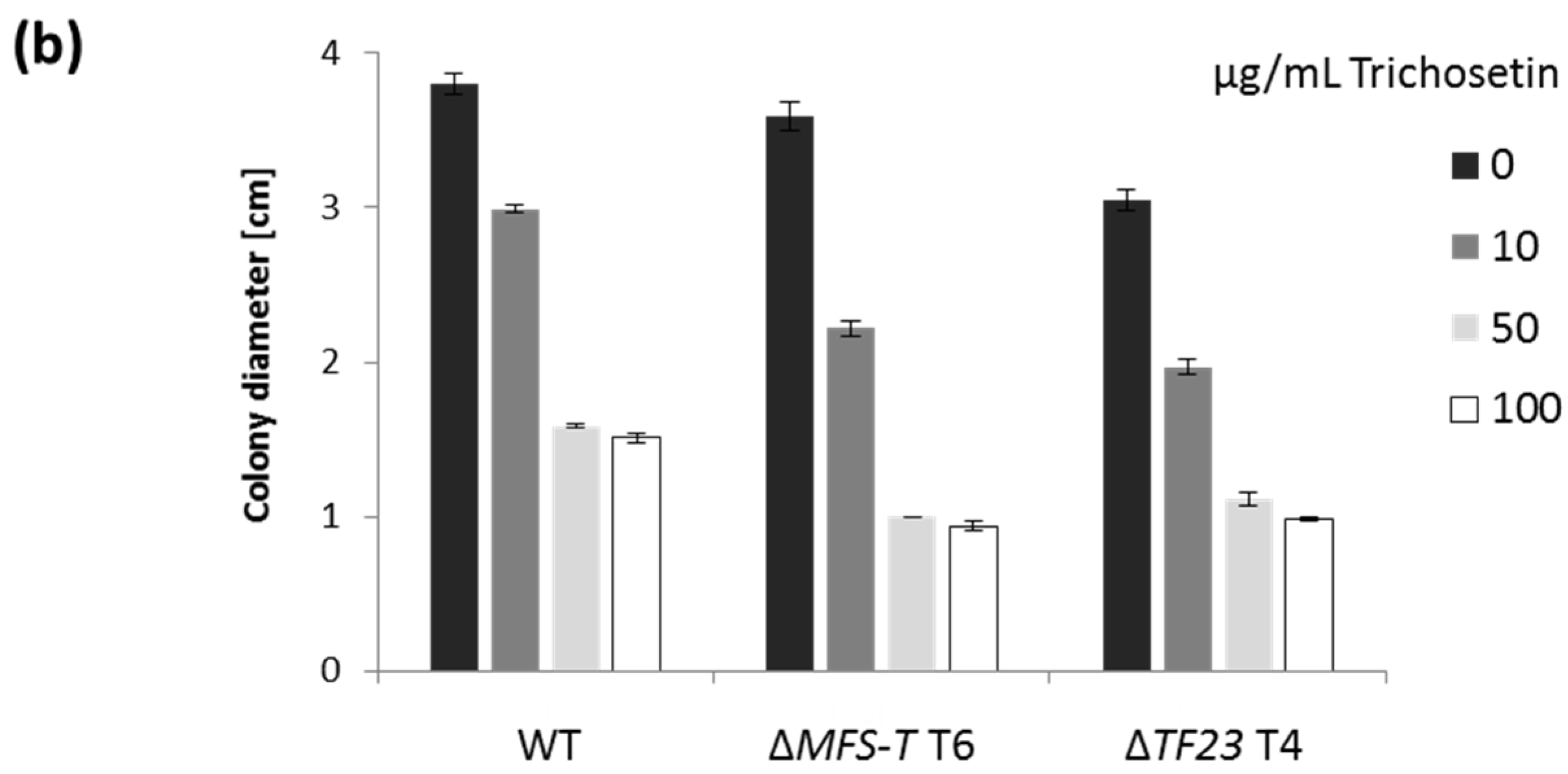
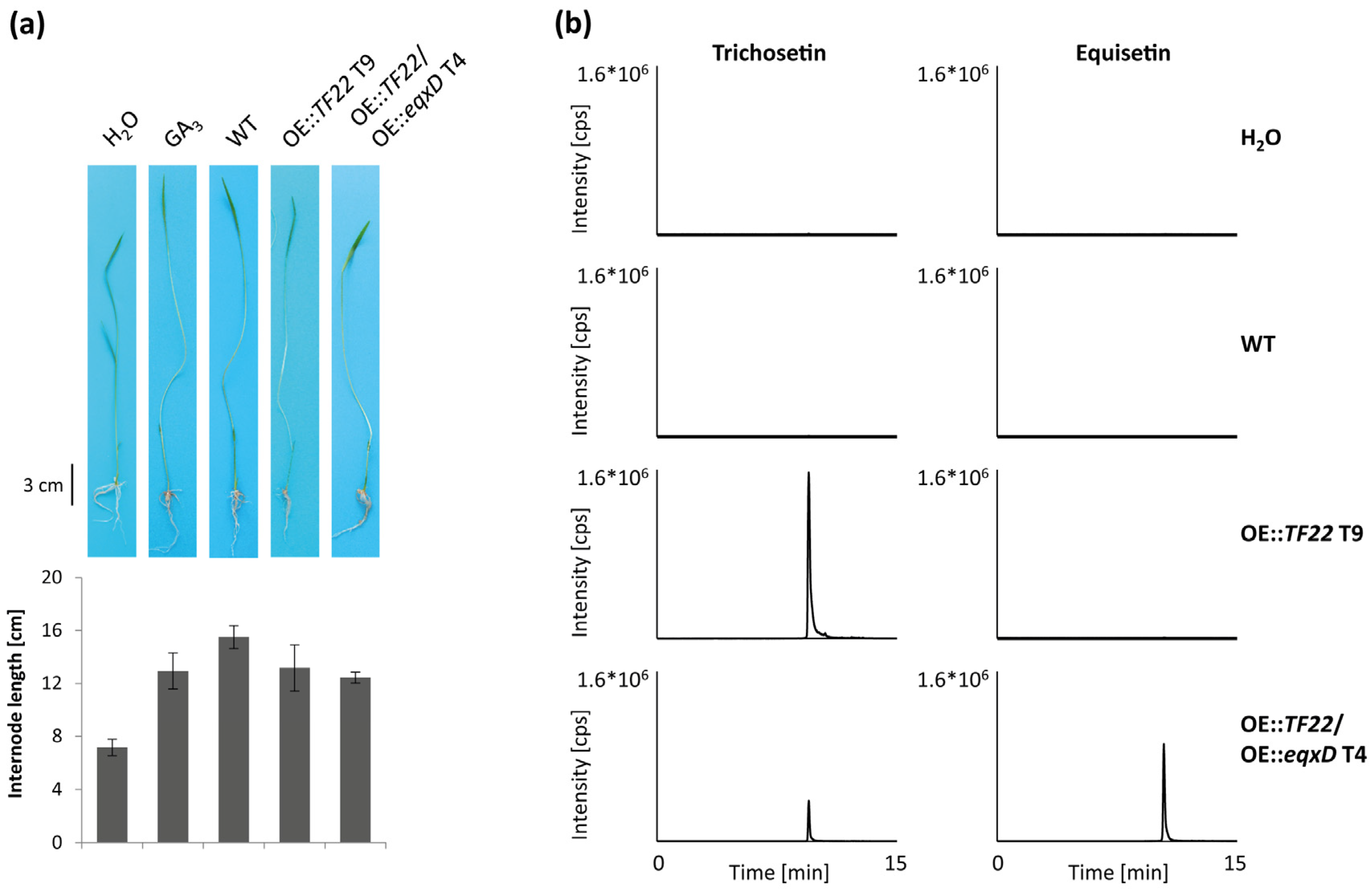
2.4. Trichosetin Is Toxic to the Producing Fungus
2.5. Pathogenicity of Trichosetin- and Equisetin-Producing Mutants on Rice
2.6. Cytotoxicity of Trichosetin and Equisetin
3. Discussion
3.1. Conservation of the Equisetin/Trichosetin Gene Cluster among the Genus Fusarium
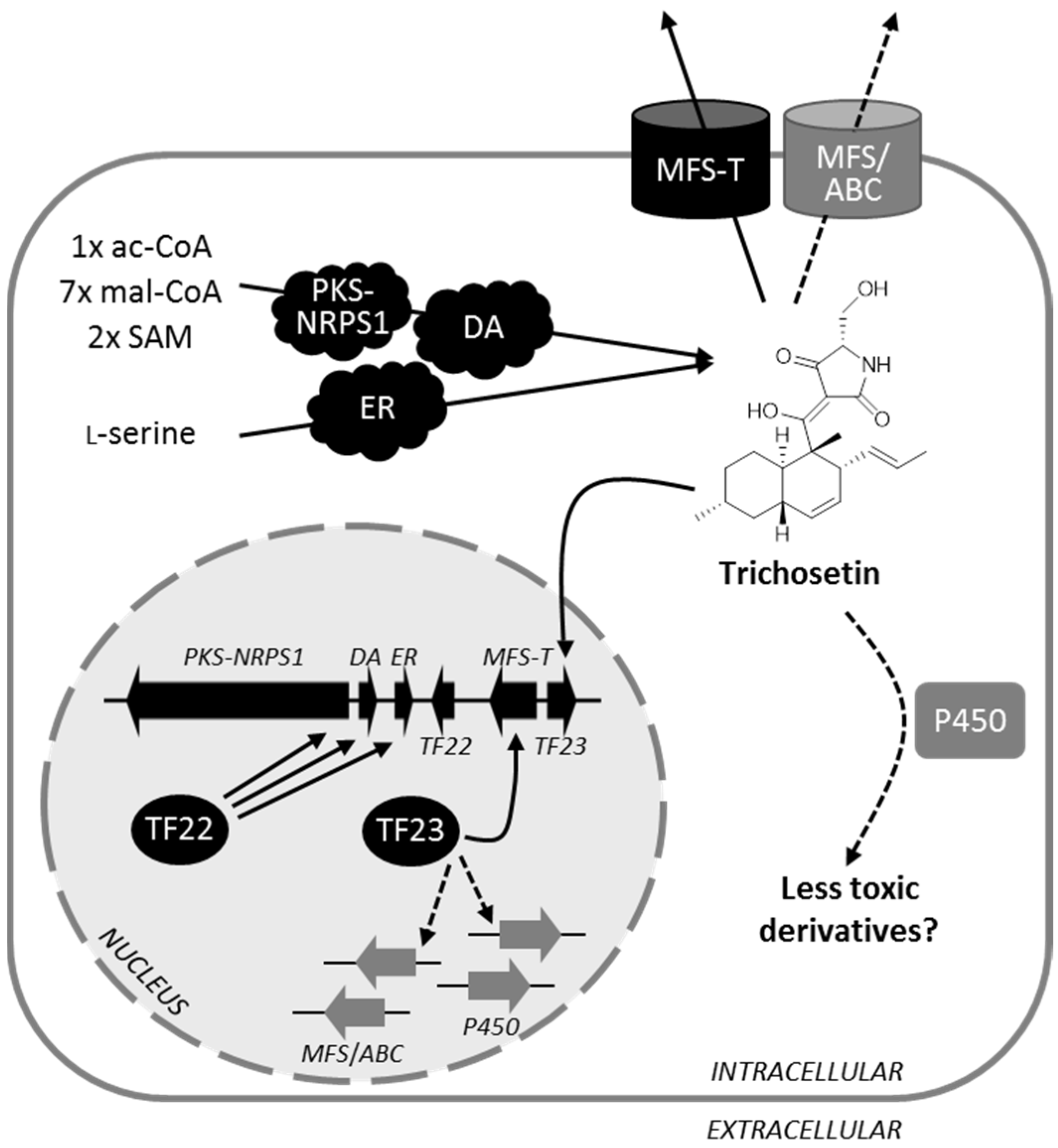
3.2. Biosynthesis of Trichosetin Is Regulated by TF22
3.3. Detoxification of Trichosetin Is Likely Regulated by TF23
3.4. Phytotoxicity and Cytotoxicity of Trichosetin
4. Materials and Methods
4.1. Fungal Strains, Media, and Growth Conditions
4.2. Plasmid Constructions
4.3. Fungal Transformations and Analysis of Transformants
4.4. Molecular Methods
4.5. Rice Virulence and Rice Germination Assays
4.6. Cytotoxicity Assay
4.7. Isolation of Trichosetin
4.8. Physico-Chemical Properties of Trichosetin
4.9. HPLC Analysis of Trichosetin and Equisetin
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nirenberg, H.I.; O’Donnell, K. New Fusarium Species and Combinations within the Gibberella fujikuroi Species Complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. Fusarium Laboratory Workshops—A Recent History. Mycotoxin Res. 2006, 22, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Snyder, W.C. The bakanae disease of the rice plant. In Fusarium: Diseases, Biology and Taxonomy; Nelson, P.E., Toussoun, T.A., Cook, R.J., Eds.; The Pennsylvania State University Press: University Park, PA, USA, 1981; pp. 104–113. [Google Scholar]
- Tudzynski, B.; Hölter, K. Gibberellin Biosynthetic Pathway in Gibberella fujikuroi: Evidence for a Gene Cluster. Fungal Genet. Biol. 1998, 25, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Bömke, C.; Tudzynski, B. Diversity, Regulation, and Evolution of the Gibberellin Biosynthetic Pathway in Fungi Compared to Plants and Bacteria. Phytochemistry 2009, 70, 1876–1893. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.; Tudzynski, B. Biosynthesis of the Red Pigment Bikaverin in Fusarium fujikuroi: Genes, their Function and Regulation. Mol. Microbiol. 2009, 72, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Arndt, B.; Studt, L.; Wiemann, P.; Osmanov, H.; Kleigrewe, K.; Köhler, J.; Krug, I.; Tudzynski, B.; Humpf, H. Genetic Engineering, High Resolution Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy Elucidate the Bikaverin Biosynthetic Pathway in Fusarium fujikuroi. Fungal Genet. Biol. 2015, 84, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Wiemann, P.; Kleigrewe, K.; Humpf, H.; Tudzynski, B. Biosynthesis of Fusarubins Accounts for Pigmentation of Fusarium fujikuroi Perithecia. Appl. Environ. Microbiol. 2012, 78, 4468–4480. [Google Scholar] [CrossRef] [PubMed]
- Von Bargen, K.W.; Niehaus, E.; Krug, I.; Bergander, K.; Würthwein, E.; Tudzynski, B.; Humpf, H. Isolation and Structure Elucidation of Fujikurins A–D: Products of the PKS19 Gene Cluster in Fusarium fujikuroi. J. Nat. Prod. 2015, 78, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Rösler, S.M.; Sieber, C.M.K.; Humpf, H.; Tudzynski, B. Interplay between Pathway-Specific and Global Regulation of the Fumonisin Gene Cluster in the Rice Pathogen Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2016, 100, 5869–5882. [Google Scholar] [CrossRef] [PubMed]
- Janevska, S.; Arndt, B.; Niehaus, E.; Burkhardt, I.; Rösler, S.M.; Brock, N.L.; Humpf, H.; Dickschat, J.S.; Tudzynski, B. Gibepyrone Biosynthesis in the Rice pathogen Fusarium fujikuroi is Facilitated by a Small Polyketide Synthase Gene Cluster. J. Biol. Chem. 2016, 291, 27403–27420. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.; Janevska, S.; von Bargen, K.W.; Sieber, C.M.K.; Harrer, H.; Humpf, H.; Tudzynski, B. Apicidin F: Characterization and Genetic Manipulation of a New Secondary Metabolite Gene Cluster in the Rice Pathogen Fusarium fujikuroi. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.; Studt, L.; von Bargen, K.W.; Kummer, W.; Humpf, H.; Reuter, G.; Tudzynski, B. Sound of Silence: The Beauvericin Cluster in Fusarium fujikuroi Is Controlled by Cluster-Specific and Global Regulators Mediated by H3K27 Modification. Environ. Microbiol. 2016, 18, 4282–4302. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.; Kleigrewe, K.; Wiemann, P.; Studt, L.; Sieber, C.M.K.; Connolly, L.R.; Freitag, M.; Güldener, U.; Tudzynski, B.; Humpf, H. Genetic Manipulation of the Fusarium fujikuroi Fusarin Gene Cluster Yields Insight into the Complex Regulation and Fusarin Biosynthetic Pathway. Chem. Biol. 2013, 20, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.; von Bargen, K.W.; Espino, J.J.; Pfannmüller, A.; Humpf, H.; Tudzynski, B. Characterization of the Fusaric Acid Gene Cluster in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2014, 98, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Janevska, S.; Niehaus, E.; Burkhardt, I.; Arndt, B.; Sieber, C.M.K.; Humpf, H.; Dickschat, J.S.; Tudzynski, B. Two Separate Key Enzymes and Two Pathway-Specific Transcription Factors Are Involved in Fusaric Acid Biosynthesis in Fusarium fujikuroi. Environ. Microbiol. 2016, 18, 936–956. [Google Scholar] [CrossRef] [PubMed]
- Brock, N.L.; Huss, K.; Tudzynski, B.; Dickschat, J.S. Genetic Dissection of Sequiterpene Biosynthesis by Fusarium fujikuroi. ChemBioChem 2013, 14, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, I.; Siemon, T.; Henrot, M.; Studt, L.; Rösler, S.; Tudzynski, B.; Christmann, M.; Dickschat, J.S. Mechanistic Characterization of Two Sesquiterpene Cyclases from the Plant Pathogenic Fungus Fusarium fujikuroi. Angew. Chem. Int. Ed. 2016, 55, 8748–8751. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Sieber, C.M.K.; von Bargen, K.W.; Studt, L.; Niehaus, E.; Espino, J.J.; Huß, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the Cryptic Genome: Genome-Wide Analyses of the Rice Pathogen Fusarium fujikuroi Reveal Complex Regulation of Secondary Metabolism and Novel Metabolites. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cui, Z.; Gu, Y.; Liu, Y.; Wang, Q. The Phytotoxicity of Natural Tetramic Acid Derivatives. Pest Manag. Sci. 2011, 67, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Li, Q.; Ju, J. Naturally Occurring Tetramic Acid Products: Isolation, Structure Elucidation and Biological Activity. RSC Adv. 2014, 4, 50566–50593. [Google Scholar] [CrossRef]
- Burmeister, H.R.; Bennett, G.A.; Vesonder, R.F.; Hesseltine, C.W. Antibiotic Produced by Fusarium equiseti NRRL 5537. Antimicrob. Agents Chemother. 1974, 5, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.H.; Stipanovic, R.D.; Puckhaber, L.S. Phytotoxicity of Equisetin and Epi-Equisetin Isolated from Fusarium equiseti and F. pallidoroseum. Mycol. Res. 1999, 103, 967–973. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Goetz, M.A.; Dombrowski, A.W.; Polishook, J.D.; Hazuda, D.J. Equisetin and a Novel Opposite Stereochemical Homolog Phomasetin, Two Fungal Metabolites as Inhibitors of HIV-1 Integrase. Tetrahedron Lett. 1998, 39, 2243–2246. [Google Scholar] [CrossRef]
- Hazuda, D.; Blau, C.U.; Felock, P.; Hastings, J.; Pramanik, B.; Wolfe, A.; Bushman, F.; Farnet, C.; Goetz, M.; Williams, M.; et al. Isolation and Characterization of Novel Human Immunodeficiency Virus Integrase Inhibitors from Fungal Metabolites. Antivir. Chem. Chemother. 1999, 10, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kakule, T.B.; Sardar, D.; Lin, Z.; Schmidt, E.W. Two Related Pyrrolidinedione Synthetase Loci in Fusarium heterosporum ATCC 74349 Produce Divergent Metabolites. ACS Chem. Biol. 2013, 8, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Nogawa, T.; Hirota, H.; Jang, J.; Takahashi, S.; Ahn, J.S.; Osada, H. A New Enzyme Involved in the Control of the Stereochemistry in the Decalin Formation during Equisetin Biosynthesis. Biochem. Biophys. Res. Commun. 2015, 460, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Marfori, E.C.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Trichosetin, a Novel Tetramic Acid Antibiotic Produced in Dual Culture of Trichoderma harzianum and Catharanthus roseus Callus. Z. Naturforsch. Sect. C J. Biosci. 2002, 57, 465–470. [Google Scholar] [CrossRef]
- Inokoshi, J.; Shigeta, N.; Fukuda, T.; Uchida, R.; Nonaka, K.; Masuma, R.; Tomoda, H. Epi-Trichosetin, a New Undecaprenyl Pyrophosphate Synthase Inhibitor, Produced by Fusarium oxysporum FKI-4553. J. Antibiot. 2013, 66, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, H. New Approaches to Drug Discovery for Combating MRSA. Chem. Pharm. Bull. 2016, 64, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Alad, P.A.O.; Cocos, A.A.S.; Balolong, E.C., Jr.; Parungao, M.M.; Marfori, E.C. Mutagenicity Potential of the Novel Drug Trichosetin Estimated by using the Rec Assay and Micronucleus Test. Philipp. J. Sci. 2009, 138, 119–124. [Google Scholar]
- Parungao-Balolong, M.M.; Caluag, M.L.B.; Catibog, I.B.D.; Cortes-Maramba, N.P.; Sia, I.C.; Sio, S.O.; Balolong, E.C.; Marfori, E.C. Acute Oral Toxicity of Trichosetin in Mice (Mus musculus L.). Philipp. J. Sci. 2014, 143, 99–106. [Google Scholar]
- Marfori, E.C.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Phytotoxicity of the Tetramic Acid Metabolite Trichosetin. Phytochemistry 2003, 62, 715–721. [Google Scholar] [CrossRef]
- Fisch, K.M. Biosynthesis of Natural Products by Microbial Iterative Hybrid PKS-NRPS. RSC Adv. 2013, 3, 18228–18247. [Google Scholar] [CrossRef]
- Hansen, F.T.; Gardiner, D.M.; Lysøe, E.; Fuertes, P.R.; Tudzynski, B.; Wiemann, P.; Sondergaard, T.E.; Giese, H.; Brodersen, D.E.; Sørensen, J.L. An Update to Polyketide Synthase and Non-Ribosomal Synthetase Genes and Nomenclature in Fusarium. Fungal Genet. Biol. 2015, 75, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Turos, E.; Audia, J.E.; Danishefsky, S.J. Total Synthesis of the Fusarium Toxin Equisetin: Proof of the Stereochemical Relationship of the Tetramate and Terpenoid Sectors. J. Am. Chem. Soc. 1989, 111, 8231–8236. [Google Scholar] [CrossRef]
- Meyer, V.; Wanka, F.; van Gent, J.; Arentshorst, M.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Fungal Gene Expression on Demand: An Inducible, Tunable, and Metabolism-Independent Expression System for Aspergillus niger. Appl. Environ. Microbiol. 2011, 77, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Berens, C.; Hillen, W. Gene Regulation by Tetracyclines: Constraints of Resistance Regulation in Bacteria Shape TetR for Application in Eukaryotes. Eur. J. Biochem. 2003, 270, 3109–3121. [Google Scholar] [CrossRef] [PubMed]
- Boettger, D.; Hertweck, C. Molecular Diversity Sculpted by Fungal PKS-NRPS Hybrids. ChemBioChem 2013, 14, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Marfori, E.C.; Bamba, T.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Biosynthetic Studies of the Tetramic Acid Antibiotic Trichosetin. Tetrahedron 2002, 58, 6655–6658. [Google Scholar] [CrossRef]
- Whitt, J.; Shipley, S.M.; Newman, D.J.; Zuck, K.M. Tetramic Acid Analogues Produced by Coculture of Saccharopolyspora erythraea with Fusarium pallidoroseum. J. Nat. Prod. 2014, 77, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvo, G.; Roper, J.A.; Chemmons, L.M.; Macdonald, K.D.; Bufton, A.W.J. The Genetics of Aspergillus nidulans. Adv. Genet. 1953, 5, 141–238. [Google Scholar] [PubMed]
- Darken, M.A.; Jensen, A.L.; Ahu, P. Production of Gibberellic Acid by Fermentation. Appl. Microbiol. 1959, 7, 301–303. [Google Scholar] [PubMed]
- Geissman, T.A.; Verbiscar, A.J.; Phinney, B.O.; Cragg, G. Studies on the Biosynthesis of Gibberellins from (−)-Kaurenoic Acid in Cultures of Gibberella fujikuroi. Phytochemistry 1966, 5, 933–947. [Google Scholar] [CrossRef]
- Colot, H.V.; Park, G.; Turner, G.E.; Ringelberg, C.; Crew, C.M.; Litvinkova, L.; Weiss, R.L.; Borkovich, K.A.; Dunlap, J.C. A High-Throughput Gene Knockout Procedure for Neurospora Reveals Functions for Multiple Transcription Factors. Proc. Natl. Acad. Sci. USA 2006, 103, 10352–10357. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J. Tools for Botrytis cinerea: New Expression Vectors Make the Gray Mold Fungus More Accessible to Cell Biology Approaches. Fungal Genet. Biol. 2012, 49, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Staben, C.; Jensen, B.; Singer, M.; Pollock, J.; Schechtmann, M.; Kinsey, J.; Selker, E. Use of a Bacterial Hygromycin B Resistance Gene as a Dominant Selectable Marker in Neurospora crassa Transformation. Fungal Genet. Newslett. 1989, 36, 79–81. [Google Scholar] [CrossRef]
- Winston, F.; Dollard, C.; Ricupero-Hovasse, S.L. Construction of a Set of Convenient Saccharomyces cerevisiae Strains that are Isogenic to S288C. Yeast 1995, 11, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Christianson, T.W.; Sikorski, R.S.; Dante, M.; Shero, J.H.; Hieter, P. Multifunctional Yeast High-Copy-Number Shuttle Vectors. Gene 1992, 110, 119–122. [Google Scholar] [CrossRef]
- Tudzynski, B.; Homann, V.; Feng, B.; Marzluf, G.A. Isolation, Characterization and Disruption of the areA Nitrogen Regulatory Gene of Gibberella fujikuroi. Mol. Gen. Genet. 1999, 261, 106–114. [Google Scholar] [PubMed]
- Cenis, J.L. Rapid Extraction of Fungal DNA for PCR Amplification. Nucleic Acids Res. 1992, 20, 2380. [Google Scholar] [CrossRef] [PubMed]
- Southern, E.M. Detection of Specific Sequences among DNA Fragments Separated by Gel Electrophoresis. J. Mol. Biol. 1975, 98, 503–517. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Current Protocols in Molecular Biology; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor: New York, NY, USA, 1989. [Google Scholar]
- Church, G.M.; Gilbert, W. Genomic Sequencing. Proc. Natl. Acad. Sci. USA 1984, 81, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29. [Google Scholar] [CrossRef]
- Wiemann, P.; Brown, D.W.; Kleigrewe, K.; Bok, J.W.; Keller, N.P.; Humpf, H.; Tudzynski, B. FfVel1 and Fflae1, Components of a Velvet-Like Complex in Fusarium fujikuroi, Affect Differentiation, Secondary Metabolism and Virulence. Mol. Microbiol. 2010, 77, 972–994. [Google Scholar] [CrossRef] [PubMed]
- Bittner, A.; Cramer, B.; Humpf, H. Matrix Binding of Ochratoxin A during Roasting. J. Agric. Food Chem. 2013, 61, 12737–12743. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janevska, S.; Arndt, B.; Baumann, L.; Apken, L.H.; Mauriz Marques, L.M.; Humpf, H.-U.; Tudzynski, B. Establishment of the Inducible Tet-On System for the Activation of the Silent Trichosetin Gene Cluster in Fusarium fujikuroi. Toxins 2017, 9, 126. https://doi.org/10.3390/toxins9040126
Janevska S, Arndt B, Baumann L, Apken LH, Mauriz Marques LM, Humpf H-U, Tudzynski B. Establishment of the Inducible Tet-On System for the Activation of the Silent Trichosetin Gene Cluster in Fusarium fujikuroi. Toxins. 2017; 9(4):126. https://doi.org/10.3390/toxins9040126
Chicago/Turabian StyleJanevska, Slavica, Birgit Arndt, Leonie Baumann, Lisa Helene Apken, Lucas Maciel Mauriz Marques, Hans-Ulrich Humpf, and Bettina Tudzynski. 2017. "Establishment of the Inducible Tet-On System for the Activation of the Silent Trichosetin Gene Cluster in Fusarium fujikuroi" Toxins 9, no. 4: 126. https://doi.org/10.3390/toxins9040126





