Occurrence of Penicillium brocae and Penicillium citreonigrum, which Produce a Mutagenic Metabolite and a Mycotoxin Citreoviridin, Respectively, in Selected Commercially Available Rice Grains in Thailand
Abstract
:1. Introduction
2. Results
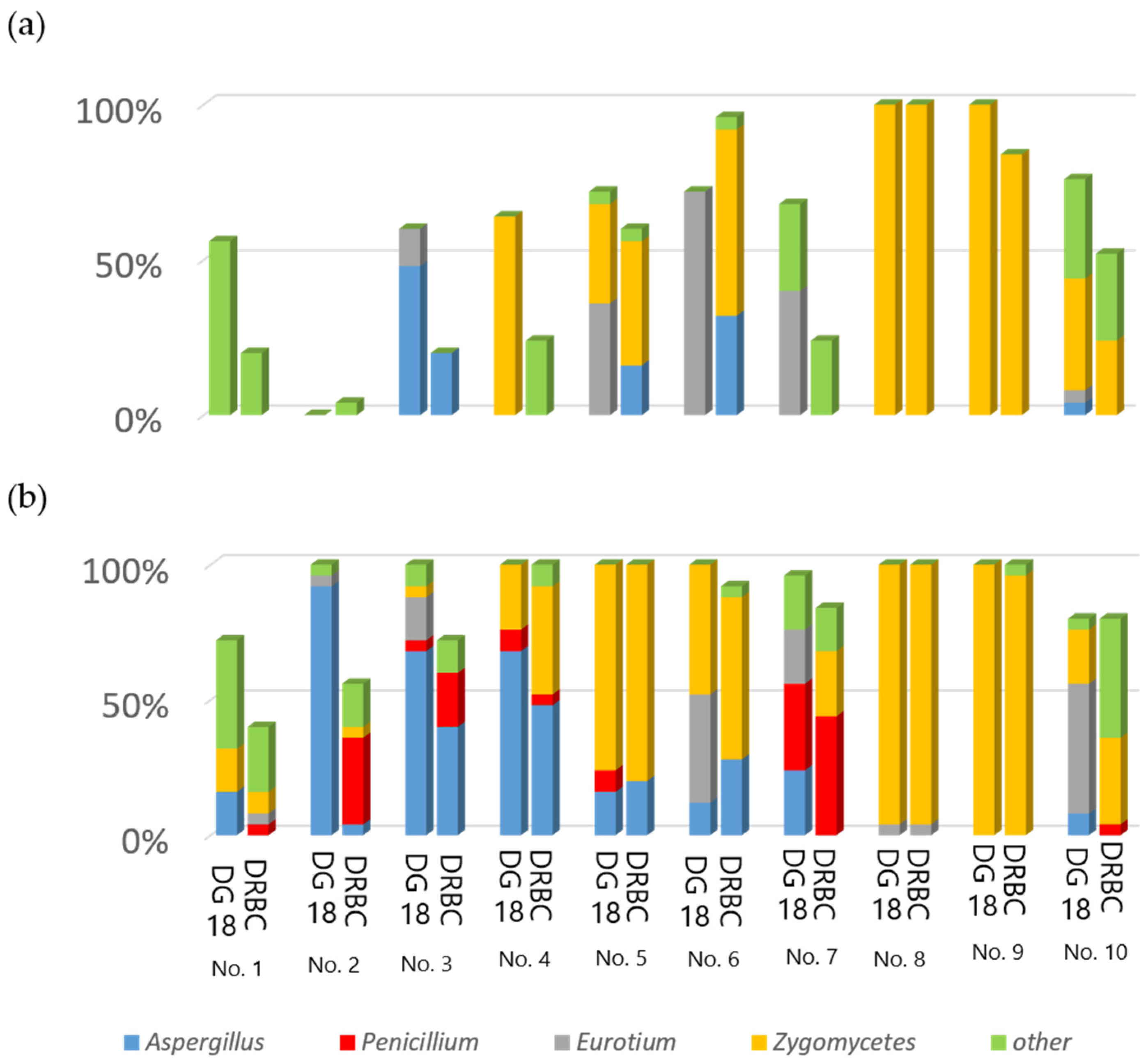
2.1. Mycoflora on Rice Grains
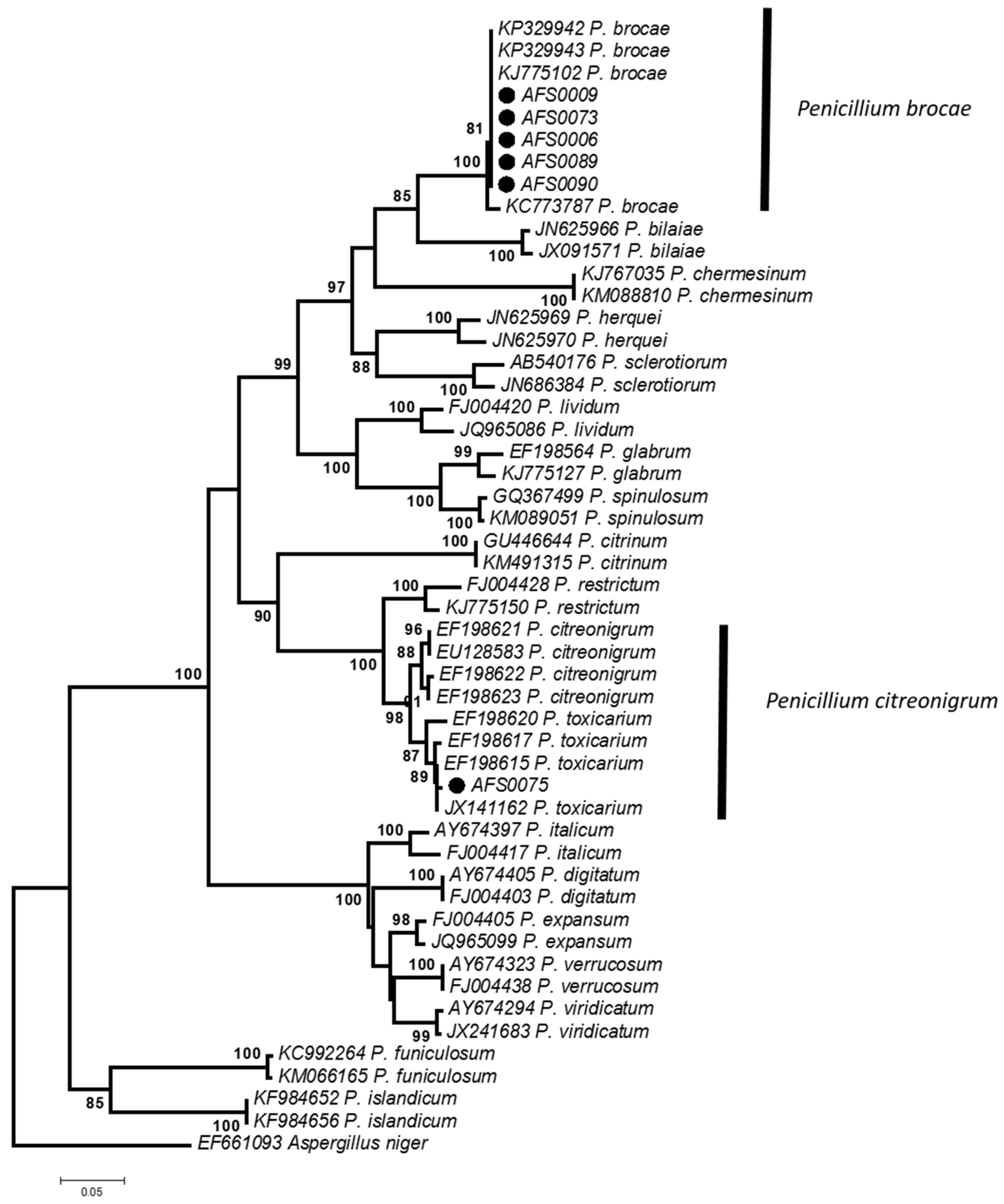
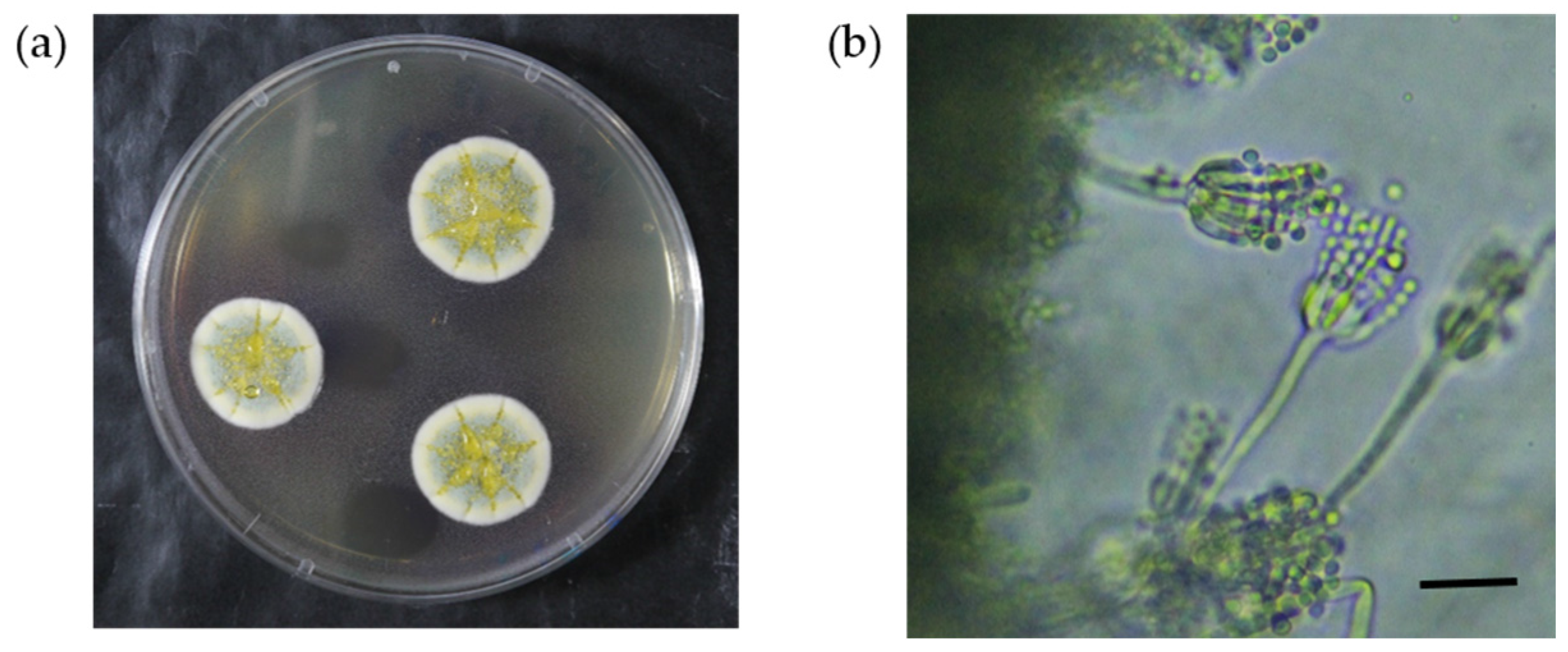
2.2. Morphological and Phylogenetic Identification
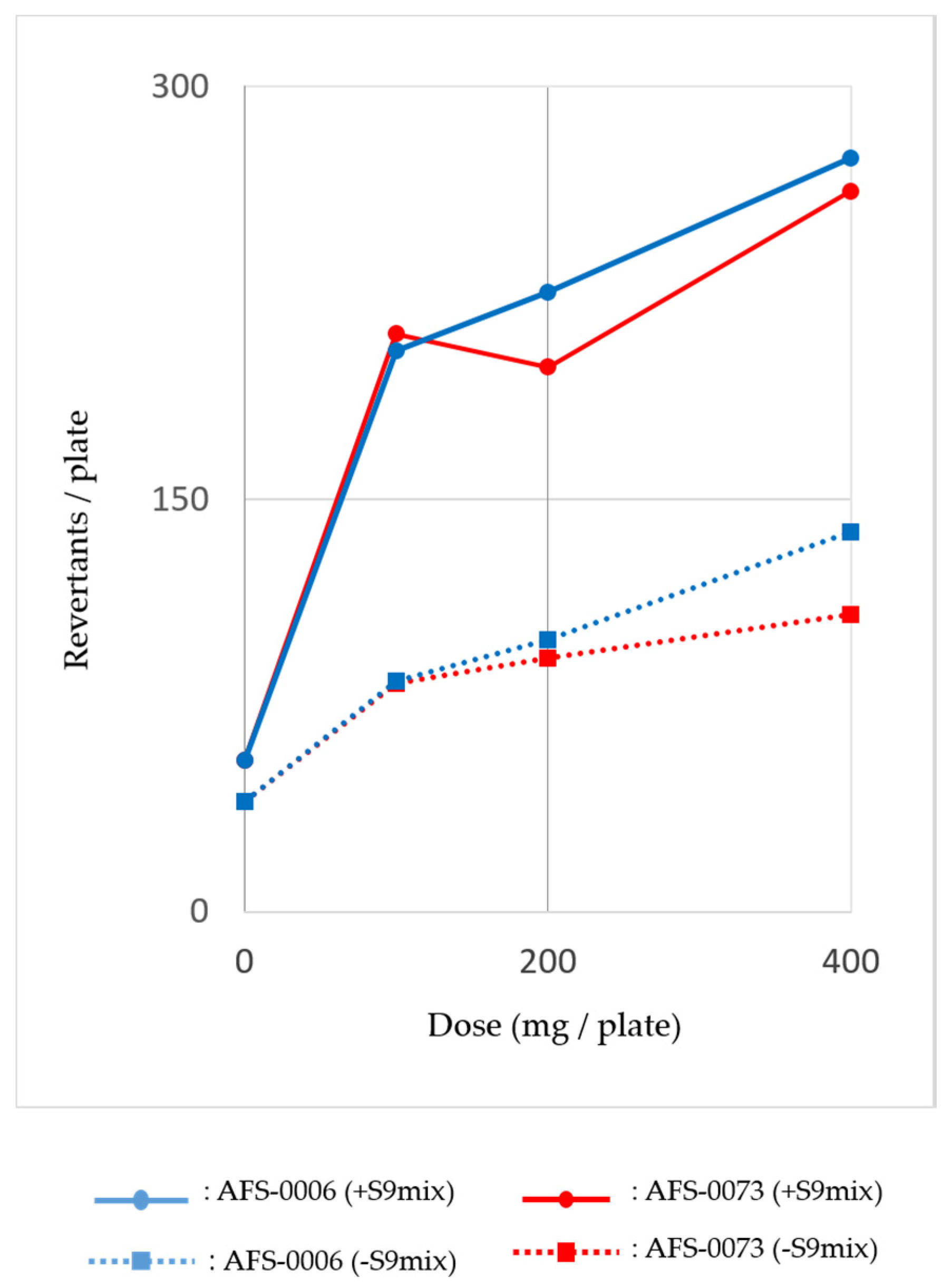
2.3. Mutagenicity of P. brocae Metabolites
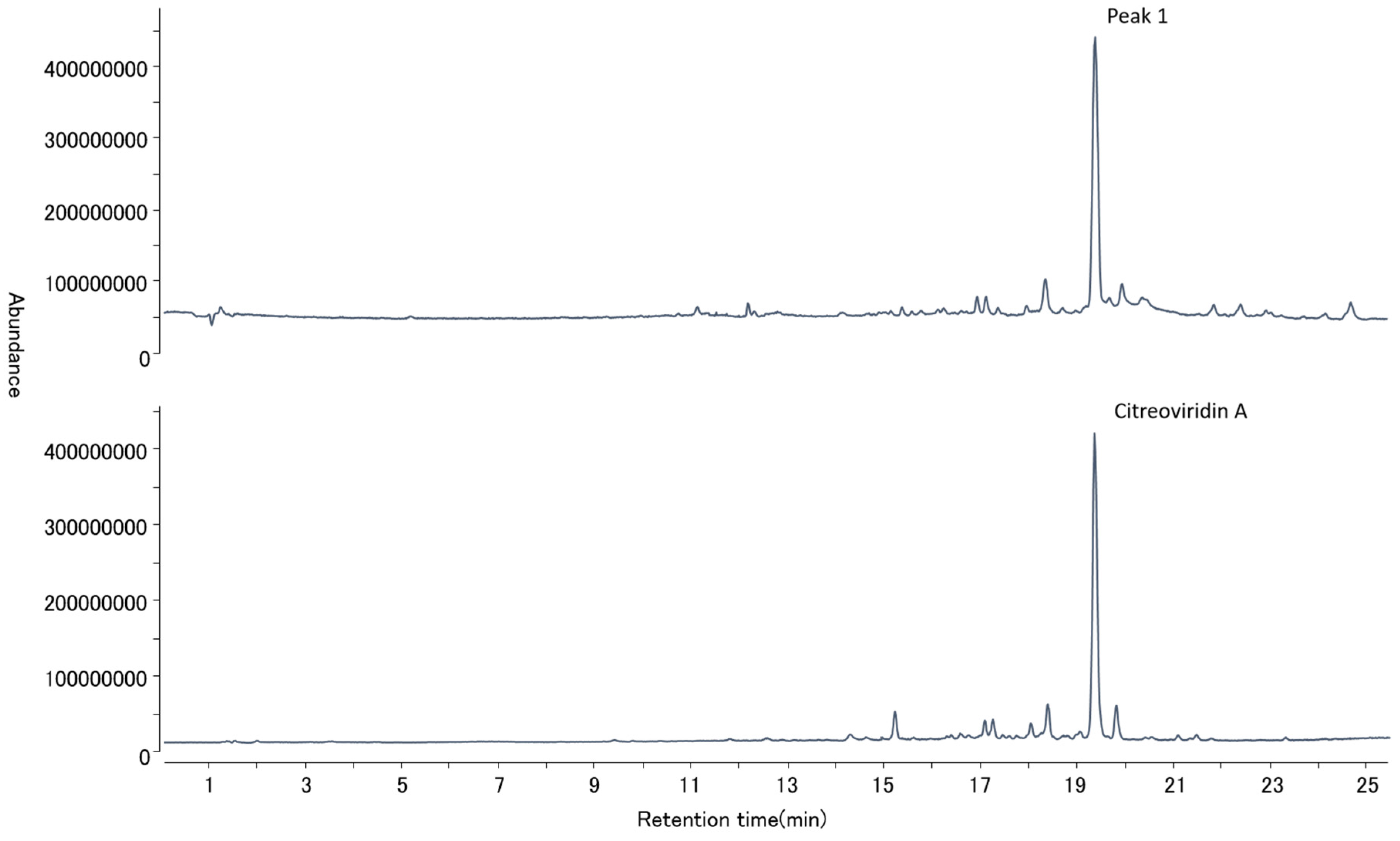
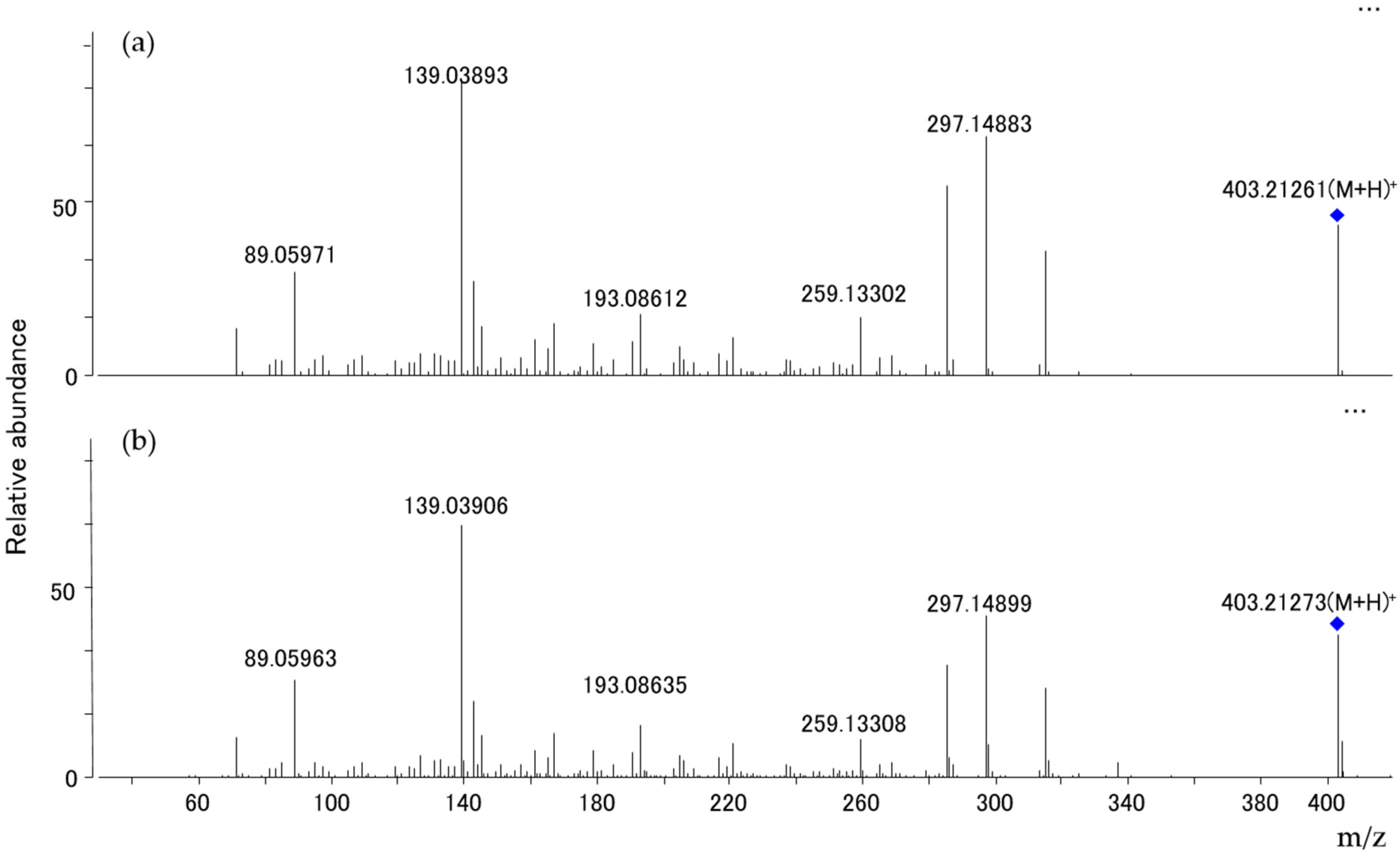
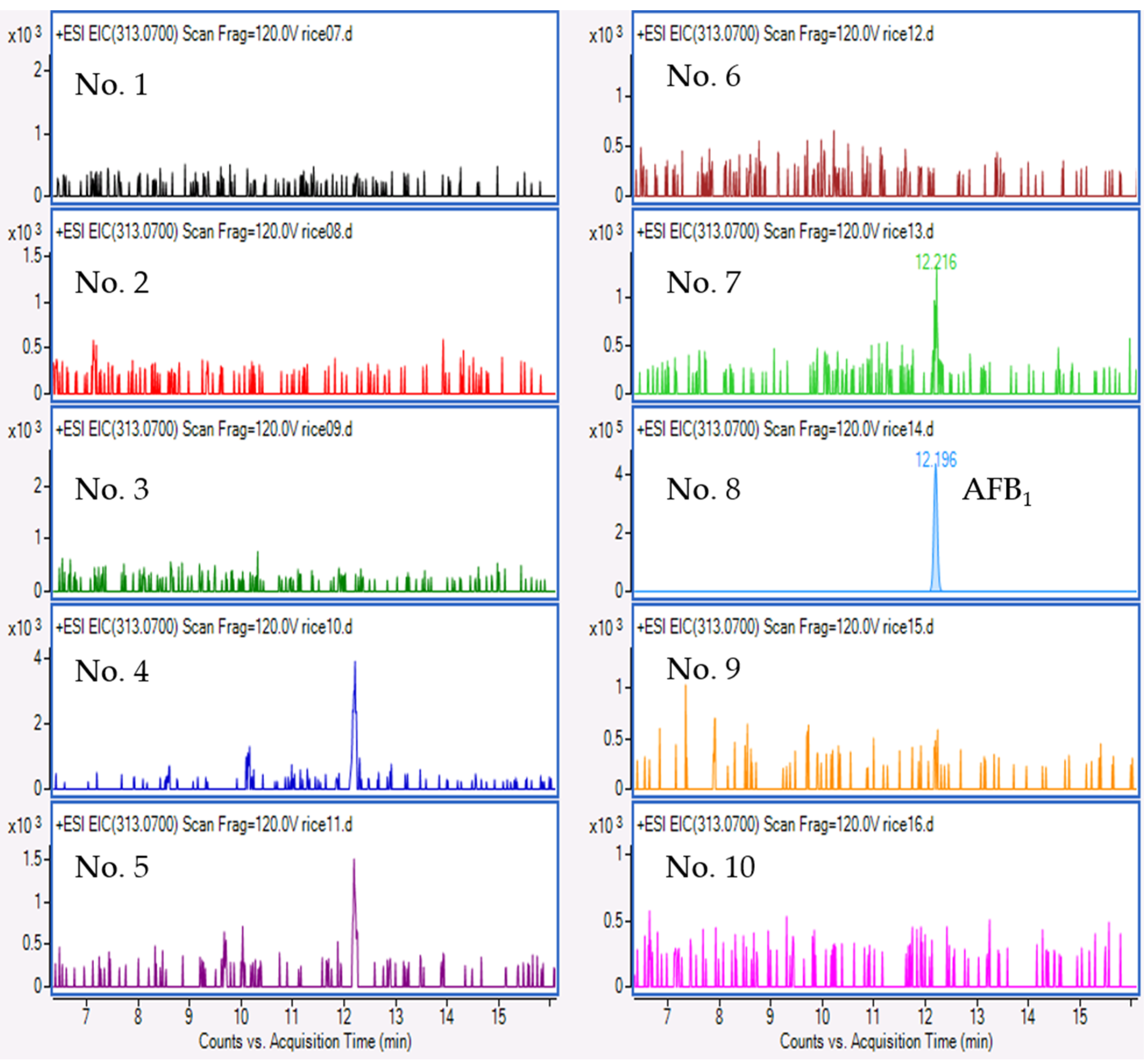
2.4. Mycotoxin Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample and Fungal Strain
5.2. Fungal Isolation and Morphological Identification
5.3. Molecular Phylogenetic Analysis
5.4. Mutagenicity Assay
5.5. Mycotoxin Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anukarahanonta, T.; Chudhabuddhi, C.; Temcharoen, P.; Sukroongreung, S. Cancer Risk from aflatoxins in Thailand. In Developments in Food Science 7 Toxigenic Fungi—Their Toxins and Health Hazard; Kurata, H., Ueno, Y., Eds.; Kodansha Ltd.: Tokyo, Japan, 1984; pp. 229–347. [Google Scholar]
- Tansakul, N.; Limsuwan, S.; Böhm, J.; Hollmann, M.; Razzazi-Fazeli, E. Aflatoxins in selected Thai commodities. Food Addit. Contam. Part B Surveill. 2013, 6, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Bansal, J.; Pantazopoulos, P.; Tam, J.; Cavlovic, P.; Kwong, K.; Turcotte, A.M.; Lau, B.P.; Scott, P.M. Surveys of rice sold in Canada for aflatoxins, ochratoxin A and fumonisins. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Suttajit, M. Current status of aflatoxin and its control in Thailand. J. Toxicol. Sci. 1998, 23, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D. Mycotoxin formation and environmental factors. In Mycotoxins in Agriculture and Food Safety; Sinha, K.K., Bhatnagar, D., Eds.; Marcel Dekker, Inc.: New York, NK, USA, 1998; pp. 255–277. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Rice and Human Nutrition. International Year of Rice 2004, Rice Is Life. Available online: http://www.fao.org/rice2004/en/f-sheet/factsheet3.pdf (accessed on 15 May 2017).
- Rohman, A.; Helmiyati, S.; Hapsari, M.; Setyaningrum, D.L. Rice in health and nutrition. Int. Food Res. J. 2014, 21, 13–24. [Google Scholar]
- Kushiro, M.; Zheng, Y.; Noriduki, H.; Sugita-Konishi, Y. Limited Surveillance of Mycoflora and Mycotoxins in Thai Rice Retailed in Japan. Asia-Pac. J. Food Saf. Secur. 2015, 1, 25–37. [Google Scholar]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, S.; Tatsuno, T. Safety of rice grains and mycotoxins—A historical review of yellow rice mycotoxicoses. Yakushigaku Zasshi 2004, 39, 321–342. [Google Scholar] [PubMed]
- Kushiro, M. Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan. JSM Mycotoxins 2015, 65, 19–23. [Google Scholar] [CrossRef]
- Tsunoda, H. Micro-organisms which deteriorate stored cereals and grains. In Proceedings of the First U.S.-Japan Conference on Toxic Micro-organisms Mycotoxins Botulism, Honolulu, Hawaii, 10 October 1968; Herzberg, M., Ed.; The UJNR Joint Panels on Toxic Micro-organisms and the U.S. Department of the Interior: Washington, DC, USA, 1970; pp. 143–162. [Google Scholar]
- Da Rocha, M.W.; Resck, I.S.; Caldas, E.D. Purification and full characterisation of citreoviridin produced by Penicillium citreonigrum in yeast extract sucrose (YES) medium. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Seifert, K.A.; Houbraken, J.; Samson, R.A.; Jacobs, K. A phylogenetic revision of Penicillium sect. Exilicaulis, including nine new species from fynbos in South Africa. IMA Fungus 2016, 7, 75–117. [Google Scholar] [PubMed]
- Pitt, J.I. Chapter 8 Subgenus Aspergilloides Dierckx. In The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces; Academic Press: New York, NY, USA, 1979; pp. 164–232. [Google Scholar]
- Peterson, S.W.; Pérez, J.; Vega, F.E.; Infante, F. Penicillium brocae, a new species associated with the coffee berry borer in Chiapas, Mexico. Mycologia 2003, 95, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, K. Mycotoxic origin of cardiac beriberi. J. Stored Prod. Res. 1969, 5, 227–236. [Google Scholar] [CrossRef]
- Rosa, C.A.; Keller, K.M.; Oliveira, A.A.; Almeida, T.X.; Keller, A.M.; Marassi, A.C.; Kruger, C.D.; Deveza, M.V.; Monteiro, B.S.; Nunes, L.M.; et al. Production of citreoviridin by Penicillium citreonigrum strains associated with rice consumption and beriberi cases in the Maranhão State, Brazil. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D.; Bhudhasamai, K.; Miscamble, B.F.; Wheeler, K.A.; Tanboon-Ek, P. The normal mycoflora of commodities from Thailand. 2. Beans, rice, small grains and other commodities. Int. J. Food Microbiol. 1994, 23, 35–43. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, M.S.; Yu, S.H. Two Species of Endophytic Penicillium from Pinus rigida in Korea. Mycobiology 2008, 36, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.W.; Vega, F.E.; Posada, F.; Nagai, C. Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia 2005, 97, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Bernan, V.S.; Greenstein, M.; Janso, J.E.; Maiese, W.M.; Mayne, C.L.; Ireland, C.M. Brocaenols A–C: Novel polyketides from a marine-derived Penicillium brocae. J. Org. Chem. 2003, 68, 2014–2017. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Li, X.M.; Lv, C.T.; Huang, C.G.; Wang, B.G. Brocazines A−F, Cytotoxic Bisthiodiketopiperazine Derivatives from Penicillium brocae MA-231, an Endophytic Fungus Derived from the Marine Mangrove Plant Avicennia marina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Dharmaputra, O.S.; Halid, H.; Sunjaya; Khim, K.S. The effect of Sitophilus zeamais on fungal infection, aflatoxin production, moisture content and damage to kernels of stored maize. In Proceedings of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R., Eds.; CAB International: Wallingford, UK, 1994; Volume 2, pp. 981–984. Available online: http://spiru.cgahr.ksu.edu/proj/iwcspp/pdf2/6/981.pdf (accessed on 16 March 2016).
- Sinha, A.K.; Sinha, K.K. Incidence of mycotoxigenic fungi on stored grain insects, Sitophilus oryzae and Tribolium casteneum. Proc. Indian Natl. Sci. Acad. 1991, B57, 77–80. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Watanabe, M.; Lee, K.; Goto, K.; Kumagai, S.; Sugita-Konishi, Y.; Hara-Kudo, Y. Rapid and effective DNA extraction method with bead grinding for a large amount of fungal DNA. J. Food Prot. 2010, 73, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [PubMed]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.B.; Whitfield, E.; Mwangen, K.; Meijer, M.; Amend, A.S.; Seifert, K.A.; Samson, R.A. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Labor Standard Bureau, Ministry of Labor. Guidebook for Mutagenicity Test Using Microorganisms; Japan Industrial Safety and Health Association: Tokyo, Japan, 1986; pp. 33–81.
- Yamada, M.; Sedgwick, B.; Sofuni, T.; Nohni, T. Construction and characterization of mutants of Salmonella typhimurium deficient in DNA repair of O6-methylguanine. J. Bacteriol. 1995, 177, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiratori, N.; Kobayashi, N.; Tulayakul, P.; Sugiura, Y.; Takino, M.; Endo, O.; Sugita-Konishi, Y. Occurrence of Penicillium brocae and Penicillium citreonigrum, which Produce a Mutagenic Metabolite and a Mycotoxin Citreoviridin, Respectively, in Selected Commercially Available Rice Grains in Thailand. Toxins 2017, 9, 194. https://doi.org/10.3390/toxins9060194
Shiratori N, Kobayashi N, Tulayakul P, Sugiura Y, Takino M, Endo O, Sugita-Konishi Y. Occurrence of Penicillium brocae and Penicillium citreonigrum, which Produce a Mutagenic Metabolite and a Mycotoxin Citreoviridin, Respectively, in Selected Commercially Available Rice Grains in Thailand. Toxins. 2017; 9(6):194. https://doi.org/10.3390/toxins9060194
Chicago/Turabian StyleShiratori, Nozomi, Naoki Kobayashi, Phitsanu Tulayakul, Yoshitsugu Sugiura, Masahiko Takino, Osamu Endo, and Yoshiko Sugita-Konishi. 2017. "Occurrence of Penicillium brocae and Penicillium citreonigrum, which Produce a Mutagenic Metabolite and a Mycotoxin Citreoviridin, Respectively, in Selected Commercially Available Rice Grains in Thailand" Toxins 9, no. 6: 194. https://doi.org/10.3390/toxins9060194
APA StyleShiratori, N., Kobayashi, N., Tulayakul, P., Sugiura, Y., Takino, M., Endo, O., & Sugita-Konishi, Y. (2017). Occurrence of Penicillium brocae and Penicillium citreonigrum, which Produce a Mutagenic Metabolite and a Mycotoxin Citreoviridin, Respectively, in Selected Commercially Available Rice Grains in Thailand. Toxins, 9(6), 194. https://doi.org/10.3390/toxins9060194





