Influence of Environmental Factors on the Production of Penitrems A–F by Penicillium crustosum
Abstract
:1. Introduction
2. Results
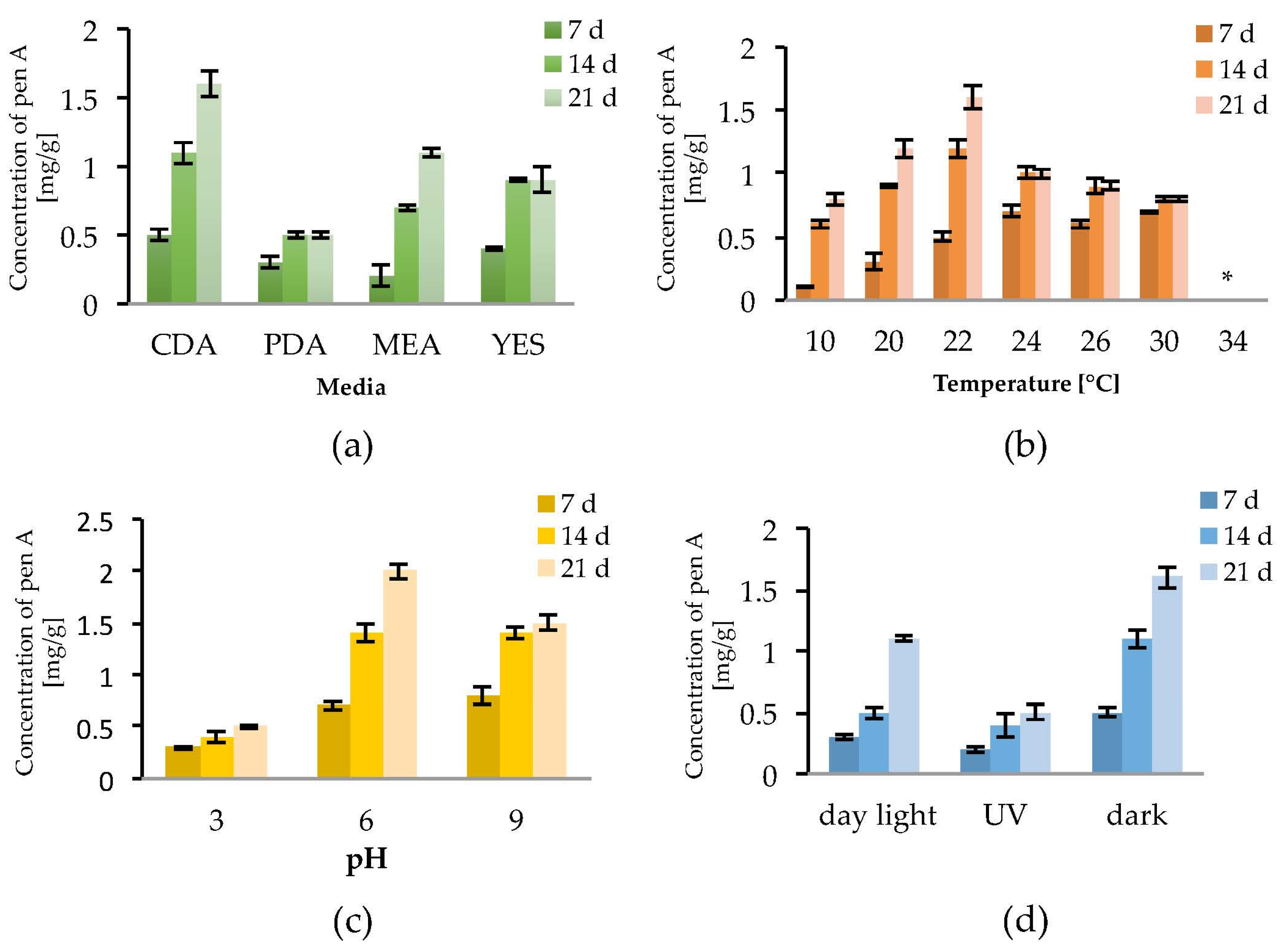
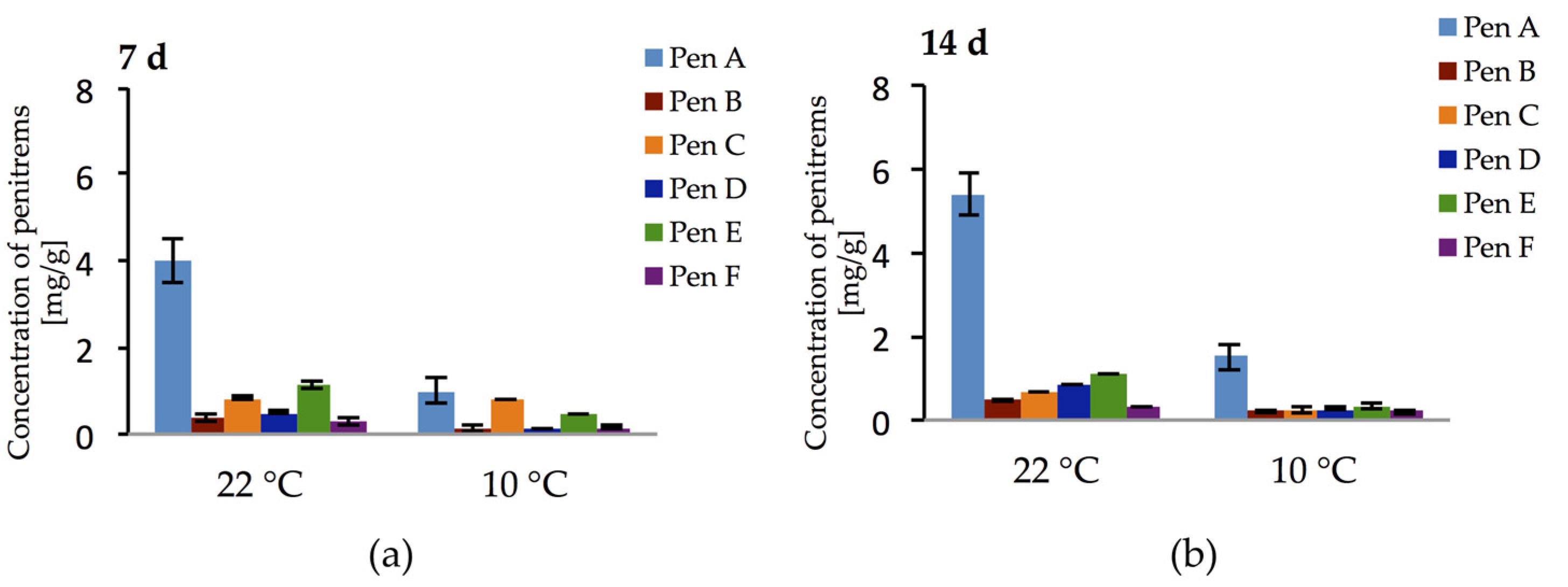
2.1. Penitrem A Production Depending on Media, Time, Temperature, pH, and Light
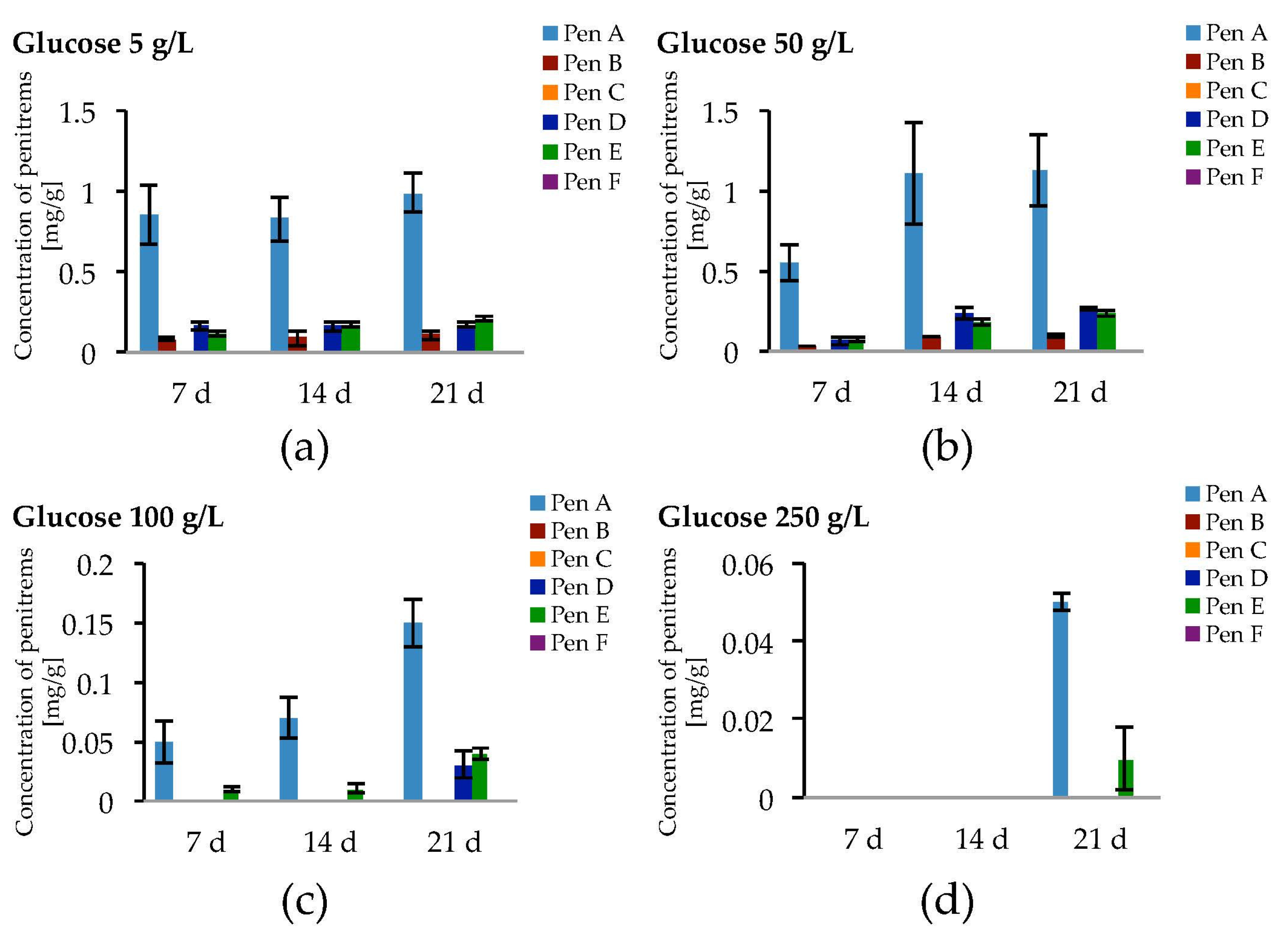
2.2. Impact of the Carbon Source on the Production of Penitrems A–F
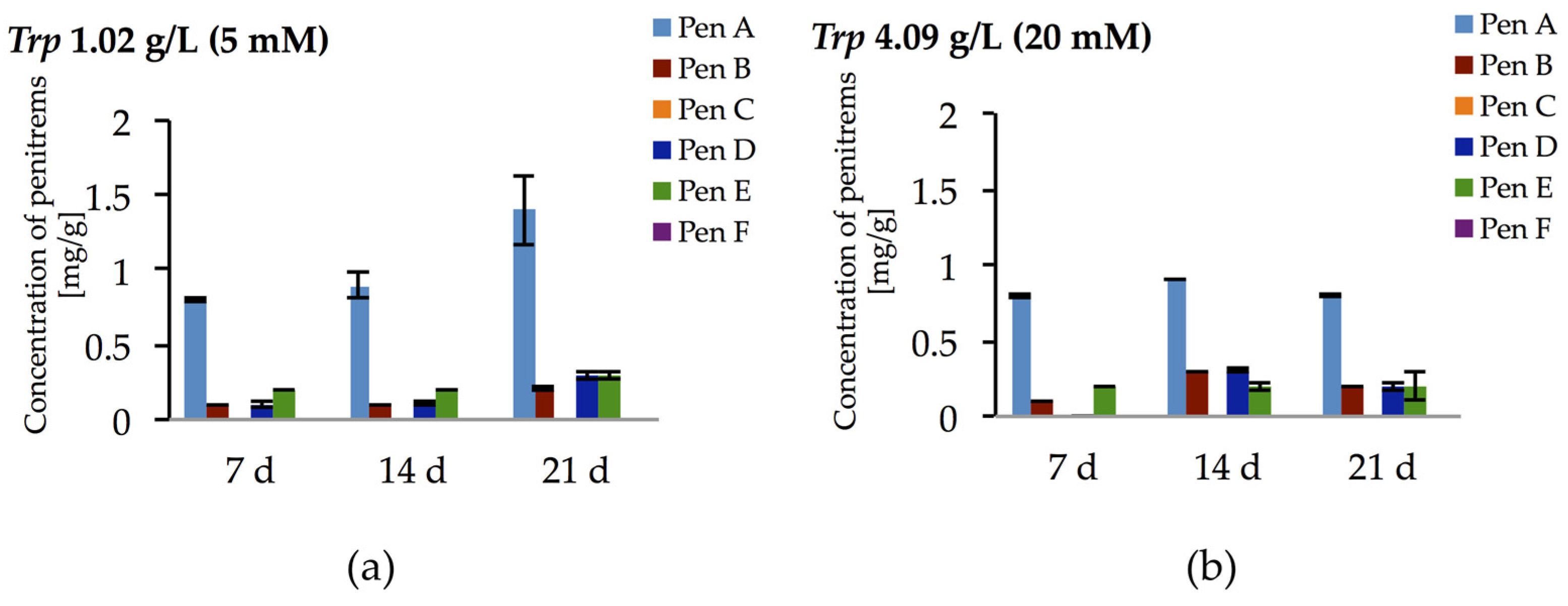
2.3. Impact of the Nitrogen Source on the Production of Penitrems A–F
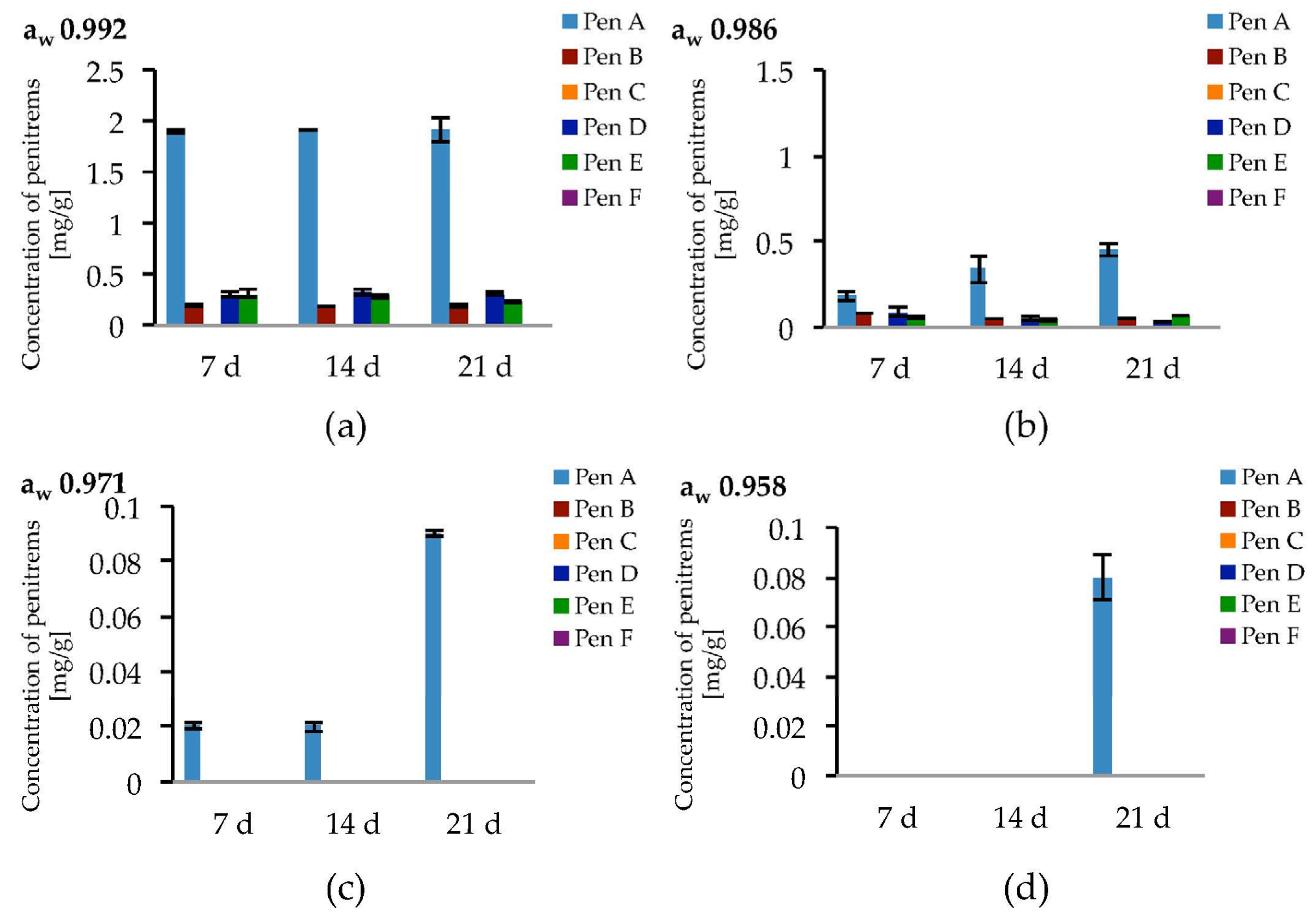
2.4. Impact of the Water Activity on the Production of Penitrems A–F
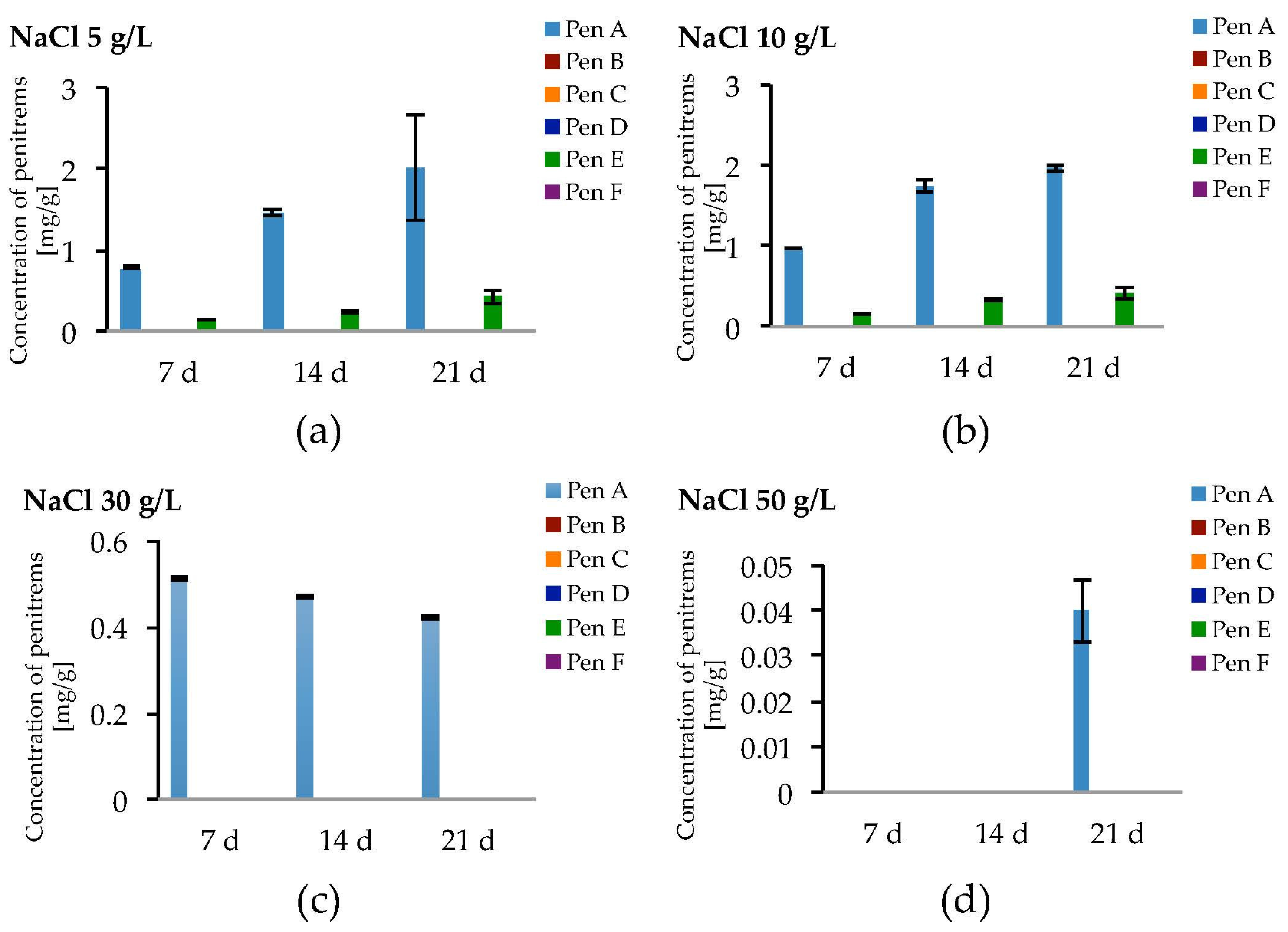
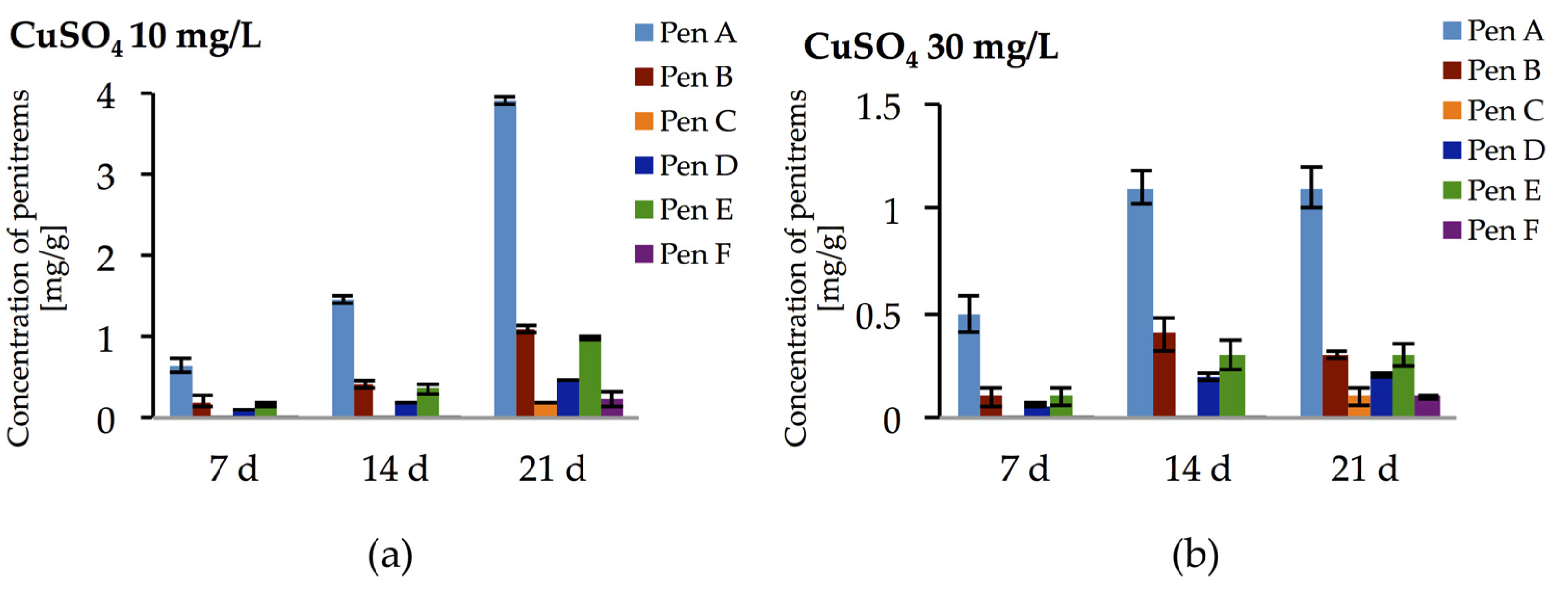
2.5. Impact of Salt Stress on the Production of Penitrems A–F
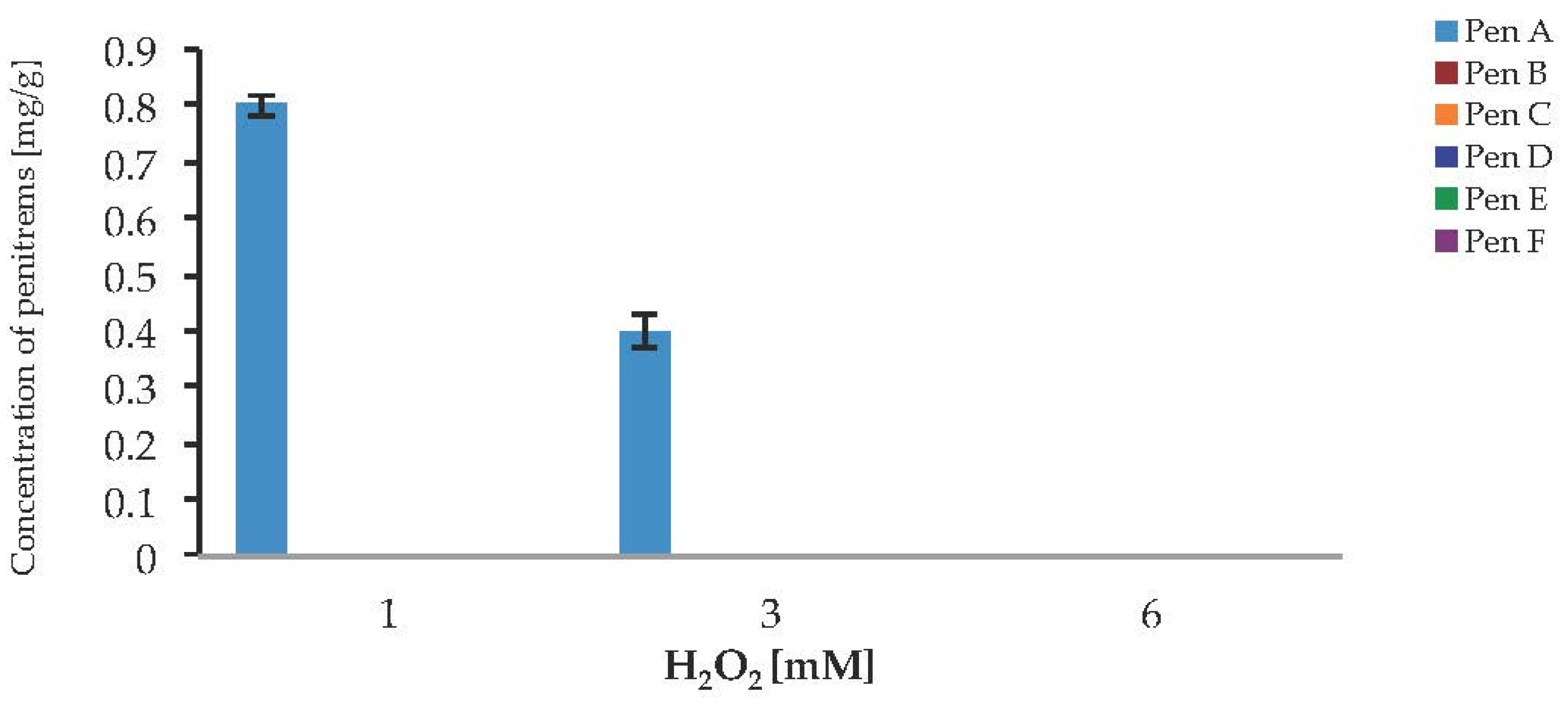
2.6. Impact of the Oxidative Stress on the Production of Penitrems A–F
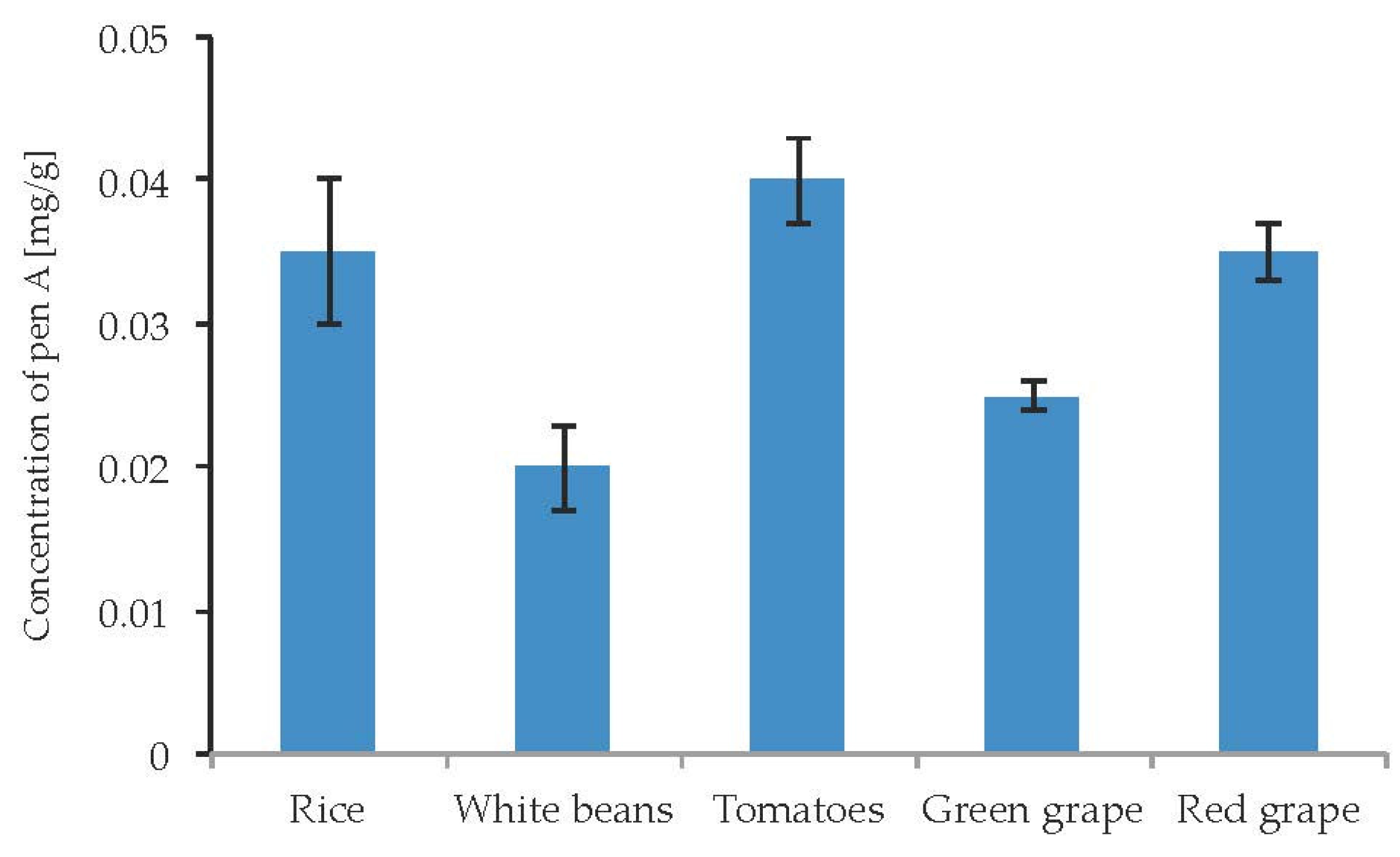
2.7. Impact of the Substrate and Food Model Media on the Production of Penitrems A–F
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungi and Media
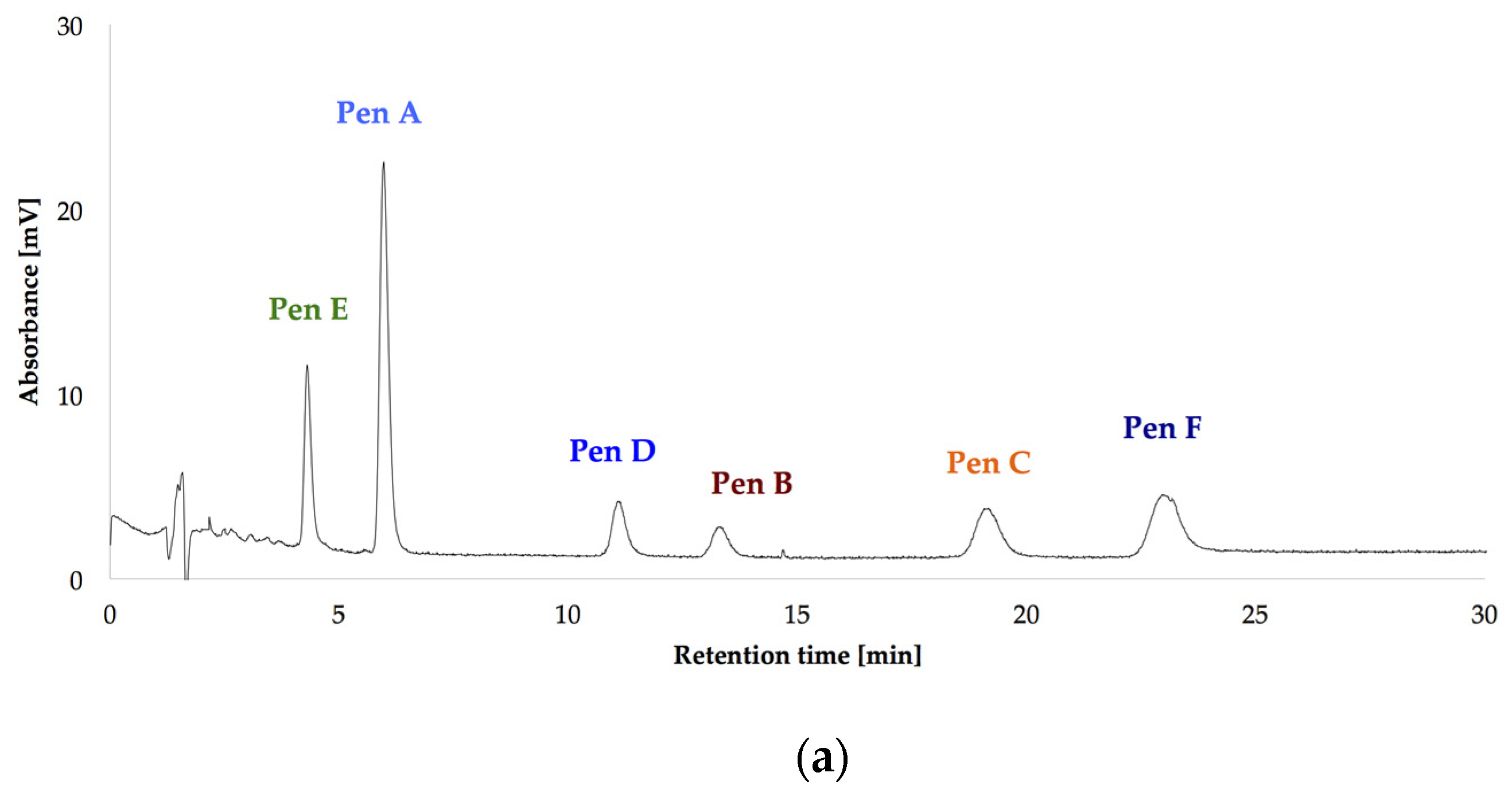
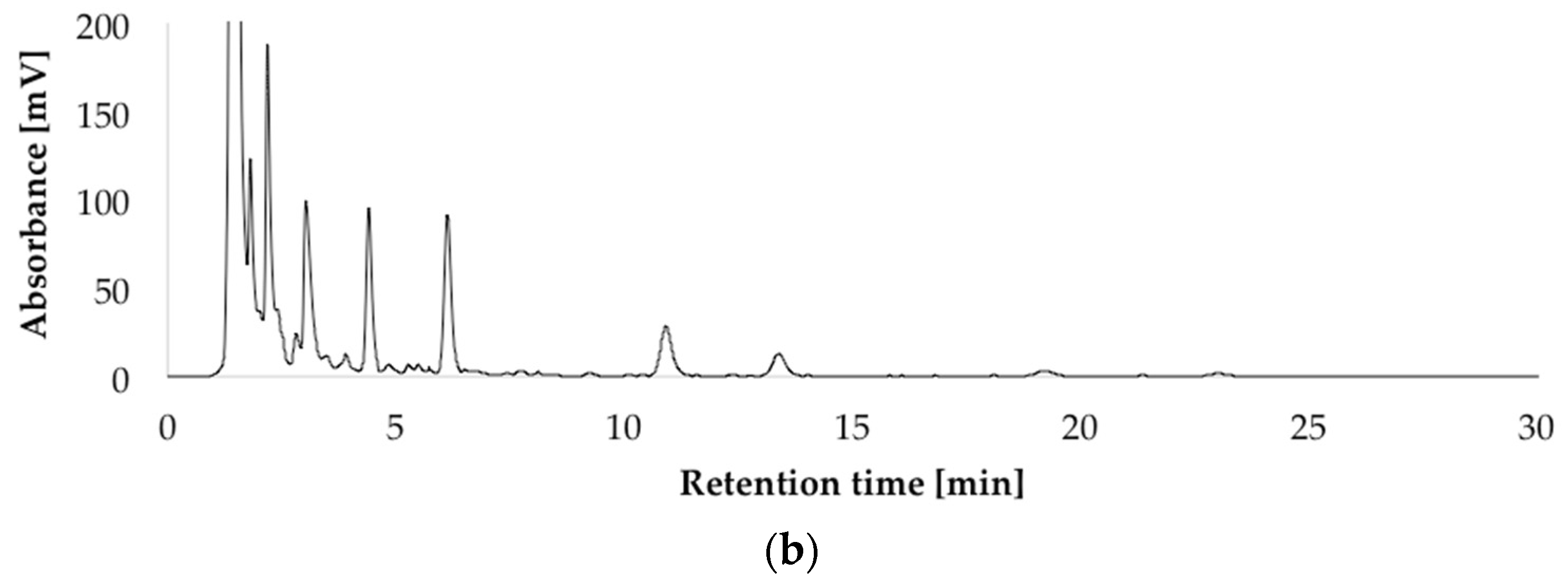
5.2. Sample Preparation and Determination of Penitrems
5.3. Isolation and Analytical Data
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GABA | gamma-aminobutyric acid |
| BK channels | big potassium channels, also called Maxi-K or slo1 |
| CNS | central nervous system |
| BBB | blood brain barrier |
| HPLC | high performance liquid chromatography |
| YES | yeast extract sucrose medium |
| CDA | Czapek–Dox Agar |
| MEA | malt extract agar |
| Pen | penitrem |
| aw | water activity |
| NP | normal phase |
References
- Steyn, P.S.; Vleggaar, R. Tremorgenic mycotoxins. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1985; pp. 1–80. [Google Scholar]
- Smith, M.M.; Warren, V.A.; Thomas, B.S.; Brochu, R.M.; Ertel, E.A.; Rohrer, S.; Schaeffer, J.; Schmatz, D.; Petuch, B.R.; Tang, Y.S.; et al. Nodulisporic acid opens insect glutamate-gated chloride channels: Identification of a new high affinity modulator. Biochemistry 2000, 39, 5543–5554. [Google Scholar] [CrossRef] [PubMed]
- Ondeyka, J.G.; Helms, G.L.; Hensens, O.D.; Goetz, M.A.; Zink, D.L.; Tsipouras, A.; Shoop, W.L.; Slayton, L.; Dombrowski, A.W.; Polishook, J.D.; et al. Nodulisporic acid A, a novel and potent insecticide from a Nodulisporium sp. Isolation, structure determination, and chemical transformations. J. Am. Chem. Soc. 1997, 119, 8809–8816. [Google Scholar] [CrossRef]
- Shoop, W.L.; Zakson-Aiken, M.; Gregory, L.M.; Michael, B.F.; Pivnichny, J.; Meinke, P.T.; Fisher, M.H.; Wyvratt, M.J.; Pikounis, B.; Schmatz, D.M. Systemic efficacy of nodulisporamides against fleas on dogs. J. Parasitol. 2001, 87, 1150–1154. [Google Scholar] [CrossRef]
- De Jesus, A.E.; Gorst-Allman, C.P.; Steyn, P.S.; van Heerden, F.R.; Vleggaar, R.; Wessels, P.L.; Hull, W.E. Tremorgenic mycotoxins from Penicillium crustosum Biosynthesis of Penitrem A. J. Chem. Soc. Perkin Trans. 1 1983, 8, 1863–1868. [Google Scholar] [CrossRef]
- Byrne, K.M.; Smith, S.K.; Ondeyka, J.G. Biosynthesis of nodulisporic acid A: Precursor studies. J. Am. Chem. Soc. 2002, 124, 7055–7060. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.J.; Scott, D.B. Indole-Diterpene Biosynthesis in Ascomycetous Fungi; Marcel Dekker: New York, NY, USA, 2004; Volume 70, pp. 405–426. [Google Scholar]
- Saikia, S.; Nicholson, M.J.; Young, C.; Parker, E.J.; Scott, B. The genetic basis for indole-diterpene chemical diversity in filamentous fungi. Mycol. Res. 2008, 112, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J. Tremorgenic mycotoxins: An update. In Antinutrients and Natural Toxins in Foods; Ory, R.L., Ed.; Food and Nutrition Press: Westport, CT, USA, 1980; pp. 17–37. [Google Scholar]
- González, M.C.; Lull, C.; Moya, P.; Ayala, I.; Primo, J.; Primo Yúfera, E. Insecticidal activity of penitrems, including penitrem G, a new member of the family isolated from Penicillium crustosum. J. Agric. Food Chem. 2003, 51, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Laakso, J.A.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. A new penitrem analog with antiinsectan activity from the sclerotia of Aspergillus sulphureus. J. Agric. Food Chem. 1993, 41, 973–975. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.; Rundberget, T.; Uhlig, S.; Rise, F.; Wilkins, A.L. Isolation and structure elucidation of secopenitrem D, an indole alkaloid from Penicillium crustosum Thom. Toxicon 2011, 57, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Rundberget, T.; Skaar, I.; O’Brien, O.; Flåøyen, A. Penitrem and thomitrem formation by Penicillium crustosum. Mycopathologia 2004, 157, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Naik, J.T.; Mantle, P.G.; Sheppard, R.N.; Waight, E.S. Penitremones A-C, Penicillium metabolites containing an oxidized penitrem carbon skeleton giving insight into structure-tremorgenic relationships. J. Chem. Soc. Perkin Trans. 1 1995, 9, 1121–1125. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nozawa, K.; Hosoe, T.; Nakajima, S.; Kawai, K. Indoloterpenes related to tremorgenic mycotoxins, penitrems, from Penicillium crustosum. Phytochemistry 1993, 32, 1177–1181. [Google Scholar] [CrossRef]
- Sallam, A.A.; Ayoub, N.M.; Foudah, A.I.; Gissendanner, C.R.; Meyer, S.A.; El Sayed, K.A. Indole diterpene alkaloids as novel inhibitors of the Wnt/β-catenin pathway in breast cancer cells. Eur. J. Med. Chem. 2013, 70, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudah, A.I.; El Sayed, K.A. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. MedChemComm 2013, 4, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.J.; Smith, C.C.T.; De Belleroche, J.; Bradford, H.F.; Mantle, P.G.; Thomas, A.J.; Penny, R.H.C. Actions of tremorgenic fungal toxins on neurotransmitter release. J. Neurochem. 1980, 34, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Knaus, H.G.; McManus, O.B.; Lee, S.H.; Schmalhofer, W.A.; Garcia-Calvo, M.; Helms, L.M.; Sanchez, M.; Giangiacomo, K.; Reuben, J.P. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 1994, 33, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, G.S.; Jäderlund, K.H.; Moldes-Anaya, A.; Schönheit, J.; Bernhoft, A.; Jaeger, G.; Rundberget, T.; Skaar, I. Poisoning of dogs with tremorgenic Penicillium toxins. Med. Mycol. 2010, 48, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Breton, P.; Bizot, J.C.; Buee, J.; De La Manche, I. Brain neurotoxicity of Penitrem A: electrophysiological, behavioral and histopathological study. Toxicon 1998, 36, 645–655. [Google Scholar] [CrossRef]
- Richard, J.L.; Arp, L.H. Natural occurrence of the mycotoxin penitrem A in moldy cream cheese. Mycopathologia 1979, 67, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L.; Bacchetti, P.; Arp, L.H. Moldy walnut toxicosis in a dog, caused by the mycotoxin, penitrem A. Mycopathologia 1981, 76, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.D.; Holds, K.; Tobin, N.F. Intoxication by tremorgenic mycotoxin (penitrem A) in a dog. Aust. Vet. J. 1988, 65, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Boysen, S.R.; Rozanski, E.A.; Chan, D.L.; Grobe, T.L.; Fallon, M.J.; Rush, J.E. Tremorgenic mycotoxicosis in four dogs from a single household. J. Am. Vet. Med. Assoc. 2002, 221, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Naude, T.W.; O’Brien, O.M.; Rundberget, T.; McGregor, A.D.G.; Roux, C.; Flåøyen, A. Tremorgenic neuromycotoxicosis in 2 dogs ascribed to the ingestion of penitrem A and possibly roquefortine in rice contaminated with Penicillium crustosum: Clinical communication. J. S. Afr. Vet. Assoc. 2002, 73, 211–215. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12665136?dopt=Abstract (accessed on 12 April 2017).
- Mantle, P.G.; Penny, R.H.C. Tremorgenic mycotoxins and neurological disorders-a review. Vet. Annu. 1981, 21, 51–62. Available online: http://agris.fao.org/agris-search/search.do?recordID=US201301309076 (accessed on 12 April 2017).
- Lewis, P.R.; Donoghue, M.B.; Hocking, A.D.; Cook, L.; Granger, L.V. Tremor syndrome associated with a fungal toxin: Sequelae of food contamination. Med. J. Aust. 2005, 182, 582–584. Available online: https://www.mja.com.au/journal/2005/182/11/tremor-syndrome-associated-fungal-toxin-sequelae-food-contamination (accessed on 12 April 2017).
- Wilson, B.J.; Wilson, C.H.; Hayes, A.W. Tremorgenic toxin from Penicillium cyclopium grown on food materials. Nature 1968, 220, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P.G.; Perera, K.P.; Maishman, N.J.; Mundy, G.R. Biosynthesis of penitrems and roquefortine by Penicillium crustosum. Appl. Environ. Microbiol. 1983, 45, 1486–1490. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6870239 (accessed on 12 April 2017).
- Dorner, J.W.; Cole, R.J.; Hill, R.A. Tremorgenic mycotoxins produced by Aspergillus fumigatus and Penicillium crustosum isolated from molded corn implicated in a natural intoxication of cattle. J. Agric. Food Chem. 1984, 32, 411–413. Available online: http://scholar.google.com/scholar_lookup?title=Tremorgenic%20mycotoxins%20produced%20by%20Aspergillus%20fumigatus%20and%20Penicillium%20crustosum%20isolated%20from%20moulded%20corn%20implicated%20in%20a%20natural%20intoxication%20of%20cattle&author=JW.%20Dorner&author=RJ.%20Cole&author=RA.%20Hill&journal=J%20Agric%20Food%20Chem&volume=32&pages=411–413&publication_year=1984 (accessed on 12 April 2017).
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Karanyi, Z.; Holb, I.; Hornok, L.; Pocsi, I.; Miskei, M. FRSD: Fungal Stress Response Database. Database 2013. Available online: http://internal.med.unideb.hu/fsrd/ (accessed on 12 April 2017). [CrossRef]
- Wagener, R.E.; Davis, N.D.; Diener, U.L. Penitrem A and roquefortine production by Penicillium commune. Appl. Environ. Microbiol. 1980, 39, 882–887. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC291438/ (accessed on 12 April 2017).
- Hou, C.T.; Ciegler, A.; Hessseltine, C.W. Tremorgenic toxins from penicillia III. Tremortin production by Penicillium species on various agricultural commodities. Appl. Microbiol. 1971, 21, 1101–1103. Available online: http://aem.asm.org/content/21/6/1101.short (accessed on 12 April 2017).
- Sumarah, M.W.; Miller, J.D.; Blackwell, B.A. Isolation and metabolite production by Penicillium roqueforti, P. paneum and P. crustosum isolated in Canada. Mycopathologia 2005, 159, 571–577. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, A.A.; Leistner, L. Production of penitrem A by Penicillium crustosum isolated from foodstuffs. Int. J. Food Microbiol. 1988, 7, 9–17. [Google Scholar] [CrossRef]
- Surekha, M.; Reddy, S.M. Effect of carbon and nitrogen sources on the production of penitrem B by Penicillium aurantiogriseum. Folia Microbiol. 1992, 37, 47–49. Available online: https://www.ncbi.nlm.nih.gov/pubmed/1505862 (accessed on 12 April 2017).
- Surekha, M.; Saini, K.; Advala, N.; Reddy, S.M. Production of penitrem B a tremogenic toxin produced by Penicillium aurantiogriseum. Int. J. Curr. Res. 2012, 4, 37–39. Available online: https://www.journalcra.com (accessed on 12 April 2017).
- Mantle, P.G.; Laws, I.; Tan, M.J.L.; Tizard, M. A novel process for the production of penitrem mycotoxins by submerged fermentation of Penicillium nigricans. Microbiology 1984, 130, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tagami, K.; Minami, A.; Matsumoto, T.; Frisvad, J.C.; Suzuki, H.; Ishikawa, J.; Gomi, K.; Oikawa, H. Reconstitution of biosynthetic machinery for the synthesis of the highly elaborated indole diterpene penitrem. Angew. Chem. Int. Ed. 2015, 54, 5748–5752. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, A.E.; Steyn, P.S.; van Heerden, F.R.; Vleggaar, R.; Wessels, P.L.; Hull, W.E. Tremorgenic mycotoxins from Penicillium crustosum: Isolation of penitrems A–F and the structure elucidation and absolute configuration of penitrem A. J. Chem. Soc. Perkin Trans. 1 1983, 1847–1856. [Google Scholar] [CrossRef]
- De Jesus, A.E.; Steyn, P.S.; van Heerden, F.R.; Vleggaar, R.; Wessels, P.L.; Hull, W.E. Structure and biosynthesis of the penitrems A–F, six novel tremorgenic mycotoxins from Penicillium crustosum. J. Chem. Soc. Chem. Commun. 1981, 6, 289–291. [Google Scholar] [CrossRef]
- Mantle, P.G. The role of tryptophan as a biosynthetic precursor of indole-diterpenoid fungal metabolites: Continuing a debate. Phytochemistry 2009, 70, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Cayley, G.R.; Lacey, J. Effect of water activity and temperature on mycotoxin production by Alternaria alternata in culture and on wheat grain. Appl. Environ. Microbiol. 1984, 47, 1113–1117. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6540067 (accessed on 12 April 2017).
- Schmidt-Heydt, M.; Graf, E.; Stoll, D.; Geisen, R. The biosynthesis of ochratoxin A by Penicillium as one mechanism for adaptation to NaCl rich foods. Food Microbiol. 2012, 29, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.L. Acute penitrem A and roquefortine poisoning in a dog. Can. Vet. J. 2002, 43, 372–374. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC339273/ (accessed on 12 April 2017).
- Felsöciova, S.; Rybarik, L.; Tancinova, D.; Maskova, Z.; Kacaniova, M. Microfungi and mycotoxins of grapes from Tokaj wine region. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 16–18. [Google Scholar] [CrossRef]
- Atoui, A.; Kastner, C.; Larey, C.M.; Thokala, R.; Etxebeste, O.; Espeso, E.A.; Fischer, R.; Calvo, A.M. Cross-talk between light and glucose regulation controls toxin production and morphogenesis in Aspergillus nidulans. Fungal Genet. Biol. 2010, 47, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Nakajima, T.; Hirayae, K. Effects of carbon sources and amines on induction of trichothecene production by Fusarium asiaticum in liquid culture. FEMS Microbiol. Lett. 2014, 352, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Mateo, E.M.; Valle-Algarra, F.M.; Mateo, F.; Mateo, R.; Jimenez, M. Influence of nitrogen and carbon sources on the production of ochratoxin A by ochratoxigenic strains of Aspergillus spp. isolated from grapes. Int. J. Food Microbiol. 2008, 122, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Stahl, H.G. Ability of various carbon sources to induce and support aflatoxin synthesis by Aspergillus parasiticus. J. Food Saf. 1984, 6, 271–279. [Google Scholar] [CrossRef]
- Brzonkalik, K.; Herrling, T.; Syldatk, C.; Neumann, A. The influence of different nitrogen and carbon sources on mycotoxin production in Alternaria alternata. Int. J. Food Micribiol. 2011, 147, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, T.; Subramanyam, C. Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Radic. Biol. Med. 2000, 29, 981–985. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Bogialli, S.; Bortolini, C.; Tapparo, A.; Causin, R. In vitro production of fumonisins by Fusarium verticillioides under oxidative stress induced by H2O2. J. Agric. Food Chem. 2015, 63, 4879–4885. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Rüfer, C.; Raupp, F.; Bruchmann, A.; Perrone, G.; Geisen, R. Influence of light on food relevant fungi with emphasis on ochratoxin producing species. Int. J. Food Microbiol. 2011, 145, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Pose, G.; Patriara, A.; Kyanko, V.; Pardo, A.; Fernández Pinto, V. Water activity and temperature effects on mycotoxin production by Alternaria alternata on a synthetic tomato medium. Int. J. Food Micribiol. 2010, 142, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; van Reenen-Hoekstra, E.S. Introduction to Food-Borne Fungi, 3rd ed.; Centraalbureau voor Schimmelcutures: Baarn, The Netherlands, 1988. [Google Scholar]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food-Borne Fungi, 5th ed.; Centraalbureau voor Schimmelcutures: Baarn, The Netherlands, 1996. [Google Scholar]
- Kokkonen, M.; Jestoi, M.; Rizzo, A. The effect of substrate on mycotoxin production of selected Penicillium strains. Int. J. Food Micribiol. 2005, 99, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Smedsgaard, J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chromatogr. A 1997, 760, 264–270. [Google Scholar] [CrossRef]


| C Source 10 g/L | Pen A | Pen B | Pen C | Pen D | Pen E | Pen F |
|---|---|---|---|---|---|---|
| None | ND | ND | ND | ND | ND | ND |
| Glucose | 1.13 ± 0.012 | 0.12 ± 0.037 | + | 0.26 ± 0.016 | 0.23 ± 0.033 | + |
| Galactose | 0.65 ± 0.013 | 0.07 ± 0.001 | + | 0.13 ± 0.002 | 0.12 ± 0.017 | + |
| Arabinose | 0.58 ± 0.072 | 0.05 ± 0.006 | + | 0.09 ± 0.001 | 0.16 ± 0.012 | + |
| Fructose | 0.62 ± 0.024 | 0.06 ± 0.002 | + | 0.11 ± 0.007 | 0.12 ± 0.019 | + |
| Sucrose | 0.71 ± 0.092 | 0.07 ± 0.001 | + | 0.14 ± 0.003 | 0.11 ± 0.026 | + |
| Xylose | 0.54 ± 0.048 | 0.11 ± 0.001 | + | 0.11 ± 0.002 | 0.16 ± 0.008 | + |
| Rhamnose | 0.95 ± 0.024 | 0.13 ± 0.017 | + | 0.19 ± 0.005 | 0.19 ± 0.002 | + |
| Maltose | 0.76 ± 0.001 | 0.12 ± 0.062 | + | 0.19 ± 0.007 | 0.16 ± 0.003 | + |
| Cellulose | 0.34 ± 0.013 | + | ND | + | 0.07 ± 0.008 | ND |
| Sorbitol | 0.45 ± 0.014 | 0.05 ± 0.011 | ND | 0.09 ± 0.001 | 0.08 ± 0.004 | ND |
| Starch | 0.28 ± 0.021 | + | ND | + | 0.05 ± 0.002 | ND |
| N Source | Pen A | Pen B | Pen C | Pen D | Pen E | Pen F |
|---|---|---|---|---|---|---|
| None | ND | ND | ND | ND | ND | ND |
| NaNO3 | 1.5 ± 0.023 | 0.25 ± 0.004 | + | 0.31 ± 0.009 | 0.38 ± 0.008 | + |
| NH4NO3 | 1.6 ± 0.055 | 0.32 ± 0.004 | + | 0.31 ± 0.080 | 0.32 ± 0.011 | + |
| (NH4)2SO4 | 1.6 ± 0.036 | 0.31 ± 0.047 | + | 0.22 ± 0.002 | 0.27 ± 0.092 | + |
| NH4Cl+NaNO3 | 1.5 ± 0.075 | 0.12 ± 0.001 | + | 0.23 ± 0.009 | 0.27 ± 0.058 | + |
| NH4Cl | 1.8 ± 0.026 | 0.17 ± 0.001 | + | 0.34 ± 0.006 | 0.36 ± 0.049 | + |
| Urea | 1.1 ± 0.061 | 0.17 ± 0.005 | + | 0.17 ± 0.008 | 0.24 ± 0.001 | + |
| Gly | 2.0 ± 0.073 | 0.30 ± 0.006 | + | 0.35 ± 0.004 | 0.32 ± 0.002 | + |
| Ser | 2.0 ± 0.089 | 0.42 ± 0.009 | + | 0.46 ± 0.069 | 0.51 ± 0.001 | + |
| Pro | 2.1 ± 0.062 | 0.36 ± 0.019 | + | 0.37 ± 0.023 | 0.42 ± 0.008 | + |
| Arg | 1.9 ± 0.089 | 0.31 ± 0.029 | + | 0.34 ± 0.044 | 0.35 ± 0.016 | + |
| Glu | 4.0 ± 0.284 | 0.79 ± 0.031 | 0.18 ± 0.053 | 0.85 ± 0.022 | 0.91 ± 0.003 | 0.15 ± 0.017 |
| Trp | 0.92 ± 0.052 | 0.28 ± 0.020 | + | 0.32 ± 0.046 | 0.38 ± 0.001 | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, S.A.; Jagels, A.; Cramer, B.; Geisen, R.; Humpf, H.-U. Influence of Environmental Factors on the Production of Penitrems A–F by Penicillium crustosum. Toxins 2017, 9, 210. https://doi.org/10.3390/toxins9070210
Kalinina SA, Jagels A, Cramer B, Geisen R, Humpf H-U. Influence of Environmental Factors on the Production of Penitrems A–F by Penicillium crustosum. Toxins. 2017; 9(7):210. https://doi.org/10.3390/toxins9070210
Chicago/Turabian StyleKalinina, Svetlana A., Annika Jagels, Benedikt Cramer, Rolf Geisen, and Hans-Ulrich Humpf. 2017. "Influence of Environmental Factors on the Production of Penitrems A–F by Penicillium crustosum" Toxins 9, no. 7: 210. https://doi.org/10.3390/toxins9070210






