Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In Vitro Envenomation Models
Abstract
:1. Introduction
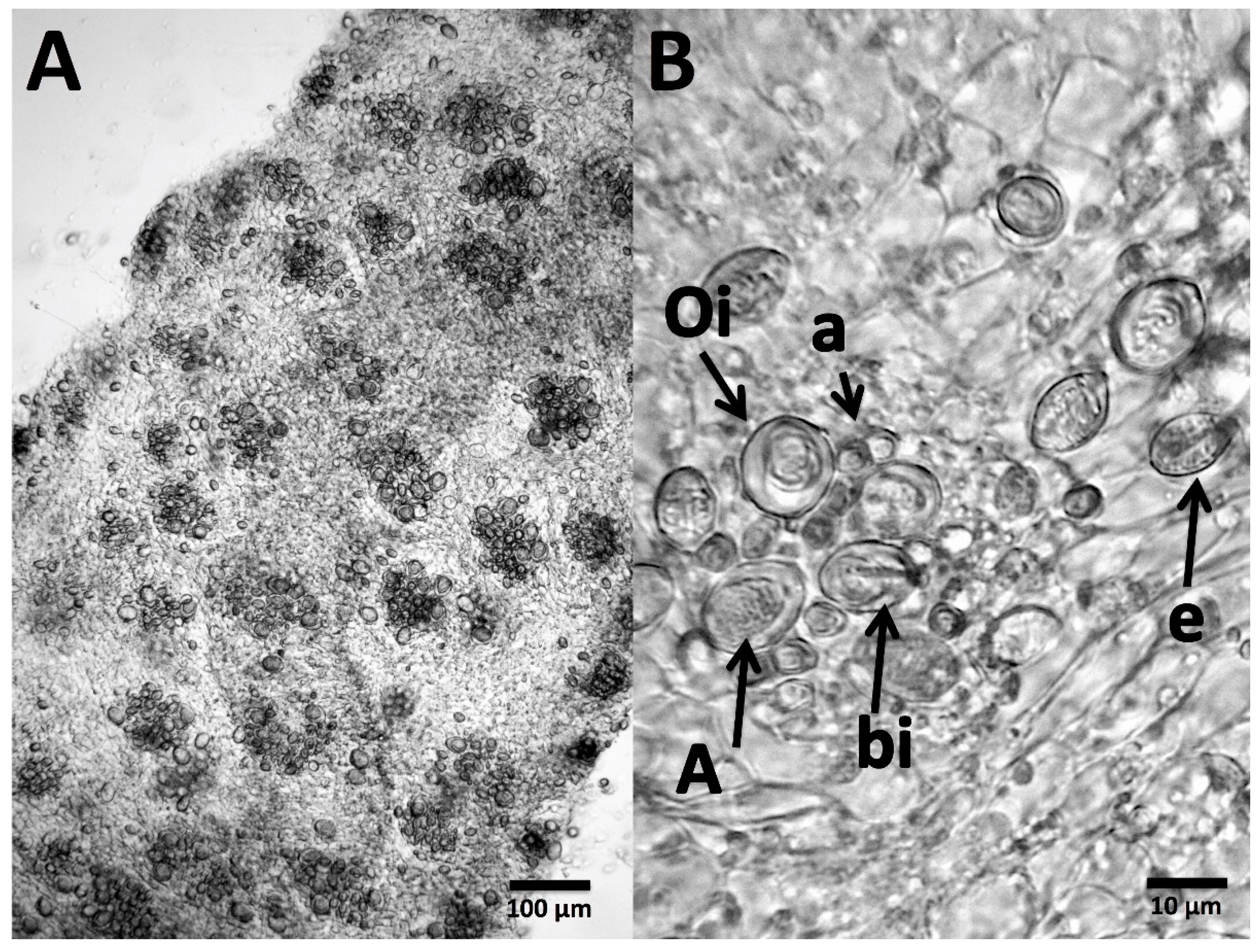
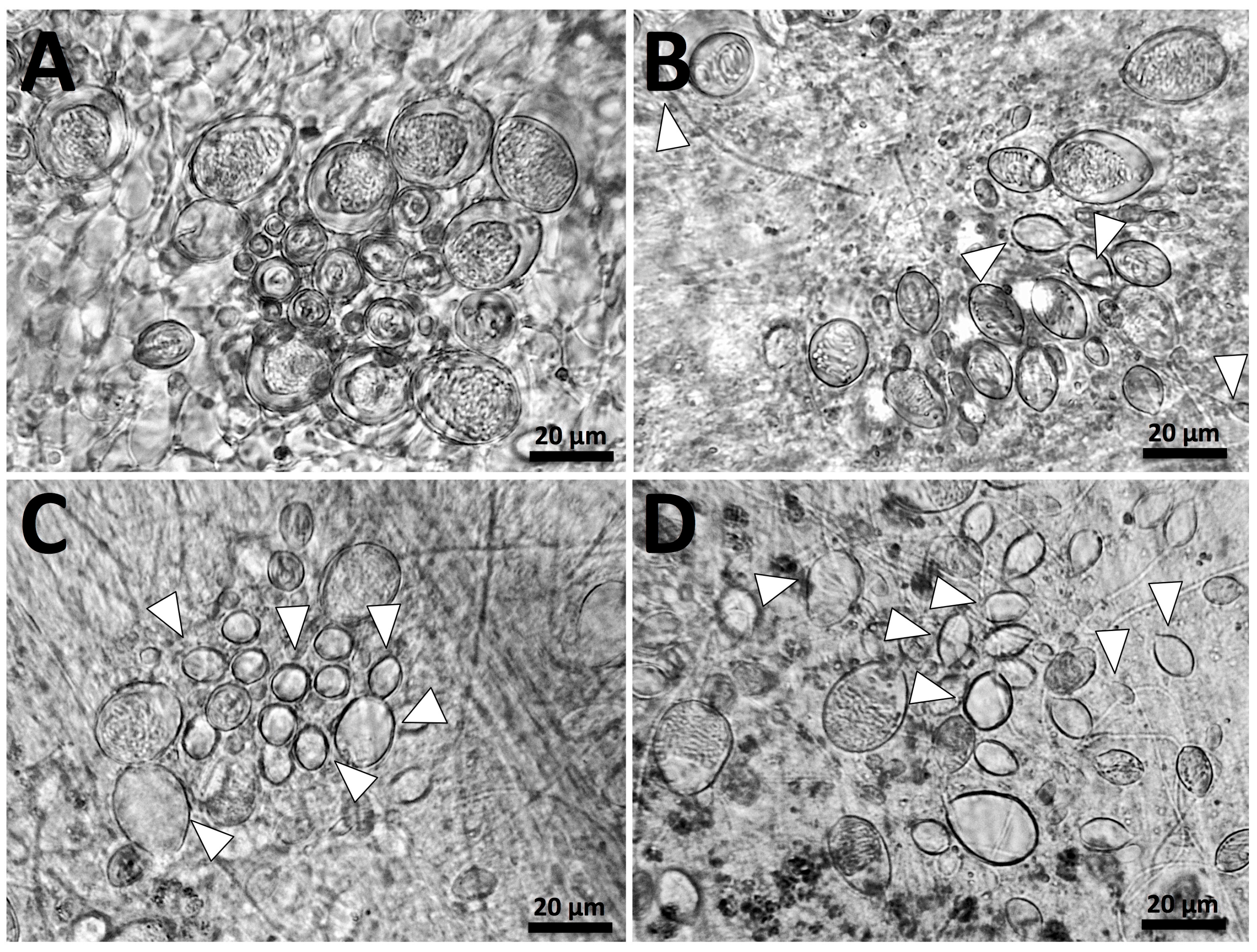
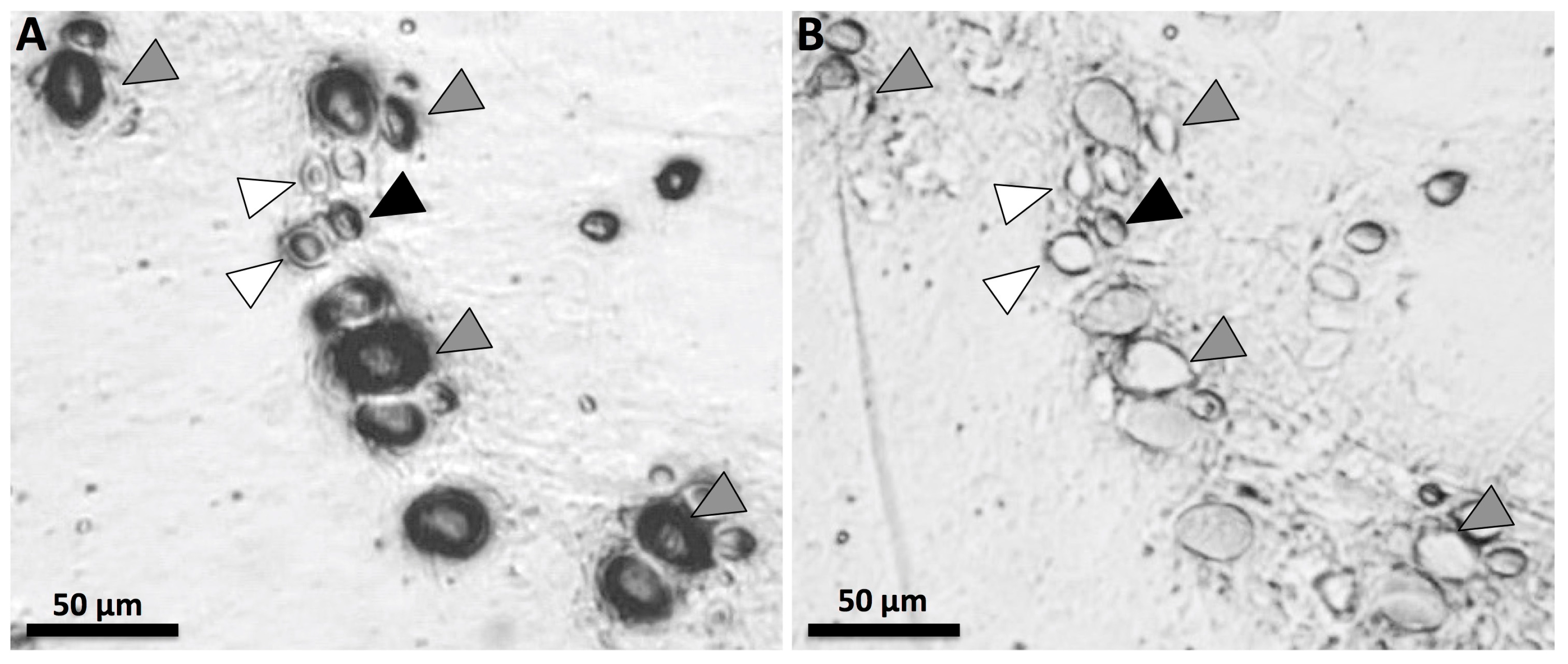
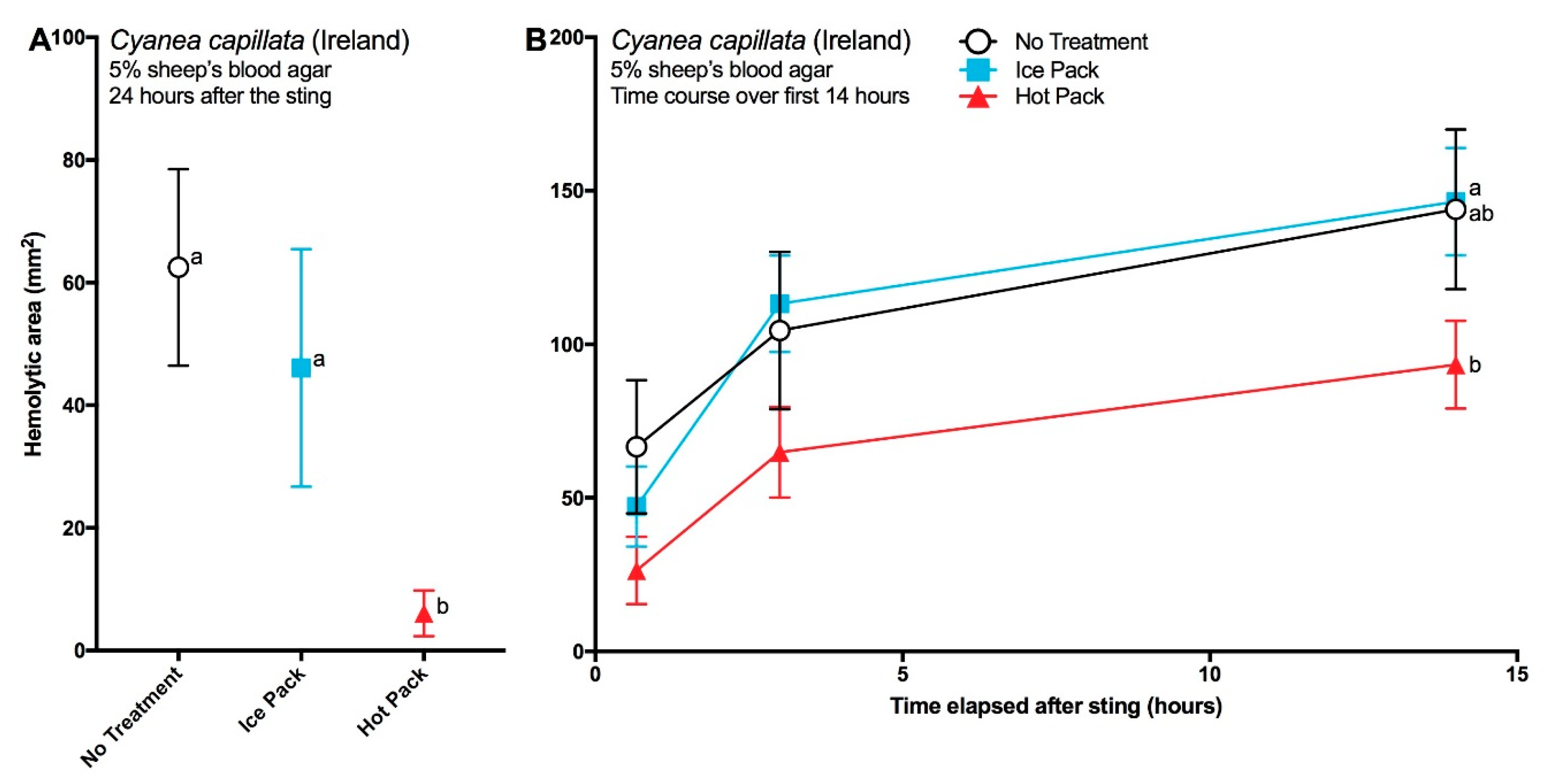
2. Results
2.1. Testing of Potential Rinse Solutions Using the Tentacle Solution Assay
2.2. Testing of First Aid Measures Using Functional Models
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Collection
5.2. Tentacle Solution Assay (TSA) and In Vitro Tests
5.3. Evaluating First Aid Measures with Functional Assays (Tentacle Skin Blood Agarose Assay, or TSBAA)
5.4. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| TSA | Tentacle Solution Assay |
| CEBM | Centre for Evidence-Based Medicine |
| TBAA | Tentacle Blood Agarose Assay |
| TSBAA | Tentacle Skin Blood Agarose Assay |
References
- Barz, K.; Hirche, H.-J. Abundance, distribution and prey composition of scyphomedusae in the southern North Sea. Mar. Biol. 2007, 151, 1021–1033. [Google Scholar] [CrossRef]
- Dawson, M.N. Cyanea capillata is not a cosmopolitan jellyfish: Morphological and molecular evidence for C. annaskala and C. rosea (Scyphozoa: Semaeostomeae: Cyaneidae) in south-eastern Australia. Invertebr. Syst. 2005, 19, 361–370. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.K.; Houghton, J.D.R.; Buckley, S.M.; Hays, G.C.; Davenport, J. The broad-scale distribution of five jellyfish species across a temperate coastal environment. Hydrobiologia 2007, 579, 29–39. [Google Scholar] [CrossRef]
- Kolbasova, G.D.; Zalevsky, A.O.; Gafurov, A.R.; Gusev, P.O.; Ezhova, M.A.; Zheludkevich, A.A.; Konovalova, O.P.; Kosobokova, K.N.; Kotlov, N.U.; Lanina, N.O.; et al. A new species of Cyanea jellyfish sympatric to C. capillata in the White Sea. Pol. Biol. 2015, 38, 1439–1451. [Google Scholar] [CrossRef]
- Lord, R.E.; Wilks, S.L.B. A case of jellyfish sting simulating an acute abdomen. Lancet 1918, 192, 390. [Google Scholar] [CrossRef]
- Russell, F.S. Vol II: Pelagic Scyphozoa with a supplement to the first volume on hydromedusae. In The Medusae of the British Isles; Cambridge University Press: Cambridge, UK, 1970. [Google Scholar]
- Bastian, T.; Haberlin, D.; Purcell, J.E.; Hays, G.C.; Davenport, J.; McAllen, R.; Doyle, T.K. Large-scale sampling reveals the spatio-temporal distributions of the jellyfish Aurelia aurita and Cyanea capillata in the Irish Sea. Mar. Biol. 2011, 158, 2639–2652. [Google Scholar] [CrossRef]
- Burnett, J.W. Medical aspects of jellyfish envenomation: Pathogenesis, case reporting and therapy. Hydrobiologia 2001, 451, 1–9. [Google Scholar] [CrossRef]
- Burnett, J.W.; Calton, G.J. Venomous pelagic coelenterates—Chemistry, toxicology, immunology and treatment of their stings. Toxicon 1987, 25, 581–602. [Google Scholar] [CrossRef]
- Suput, D. In vivo effects of cnidarian toxins and venoms. Toxicon 2009, 54, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon 2006, 48, 830–859. [Google Scholar] [CrossRef] [PubMed]
- Tønseth, A. Health damage after jellyfish stings. J. Nor. Med. Assoc. 2007, 127, 1777–1778. (In Norwegian) [Google Scholar]
- Williamson, J.A.; Fenner, P.J.; Burnett, J.W.; Rifkin, J.F. Venomous & Poisonous Marine Animals: A Medical and Biological Handbook; Surf Life Saving Australia and University of New South Wales Press: Sydney, Australia, 1996. [Google Scholar]
- Little, M.; Pereira, P.; Carrette, T.; Seymour, J. Jellyfish responsible for irukandji syndrome. QJM Int. J. Med. 2006, 99, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.R.; Jungblut, S.; Holst, S.; Kappertz, G.; Berlitz, P.; Ohmann, T. Therapieoptionen bei vernesselungen durch quallen an deutschen küstengewässern. Notf. Rett. 2016, 1–7. (In German) [Google Scholar] [CrossRef]
- Centre for Evidence-Based Medicine. Oxford Centre for Evidence-Based Medicine—Levels of Evidence. Available online: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed on 8 February 2017).
- Cegolon, L.; Heymann, W.C.; Lange, J.H.; Mastrangelo, G. Jellyfish stings and their management: A review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.T.; Darracq, M.A.; Tomaszewski, C.; Clark, R.F. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann. Emerg. Med. 2012, 60, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Exton, D.R.; Fenner, P.J.; Williamson, J.A. Cold packs—Effective topical analgesia in the treatment of painful stings by Physalia and other jellyfish. Med. J. Aust. 1989, 151, 625–626. [Google Scholar] [PubMed]
- Fenner, P.J.; Fitzpatrick, P.F. Experiments with the nematocysts of Cyanea capillata. Med. J. Aust. 1986, 145, 174. [Google Scholar] [PubMed]
- Yanagihara, A.A.; Wilcox, C.L. Cubozoan sting-site seawater rinse, scraping, and ice can increase venom load: Upending current first aid recommendations. Toxins 2017, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- DeClerck, M.P.; Bailey, Y.; Craig, D.; Lin, M.; Auerbach, L.J.; Linney, O.; Morrison, D.E.; Patry, W.; Auerbach, P.S. Efficacy of topical treatments for Chrysaora chinensis species: A human model in comparison with an in vitro model. Wilderness Environ. Med. 2016, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Berling, I.; Isbister, G. Marine envenomations reply. Aust. Fam. Physician 2015, 44, 168. [Google Scholar]
- Burnett, J.W. Treatment of Atlantic cnidarian envenomations. Toxicon 2009, 54, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- TOXBASE Entry for Jelly Fish Sting (UK Waters). Available online: http://toxbase.org (accessed on 28 July 2016).
- Auerbach, P.S. Envenomations from jellyfish and related species. J. Emerg. Nurs. 1997, 23, 555–565. [Google Scholar] [CrossRef]
- Honeycutt, J.D.; Jonas, C.E.; Smith, R.F. FPIN’s Clinical Inquiries: Treatment of jellyfish envenomation. Am. Fam. Physician 2014, 89, 823A–823C. Available online: http://www.aafp.org/afp/2014/0515/od1.pdf (accessed on 8 February 2017).
- Auerbach, P.S. Marine envenomations. N. Engl. J. Med. 1991, 325, 486–493. [Google Scholar] [PubMed]
- Markenson, D.; Ferguson, J.D.; Chameides, L.; Cassan, P.; Chung, K.-L.; Epstein, J.; Gonzales, L.; Herrington, R.A.; Pellegrino, J.L.; Ratcliff, N.; et al. Part 17: First Aid. Circulation 2010, 122, S934–S946. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.A.; Dart, R.C.; Staples, A.; White, J. Envenomations: An overview of clinical toxinology for the primary care physician. Am. Fam. Physician 2009, 80, 793–802. [Google Scholar] [PubMed]
- McGoldrick, J.; Marx, J.A. Marine envenomations Part 2: Invertebrates. J. Emerg. Med. 1992, 10, 71–77. [Google Scholar] [CrossRef]
- Australian Resuscitation Council. Guideline 9.4.5: Envenomation—Jellyfish Stings. Available online: https://resus.org.au/?wpfb_dl=41 (accessed on 8 February 2017).
- Gershwin, L.-A. The Jellyfish App: Sting Treatment. Available online: http://thejellyfishapp.com/treatment (accessed on 8 February 2017).
- Fenner, P.J. Marine envenomation: An update—A presentation on the current status of marine envenomation first aid and medical treatments. Emerg. Med. Aust. 2000, 12, 295–302. [Google Scholar] [CrossRef]
- Yanagihara, A.A.; Wilcox, C.; King, R.; Hurwitz, K.; Castelfranco, A.M. Experimental assays to assess the efficacy of vinegar and other topical first-aid approaches on cubozoan (Alatina alata) tentacle firing and venom toxicity. Toxins 2016, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Östman, C.; Hydman, J. Nematocyst analysis of Cyanea capillata and Cyanea lamarckii (Scyphozoa, Cnidaria). Sci. Mar. 1997, 61, 313–344. [Google Scholar]
- Burnett, J.W.; Ordonez, J.V.; Calton, G.J. Differential toxicity of Physalia physalis (Portuguese man o’ war) nematocysts separated by flow cytometry. Toxicon 1986, 24, 514–518. [Google Scholar] [CrossRef]
- Endean, R.; Rifkin, J. Isolation of different types of nematocyst from cubomedusan Chironex fleckeri. Toxicon 1975, 13, 375–376. [Google Scholar] [CrossRef]
- Helmholz, H.; Wiebring, A.; Lassen, S.; Ruhnau, C.; Schuett, C.; Prange, A. Cnidom analysis combined with an in vitro evaluation of the lytic, cyto- and neurotoxic potential of Cyanea capillata (Cnidaria: Scyphozoa). Sci. Mar. 2012, 76, 339–348. [Google Scholar] [CrossRef]
- Auerbach, P.S. In reply to evidence-based treatment of jellyfish stings in North America and Hawaii. Ann. Emerg. Med. 2013, 61, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.L.; Headlam, J.L.; Doyle, T.K.; Yanagihara, A.A. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins 2017, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.L.; Yanagihara, A.A. Heated debates: Hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins 2016, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Loten, C.; Stokes, B.; Worsley, D.; Seymour, J.E.; Jiang, S.; Isbister, G.K. Randomised controlled trial of hot water (45°C) immersion versus ice packs for pain relief in bluebottle stings. Med. J. Aust. 2006, 184, 329–333. [Google Scholar] [PubMed]
- Nomura, J.T.; Sato, R.L.; Ahern, R.M.; Snow, J.L.; Kuwaye, T.T.; Yamamoto, L.G. A randomized paired comparison trial of cutaneous treatments for acute jellyfish (Carybdea alata) stings. Am. J. Emerg. Med. 2002, 20, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.S.; Scott, S.A.; Galanis, D.J.; Goto, R.S. Box jellyfish (Carybdea alata) in Waikiki: Their influx cycle plus the analgesic effect of hot and cold packs on their stings to swimmers at the beach: A randomized, placebo-controlled, clinical trial. Hawaii Med. J. 2001, 60, 100–107. [Google Scholar] [PubMed]
- Muirhead, D. Applying pain theory in fish spine envenomation. J. S. Pac. Underw. Med. Soc. 2002, 32, 150–153. [Google Scholar]
- Marino, A.; Crupi, R.; Rizzo, G.; Morabito, R.; Musci, G.; La Spada, G. The unusual toxicity and stability properties of crude venom from isolated nematocysts of Pelagia noctiluca (Cnidaria, Scyphozoa). Cell. Mol. Biol. 2007, 53, 994–1002. [Google Scholar]
- Morabito, R.; Costa, R.; Rizzo, V.; Remigante, A.; Nofziger, C.; La Spada, G.; Marino, A.; Paulmichl, M.; Dossena, S. Crude venom from nematocysts of Pelagia noctiluca (Cnidaria: Scyphozoa) elicits a sodium conductance in the plasma membrane of mammalian cells. Sci. Rep. 2017, 7, 41065. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.H.; Marr, A.G.M. Sea wasp (Chironex fleckeri) venom: Lethal, haemolytic and dermonecrotic properties. Toxicon 1969, 7, 195–210. [Google Scholar] [CrossRef]
- Carrette, T.J.; Cullen, P.; Little, M.; Peiera, P.L.; Seymour, J.E. Temperature effects on box jellyfish venom: A possible treatment for envenomed patients? Med. J. Aust. 2002, 177, 654–655. [Google Scholar] [PubMed]
- Chung, J.J.; Ratnapala, L.A.; Cooke, I.M.; Yanagihara, A.A. Partial purification and characterization of a hemolysin (CAH1) from Hawaiian box jellyfish (Carybdea alata) venom. Toxicon 2001, 39, 981–990. [Google Scholar] [CrossRef]
- Endean, R.; Henderson, L. Further studies of toxic material from nematocysts of cubomedusan Chironex fleckeri Southcott. Toxicon 1969, 7, 303–314. [Google Scholar] [CrossRef]
- Koyama, T.; Noguchi, K.; Matsuzaki, T.; Sakanashi, M.; Nakasone, J.; Miyagi, K.; Sakanashi, M.; Sakanashi, M. Haemodynamic effects of the crude venom from nematocysts of the box-jellyfish Chiropsalmus quadrigatus (Habu-kurage) in anaesthetized rabbits. Toxicon 2003, 41, 621–631. [Google Scholar] [CrossRef]
- Monastyrnaya, M.M.; Zykova, T.A.; Apalikova, O.V.; Shwets, T.V.; Kozlovskaya, E.P. Biologically active polypeptides from the tropical sea anemone Radianthus macrodactylus. Toxicon 2002, 40, 1197–1217. [Google Scholar] [CrossRef]
- Cuiping, L.; Pengcheng, L.; Jinhua, F.; Rongfeng, L.; Huahua, Y. Cytotoxicity of the venom from the nematocysts of jellyfish Cyanea nozakii Kishinouye. Toxicol. Ind. Health 2012, 28, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, H.; Li, C.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Isolation and characterization of lethal proteins in nematocyst venom of the jellyfish Cyanea nozakii Kishinouye. Toxicon 2010, 55, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arredondo, A.; Murillo-Esquivel, L.J.; Rojas, A.; Sanchez-Rodriguez, J. Characteristics of hemolytic activity induced by the aqueous extract of the Mexican fire coral Millepora complanata. J. Venom Anim. Toxins Incl. Trop. Dis. 2014, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Matehuala, R.; Rojas-Molina, A.; Vuelvas-Solorzano, A.A.; Garcia-Arredondo, A.; Ibarra Alvarado, C.; Olguin-Lopez, N.; Aguilar, M. Cytolytic and systemic toxic effects induced by the aqueous extract of the fire coral Millepora alcicornis collected in the Mexican Caribbean and detection of two types of cytolisins. J. Venom Anim. Toxins Incl. Trop. Dis. 2015, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.-T.; Lee, H.; Kim, J.-S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. C 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Isolation and in vitro partial characterization of hemolytic proteins from the nematocyst venom of the jellyfish Stomolophus meleagris. Toxicol. In Vitro 2013, 27, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Valveri, N.; Muia, C.; Crupi, R.; Rizzo, G.; Musci, G.; La Spada, G. Cytotoxicity of the nematocyst venom from the sea anemone Aiptasia mutabilis. Comp. Biochem. Physiol. C 2004, 139, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Sakanashi, M.; Matsuzaki, T.; Nakasone, J.; Sakanashi, M.; Koyama, T.; Hamadate, N.; Sakanashi, M. Cardiovascular effects and lethality of venom from nematocysts of the box-jellyfish Chiropsalmus quadrigatus (Habu-kurage) in anaesthetized rats. Toxicon 2005, 45, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Seymour, J.E. In vitro effects on human heart and skeletal cells of the venom from two cubozoans, Chironex fleckeri and Carukia barnesi. Toxicon 2013, 76, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J. The Global Problem of Cnidarian (Jellyfish) Stings. M.D. Thesis, University of London, London, UK, 1997. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyle, T.K.; Headlam, J.L.; Wilcox, C.L.; MacLoughlin, E.; Yanagihara, A.A. Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In Vitro Envenomation Models. Toxins 2017, 9, 215. https://doi.org/10.3390/toxins9070215
Doyle TK, Headlam JL, Wilcox CL, MacLoughlin E, Yanagihara AA. Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In Vitro Envenomation Models. Toxins. 2017; 9(7):215. https://doi.org/10.3390/toxins9070215
Chicago/Turabian StyleDoyle, Thomas K., Jasmine L. Headlam, Christie L. Wilcox, Eoin MacLoughlin, and Angel A. Yanagihara. 2017. "Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In Vitro Envenomation Models" Toxins 9, no. 7: 215. https://doi.org/10.3390/toxins9070215
APA StyleDoyle, T. K., Headlam, J. L., Wilcox, C. L., MacLoughlin, E., & Yanagihara, A. A. (2017). Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In Vitro Envenomation Models. Toxins, 9(7), 215. https://doi.org/10.3390/toxins9070215





