The Putative Histone Methyltransferase DOT1 Regulates Aflatoxin and Pathogenicity Attributes in Aspergillus flavus
Abstract
:1. Introduction
2. Results
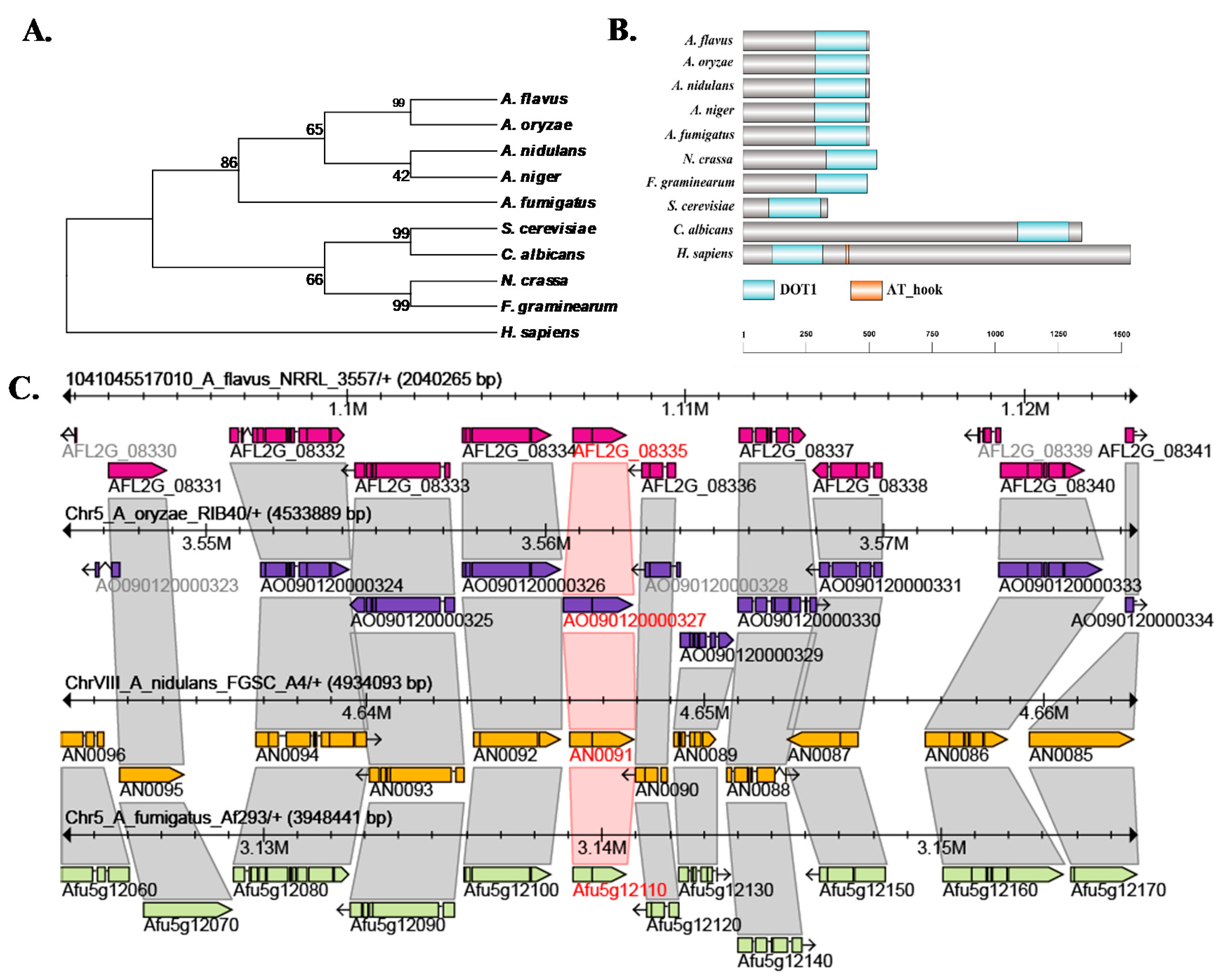
2.1. Characterization of Histone H3K79 Methyltransferase in A. flavus
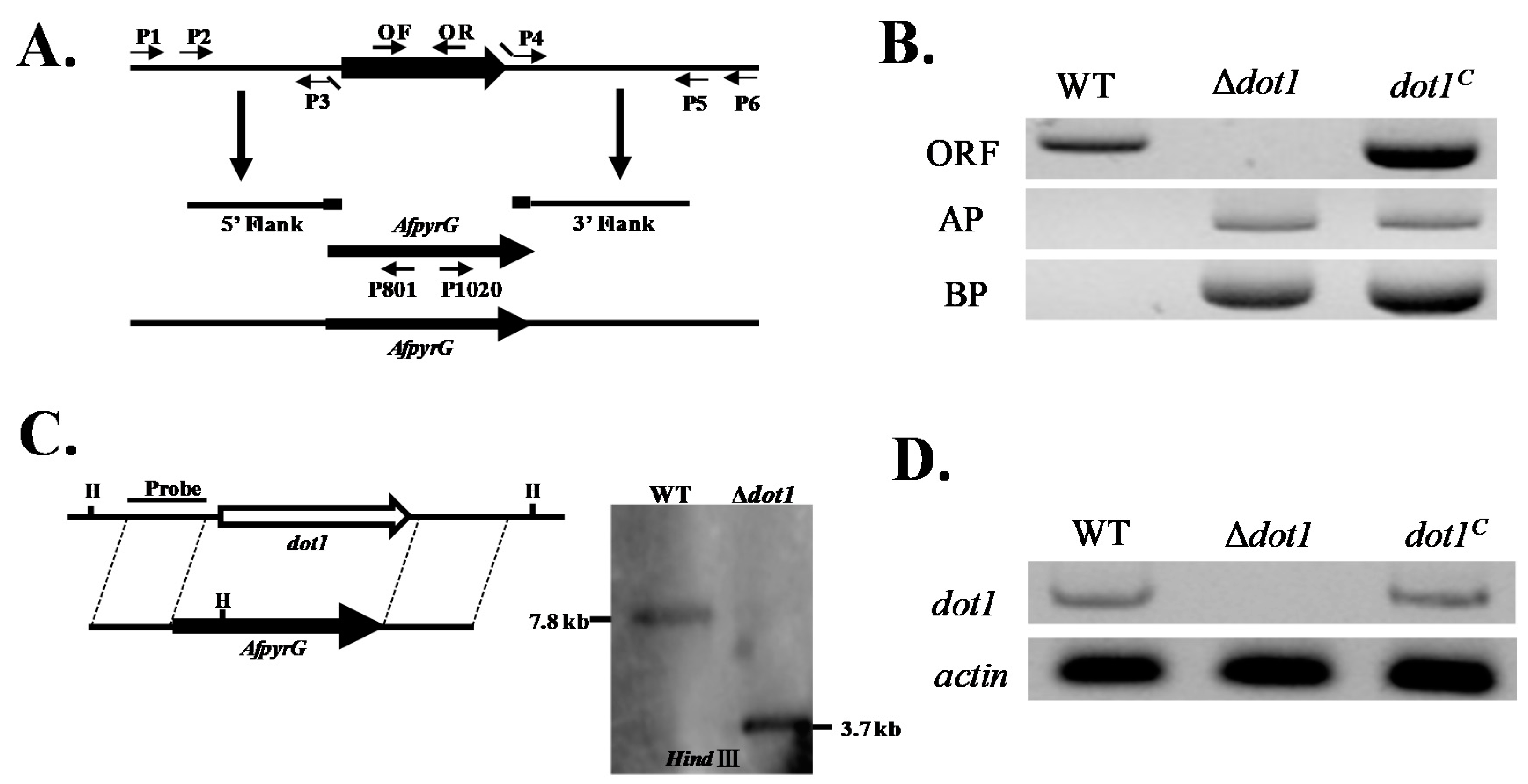
2.2. Construction of the Deleted (Δdot1) and Complemented (dot1C) Mutant Strains
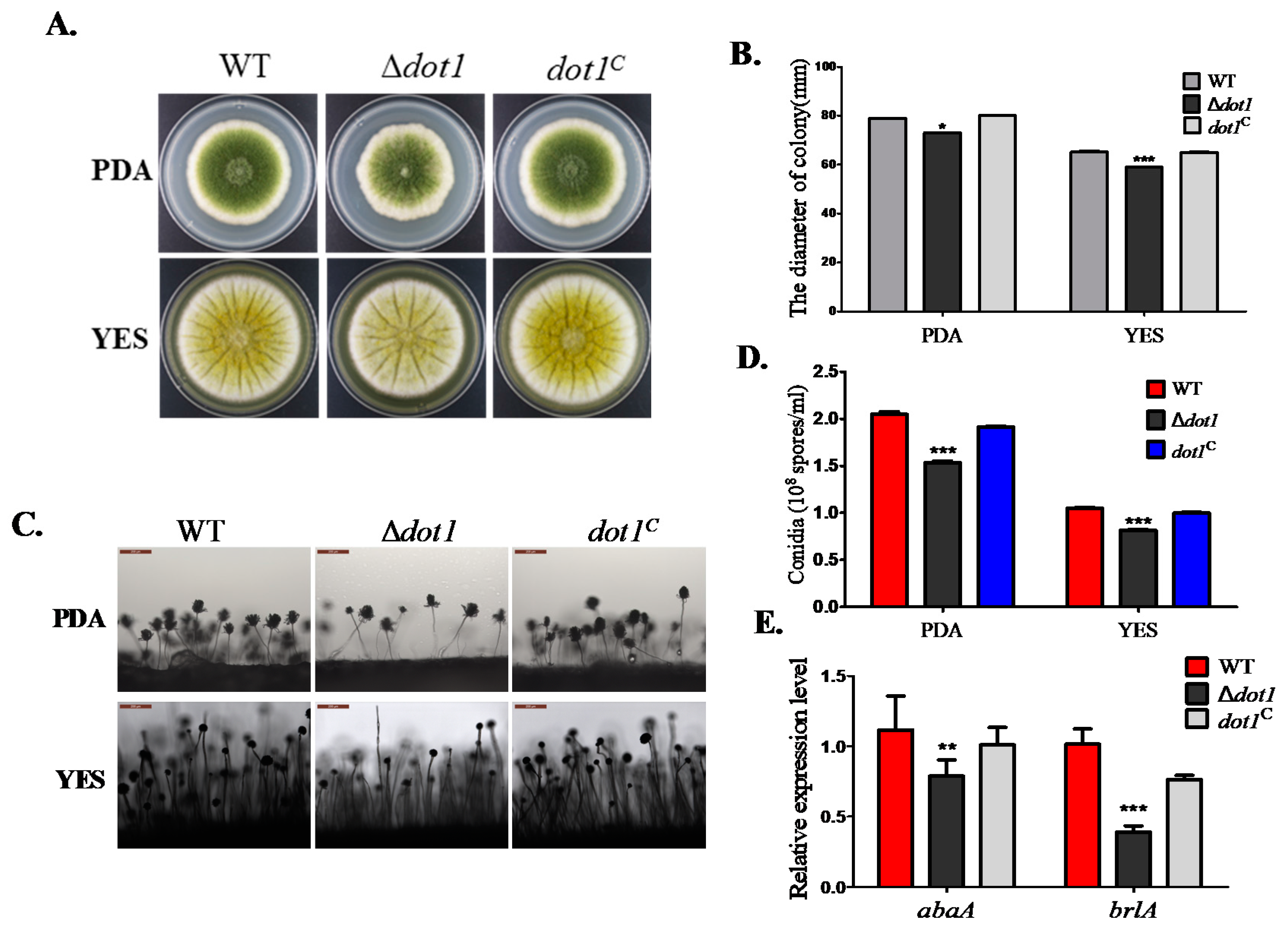
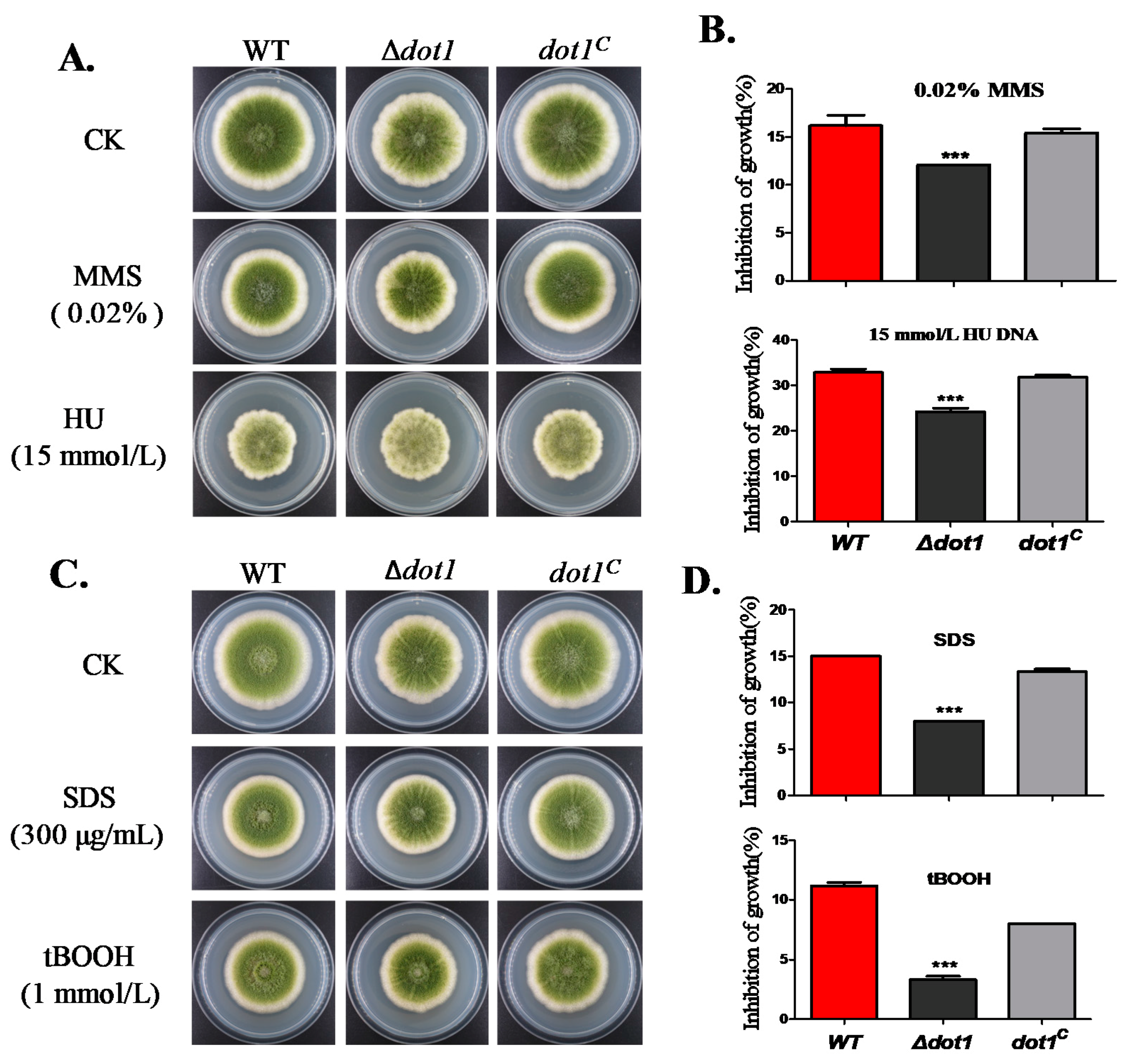
2.3. Dot1 Is Involved in Fungal Growth and Sporulation
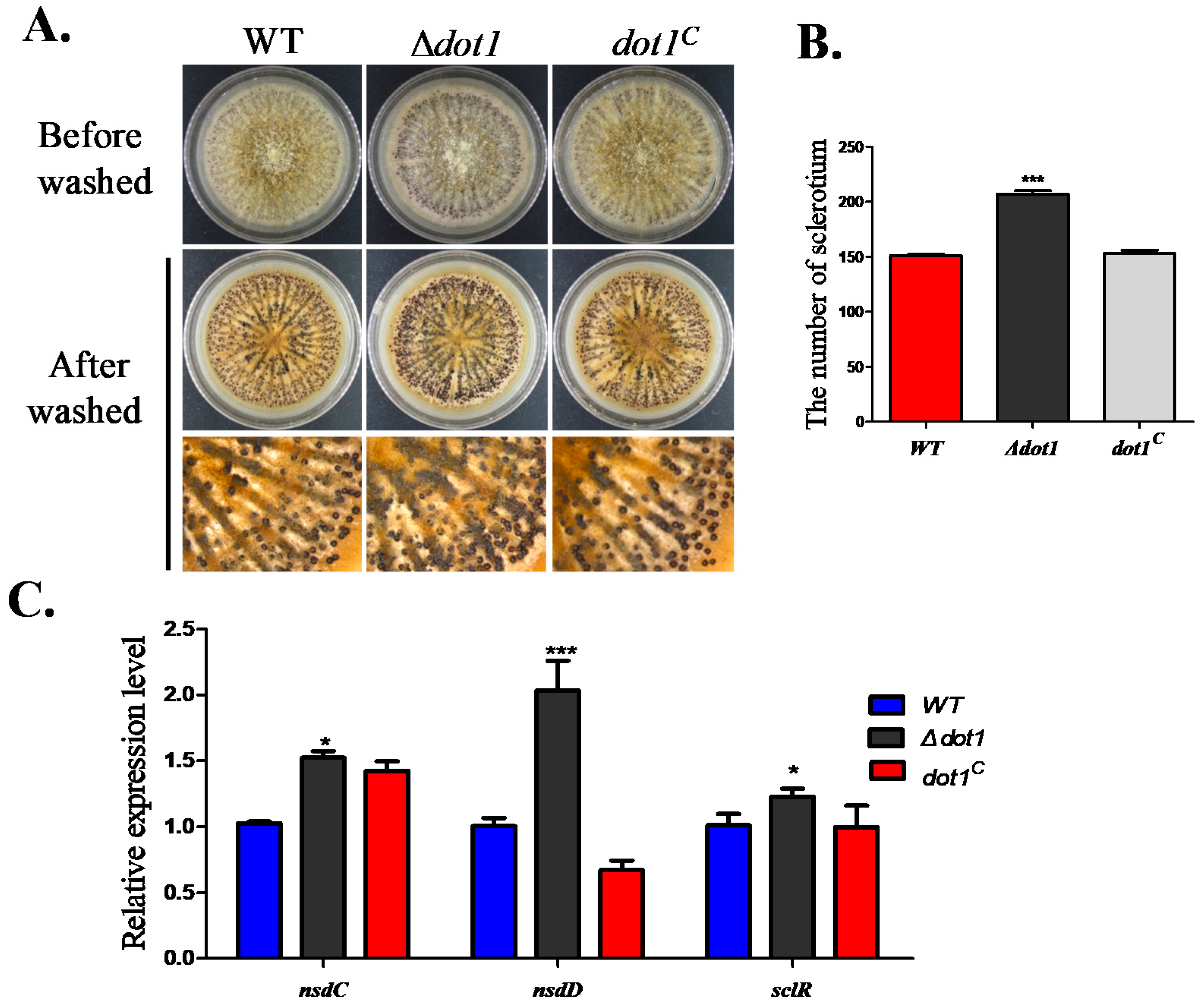
2.4. Dot1 Plays a Negative Role in Sclerotial Reproduction in A. flavus
2.5. Dot1 Response to Multiple Stresses in A. flavus
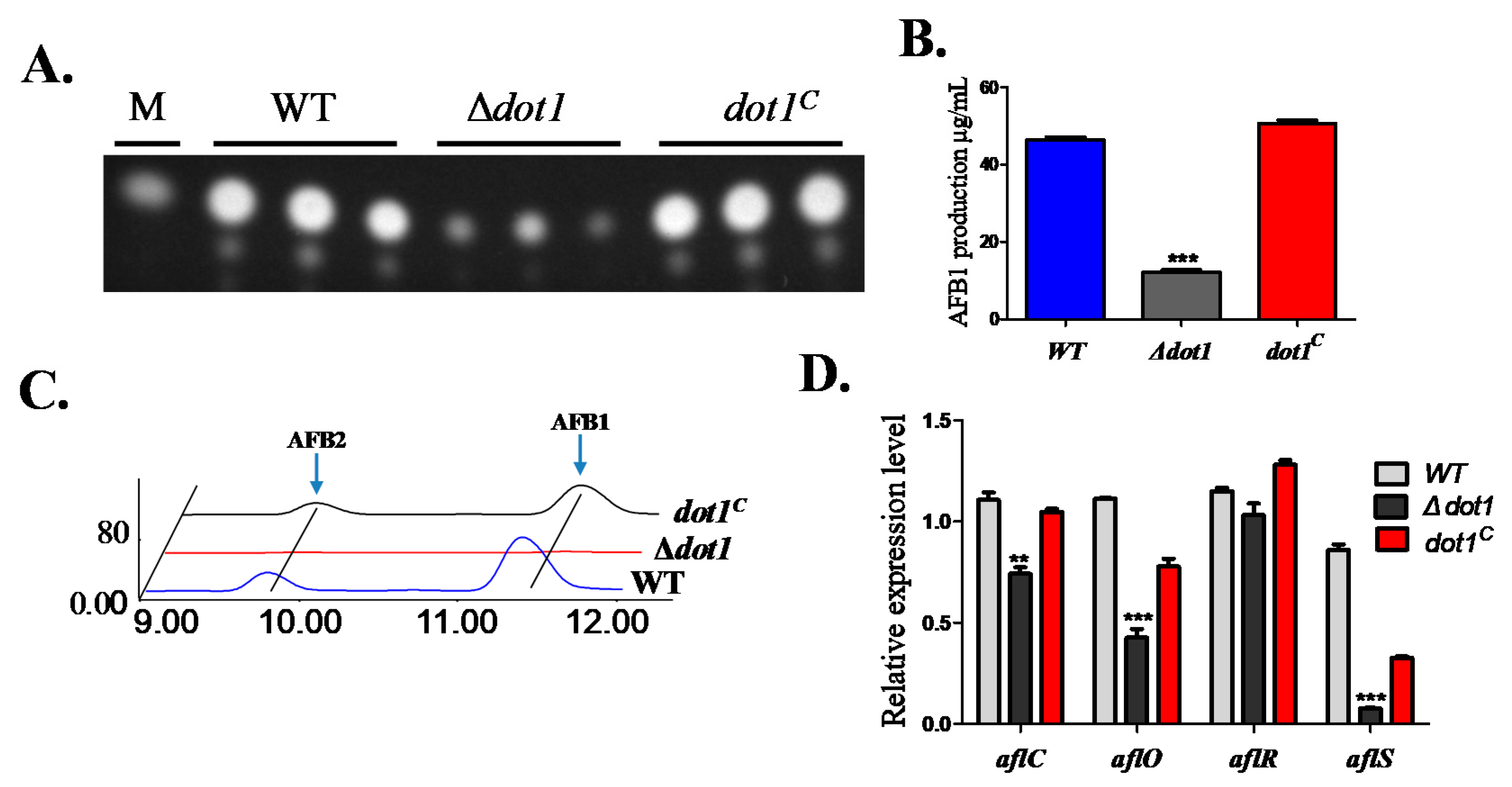
2.6. Dot1 Contributes to Aflatoxin Biosynthesis
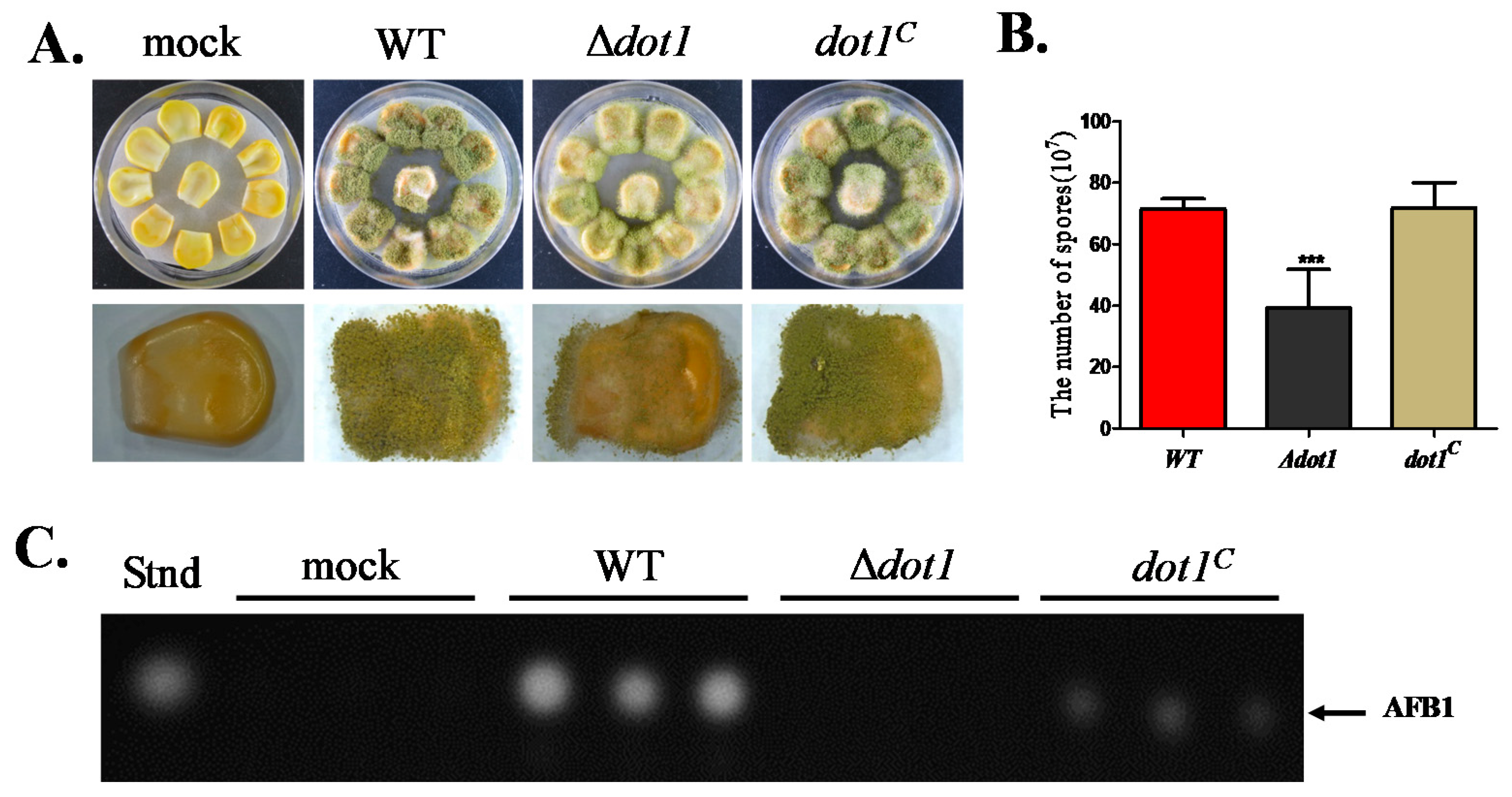
2.7. Dot1 Contributes to t Crop Seeds Colonization
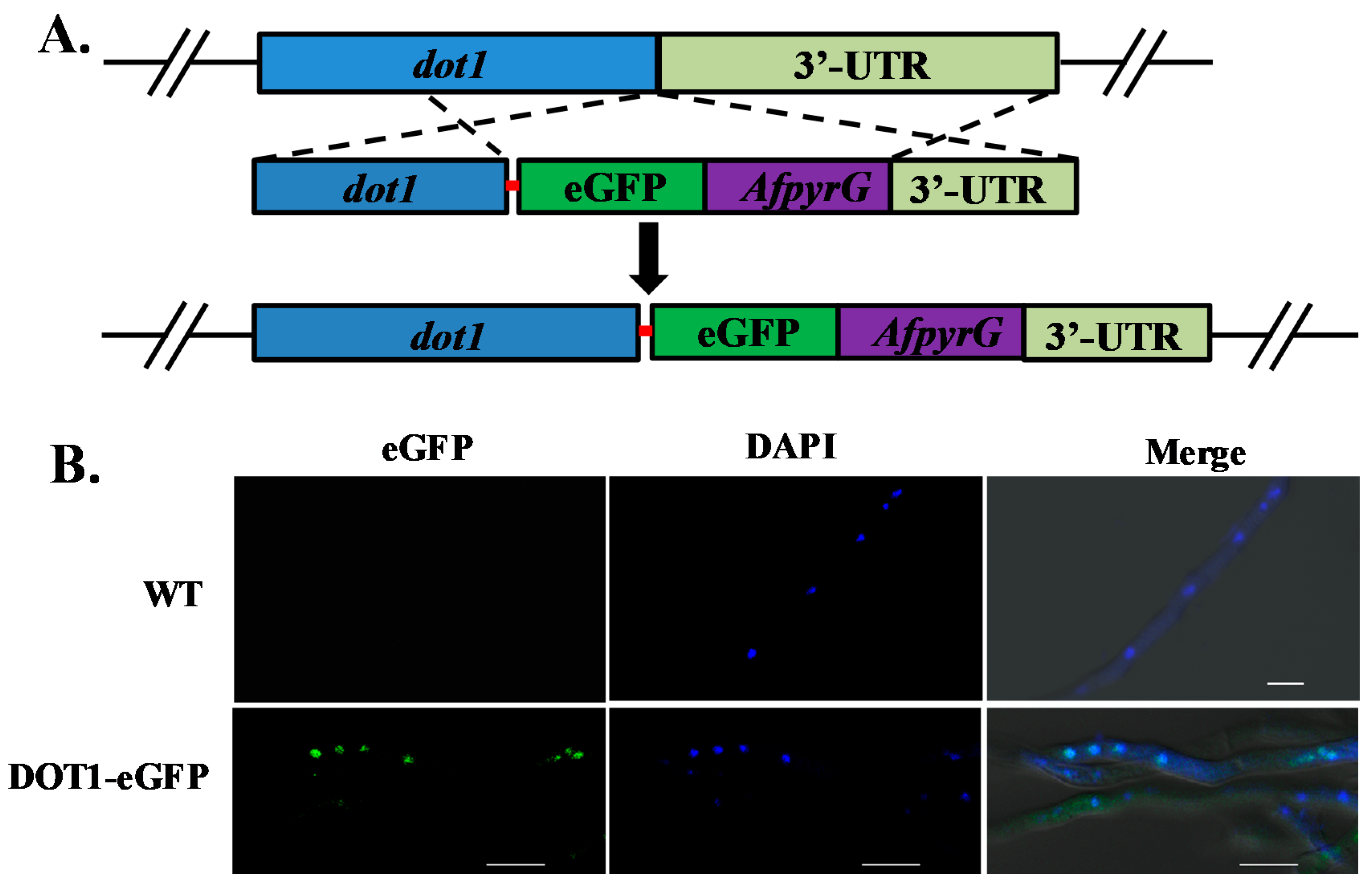
2.8. Subcellular Localization of Dot1 in A. flavus
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strain and Culture Conditions
5.2. Generation of Gene Deletion and Complementation Strains
5.3. Aflatoxin Analysis
5.4. Maize Corn Infection Assay
5.5. Gene Expression Detection
5.6. Generation of Dot1-eGFP Strain
5.7. Microscopic Determination of Dot1-eGFP Location
5.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amaike, S.; Keller, N.P. Aspergillus flavus. Ann. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhuang, Z.; Zhang, F.; Song, F.; Zhong, H.; Ran, F.; Yu, S.; Xu, G.; Lan, F.; Wang, S. Inhibition of aflatoxin metabolism and growth of Aspergillus flavus in liquid culture by a DNA methylation inhibitor. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Shier, W.T.; Plasencia, J.; Weaver, M.A.; Bellaloui, N.; Kotowicz, J.K.; Butler, A.M.; Accinelli, C.; de la Torre-Hernandez, M.E.; Zablotowicz, R.M. Mycotoxin contamination in corn smut (ustilago maydis) galls in the field and in the commercial food products. Food Control 2017, 71, 57–63. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Yu, S.; Qiu, M.; Wang, X.; Wang, Y.; Bai, Y.; Zhang, F.; Wang, S. Aspergillus flavus sumo contributes to fungal virulence and toxin attributes. J. Agric. Food Chem. 2016, 64, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, Y.; Liang, L.; Li, Z.; Qin, Q.; Nie, X.; Wang, S. The high-affinity phosphodiesterase pdeh regulates development and aflatoxin biosynthesis in Aspergillus flavus. Fungal Genet. Biol. FG B 2017, 101, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Chalivendra, S.C.; DeRobertis, C.; Chang, P.K.; Damann, K.E. Cyclopiazonic acid is a pathogenicity factor for Aspergillus flavus and a promising target for screening germplasm for ear rot resistance. Mol. Plant Microbe Interact. MPMI 2017, 30, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Bosso, L.; Lacatena, F.; Varlese, R.; Nocerino, S.; Cristinzio, G.; Russo, D. Plant pathogens but not antagonists change in soil fungal communities across a land abandonment gradient in a mediterranean landscape. Acta Oecol. 2017, 78, 1–6. [Google Scholar] [CrossRef]
- Gacek-Matthews, A.; Berger, H. Kdmb, a jumonji histone h3 demethylase, regulates genome-wide h3k4 trimethylation and is required for normal induction of secondary metabolism in aspergillus nidulans. PLoS Genet. 2016, 12, e1006222. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Sun, R.; Fan, K.; Yang, K.; Zhang, F.; Nie, X.Y.; Wang, X.; Zhuang, Z.; Wang, S. The Aspergillus flavus histone acetyltransferase aflgcne regulates morphogenesis, aflatoxin biosynthesis, and pathogenicity. Front. Microbiol. 2016, 7, 1324. [Google Scholar] [CrossRef] [PubMed]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Connolly, L.R.; Smith, K.M.; Freitag, M. The fusarium graminearum histone h3 k27 methyltransferase kmt6 regulates development and expression of secondary metabolite gene clusters. PLoS Genet. 2013, 9, e1003916. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Ku, M.; Jaffe, D.B.; Issac, B.; Lieberman, E.; Giannoukos, G.; Alvarez, P.; Brockman, W.; Kim, T.K.; Koche, R.P.; et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 2007, 448, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Gacek, A.; Strauss, J. The chromatin code of fungal secondary metabolite gene clusters. Appl. Microbiol. Biotechnol. 2012, 95, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Reyes-Dominguez, Y. Regulation of secondary metabolism by chromatin structure and epigenetic codes. Fungal Genet. Biol. FG B 2011, 48, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lewis, Z.A.; Honda, S.; Khlafallah, T.K.; Jeffress, J.K.; Freitag, M.; Mohn, F.; Schubeler, D.; Selker, E.U. Relics of repeat-induced point mutation direct heterochromatin formation in neurospora crassa. Genome Res. 2009, 19, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Adhvaryu, K.K.; Morris, S.A.; Strahl, B.D.; Selker, E.U. Methylation of histone h3 lysine 36 is required for normal development in neurospora crassa. Eukaryot. Cell 2005, 4, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.M.; Mallaredy, S.; Perry, D.W.; Sanchez, J.F.; Theisen, J.M.; Szewczyk, E.; Oakley, B.R.; Wang, C.C.; Keller, N.P.; Mirabito, P.M. Telomere position effect is regulated by heterochromatin-associated proteins and nkua in aspergillus nidulans. Microbiology 2010, 156, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.H.; Schulze, J.M.; Jackson, J.; Hentrich, T.; Seidel, C.; Jaspersen, S.L.; Kobor, M.S.; Shilatifard, A. Dot1 and histone h3k79 methylation in natural telomeric and hm silencing. Mol. Cell 2011, 42, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Osborne, E.A.; Dudoit, S.; Rine, J. The establishment of gene silencing at single-cell resolution. Nat. Genet. 2009, 41, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, N.; Utley, R.T.; Hunter, J.M.; Poirier, G.G.; Cote, J. Disruptor of telomeric silencing-1 is a chromatin-specific histone h3 methyltransferase. J. Biol. Chem. 2002, 277, 30421–30424. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Feng, Q.; Wang, H.; Erdjument-Bromage, H.; Tempst, P.; Zhang, Y.; Struhl, K. Lysine methylation within the globular domain of histone h3 by dot1 is important for telomeric silencing and sir protein association. Genes Dev. 2002, 16, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Y.; Li, X.; Fasoyin, O.E.; Hu, Y.; Liu, Y.; Yuan, J.; Zhuang, Z.; Wang, S. Histone methyltransferase aflrmta gene is involved in the morphogenesis, mycotoxin biosynthesis, and pathogenicity of Aspergillus flavus. Toxicon 2017, 127, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Gacek-Matthews, A.; Noble, L.M.; Gruber, C.; Berger, H.; Sulyok, M.; Marcos, A.T.; Strauss, J.; Andrianopoulos, A. Kdma, a histone h3 demethylase with bipartite function, differentially regulates primary and secondary metabolism in aspergillus nidulans. Mol. Microbiol. 2015, 96, 839–860. [Google Scholar] [CrossRef] [PubMed]
- Govindaraghavan, M.; Anglin, S.L.; Osmani, A.H.; Osmani, S.A. The set1/compass histone h3 methyltransferase helps regulate mitosis with the cdk1 and nima mitotic kinases in aspergillus nidulans. Genetics 2014, 197, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Rossodivita, A.A.; Boudoures, A.L.; Mecoli, J.P.; Steenkiste, E.M.; Karl, A.L.; Vines, E.M.; Cole, A.M.; Ansbro, M.R.; Thompson, J.S. Histone h3 k79 methylation states play distinct roles in uv-induced sister chromatid exchange and cell cycle checkpoint arrest in saccharomyces cerevisiae. Nucleic Acids Res. 2014, 42, 6286–6299. [Google Scholar] [CrossRef] [PubMed]
- Croken, M.M.; Nardelli, S.C.; Kim, K. Chromatin modifications, epigenetics, and how protozoan parasites regulate their lives. Trends Parasitol. 2012, 28, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Ontoso, D.; Acosta, I.; van Leeuwen, F.; Freire, R.; San-Segundo, P.A. Dot1-dependent histone h3k79 methylation promotes activation of the mek1 meiotic checkpoint effector kinase by regulating the hop1 adaptor. PLoS Genet. 2013, 9, e1003262. [Google Scholar] [CrossRef] [PubMed]
- Bani Ismail, M.; Shinohara, M.; Shinohara, A. Dot1-dependent histone h3k79 methylation promotes the formation of meiotic double-strand breaks in the absence of histone h3k4 methylation in budding yeast. PLoS ONE 2014, 9, e96648. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Adams, T.H. Complex control of the developmental regulatory locus brla in aspergillus nidulans. Mol. Genet. Genom. MGG 2001, 266, 260–270. [Google Scholar]
- Yang, K.; Liang, L.; Ran, F.; Liu, Y.; Li, Z.; Lan, H.; Gao, P.; Zhuang, Z.; Zhang, F.; Nie, X.; et al. The dmta methyltransferase contributes to Aspergillus flavus conidiation, sclerotial production, aflatoxin biosynthesis and virulence. Sci. Rep. 2016, 6, 23259. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Dominguez, Y.; Bok, J.W.; Berger, H.; Shwab, E.K.; Basheer, A.; Gallmetzer, A.; Scazzocchio, C.; Keller, N.; Strauss, J. Heterochromatic marks are associated with the repression of secondary metabolism clusters in aspergillus nidulans. Mol. Microbiol. 2010, 76, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.T.; Inoue, Y.; Vu, B.V.; Nguyen, H.H.; Nakayashiki, T.; Ikeda, K.; Nakayashiki, H. Moset1 (histone h3k4 methyltransferase in magnaporthe oryzae) regulates global gene expression during infection-related morphogenesis. PLoS Genet. 2015, 11, e1005385. [Google Scholar]
- Liu, Y.; Liu, N.; Yin, Y.; Chen, Y.; Jiang, J.; Ma, Z. Histone h3k4 methylation regulates hyphal growth, secondary metabolism and multiple stress responses in fusarium graminearum. Environ. Microbiol. 2015, 17, 4615–4630. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.; Scharfenstein, L.L.; Mack, B.; Ehrlich, K.C. Deletion of the Aspergillus flavus orthologue of a. Nidulans flug reduces conidiation and promotes production of sclerotia but does not abolish aflatoxin biosynthesis. Appl. Environ. Microbiol. 2012, 78, 7557–7563. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.; Scharfenstein, L.L.; Wei, Q.; Bhatnagar, D. Development and refinement of a high-efficiency gene-targeting system for Aspergillus flavus. J. Microbiol. Methods 2010, 81, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Tsitsigiannis, D.I.; Keller, N.P. Oxylipins act as determinants of natural product biosynthesis and seed colonization in aspergillus nidulans. Mol. Microbiol. 2006, 59, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qin, Q.; Liu, Y.; Zhang, L.; Liang, L.; Lan, H.; Chen, C.; You, Y.; Zhang, F.; Wang, S. Adenylate cyclase acya regulates development, aflatoxin biosynthesis and fungal virulence in Aspergillus flavus. Front. Cell. Infect. Microbiol. 2016, 6, 190. [Google Scholar] [CrossRef] [PubMed]
| Strain | Genotype Description | Reference |
|---|---|---|
| A. flavus CA14 PTs | Δku70, ΔpyrG | [38] |
| wild-type | Δku70, ΔpyrG::AfpyrG | This study |
| Δdot1 | Δku70, Δdot1::AfpyrG | This study |
| Δdot1C | Δku70, Δdot1:: AfpyrG, dot1(p)::dot1::ptrA | This study |
| Gdot1 | Δku70, dot1(p)::dot1-egfp::AfpyrG | This study |
| Primers | Sequence (5′-3′) | Application |
|---|---|---|
| dot1/P1 | CATTGAGATGGACGAGGACG | dot1 deletion and probe |
| dot1/P3 | GGGTGAAGAGCATTGTTTGAGGCGTTGACCGAGCGGAAATGT | |
| dot1/P4 | GCATCAGTGCCTCCTCTCAGACCACTACGCGGTTACCGAGAC | |
| dot1/P6 | CGACAGAAAGCCATAATGAAAT | |
| dot1/P2 | CGAGGACGACAATGACTACAC | |
| dot1/P5 | GAGTTGAAGGGAAAGGCTAAA | |
| PyrG/F | GCCTCAAACAATGCTCTTCACCC | pyrG selective marker |
| PyrG/R | GTCTGAGAGGAGGCACTGATGC | |
| P801/R | CAGGAGTTCTCGGGTTGTCG | |
| dot1-ORF/F | TTCTTACAGTATCCGAGTGC | dot1 mutant screen |
| dot1-ORF/R | TTCATGTCCAGGAAGTGGTT | |
| CM-dot1/F | CTATGACCATGATTACGCCAACTATGACCATGATTACGCCAAGCTTAGCCTAAGCAGCAGGTGAAGC | dot1 complementation construct |
| CM-dot1/R | CCAGTGAATTCGAGCTCGGTACCGATGGCAAGGATGGGCAAAG | |
| eGFP-pyrG/F | GGAGCTGGTGCAGGCGCTGGAGCCGGTGCCATGGTGAGCAAGGGCGAGGA | egfp |
| eGFP-pyrG/R | GGGTGAAGAGCATTGTTTGAGGCTTACTTGTACAGCTCGTCCATG | |
| dot1-eGFP/P1 | ATTCTTACAGTATCCGAGTGC | dot1-gfp tag construct |
| dot1-eGFP/P3 | GGCTCCAGCGCCTGCACCAGCTCCACCCATGCTCTCTGCAAAAG | |
| dot1-eGFP/P2 | AAAATTACATACCGGAGGACG |
| Primers | Sequence (5′-3′) | Application |
|---|---|---|
| brlA/QF | GCCTCCAGCGTCAACCTTC | brlA qRT-PCR |
| brlA/QR | TCTCTTCAAATGCTCTTGCCTC | |
| abaA/QF | CACGGAAATCGCCAAAGAC | abaA qRT-PCR |
| abaA/QR | TGCCGGAATTGCCAAAG | |
| nsdC/QF | GCCAGACTTGCCAATCAC | nsdC qRT-PCR |
| nsdC/QR | CATCCACCTTGCCCTTTA | |
| nsdD/QF | GGACTTGCGGGTCGTGCTA | nsdD qRT-PCR |
| nsdD/QR | AGAACGCTGGGTCTGGTGC | |
| sclR/QF | CAATGAGCCTATGGGAGTGG | sclR qRT-PCR |
| sclR/QR | ATCTTCGCCCGAGTGGTT | |
| aflC/QF | GTGGTGGTTGCCAATGCG | aflC qRT-PCR |
| aflC/QR | CTGAAACAGTAGGACGGGAGC | |
| aflR/QF | AAAGCACCCTGTCTTCCCTAAC | aflR qRT-PCR |
| aflR/QR | GAAGAGGTGGGTCAGTGTTTGTAG | |
| aflS/QF | CGAGTCGCTCAGGCGCTCAA | aflS qRT-PCR |
| aflS/QR | GCTCAGACTGACCGCCGCTC | |
| aflO/QF | GATTGGGATGTGGTCATGCGATT | aflO qRT-PCR |
| aflO/QR | GCCTGGGTCCGAAGAATGC | |
| Actin/QF | ACGGTGTCGTCACAAACTGG | actin qRT-PCR |
| Actin/QR | CGGTTGGACTTAGGGTTGATAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Liu, Y.; Yang, K.; Lin, G.; Xu, Z.; Lan, H.; Wang, X.; Wang, S. The Putative Histone Methyltransferase DOT1 Regulates Aflatoxin and Pathogenicity Attributes in Aspergillus flavus. Toxins 2017, 9, 232. https://doi.org/10.3390/toxins9070232
Liang L, Liu Y, Yang K, Lin G, Xu Z, Lan H, Wang X, Wang S. The Putative Histone Methyltransferase DOT1 Regulates Aflatoxin and Pathogenicity Attributes in Aspergillus flavus. Toxins. 2017; 9(7):232. https://doi.org/10.3390/toxins9070232
Chicago/Turabian StyleLiang, Linlin, Yinghang Liu, Kunlong Yang, Guinan Lin, Zhangling Xu, Huahui Lan, Xiuna Wang, and Shihua Wang. 2017. "The Putative Histone Methyltransferase DOT1 Regulates Aflatoxin and Pathogenicity Attributes in Aspergillus flavus" Toxins 9, no. 7: 232. https://doi.org/10.3390/toxins9070232





