A Review of Automated Microinjection of Zebrafish Embryos
Abstract
:1. Introduction
1.1. Background of Automated Microinjection
1.2. Key Issues in ZEM
1.3. Current State of Experimental Research on ZEM
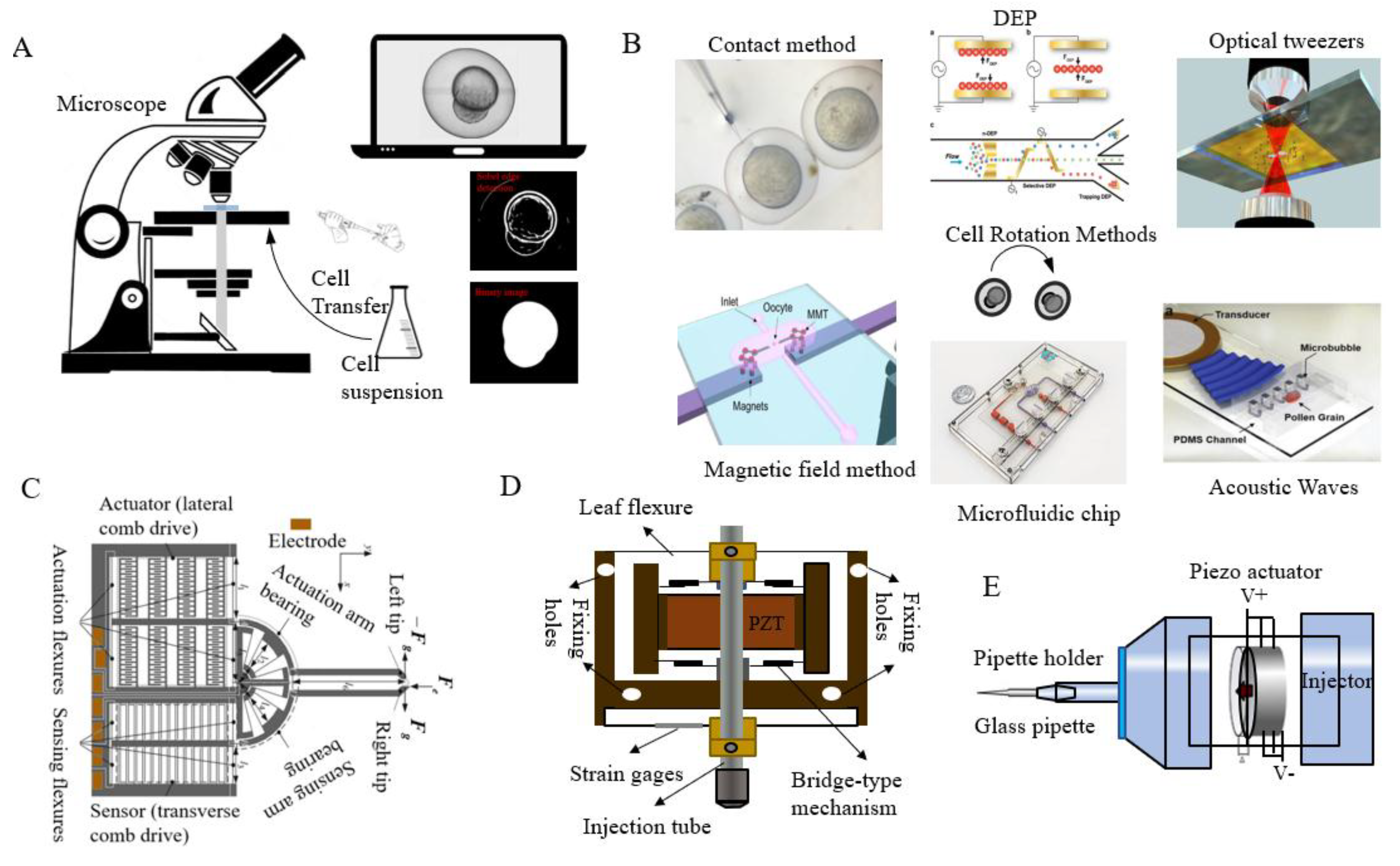
2. Cell Searching and Positioning
2.1. Cell and Injection Needle Identification
2.2. Autofocusing
- There are local extreme points. The actual focusing process is prone to falling into the local extremum and thus causes the focusing to fail. This problem is usually solved by improving the focus search strategy and finding an ideal focus evaluation function.
- Objective quantitative evaluation metrics are unavailable for evaluating the focusing function, and the specific value cannot be reflected by the performance of the focus curve function.
- It is difficult to balance speed and accuracy in the focus search strategy. The small size of the target object requires high accuracy, which is difficult to meet while operating at the required speed.
2.3. Cell Posture Adjustment Methods
2.3.1. The Contact Method
2.3.2. The Non-Contact Method
2.4. The Microfluidic Method
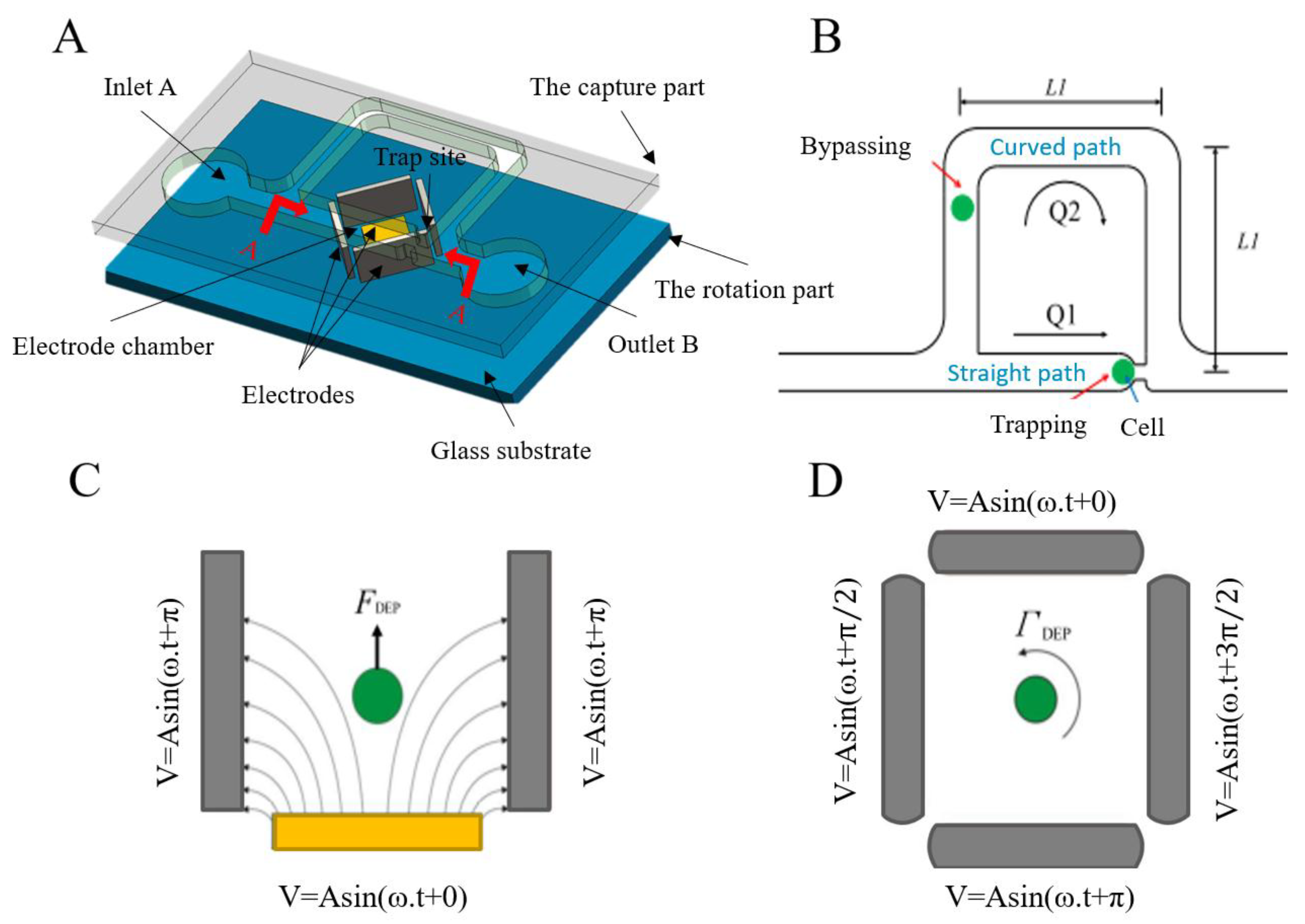
2.5. DEP
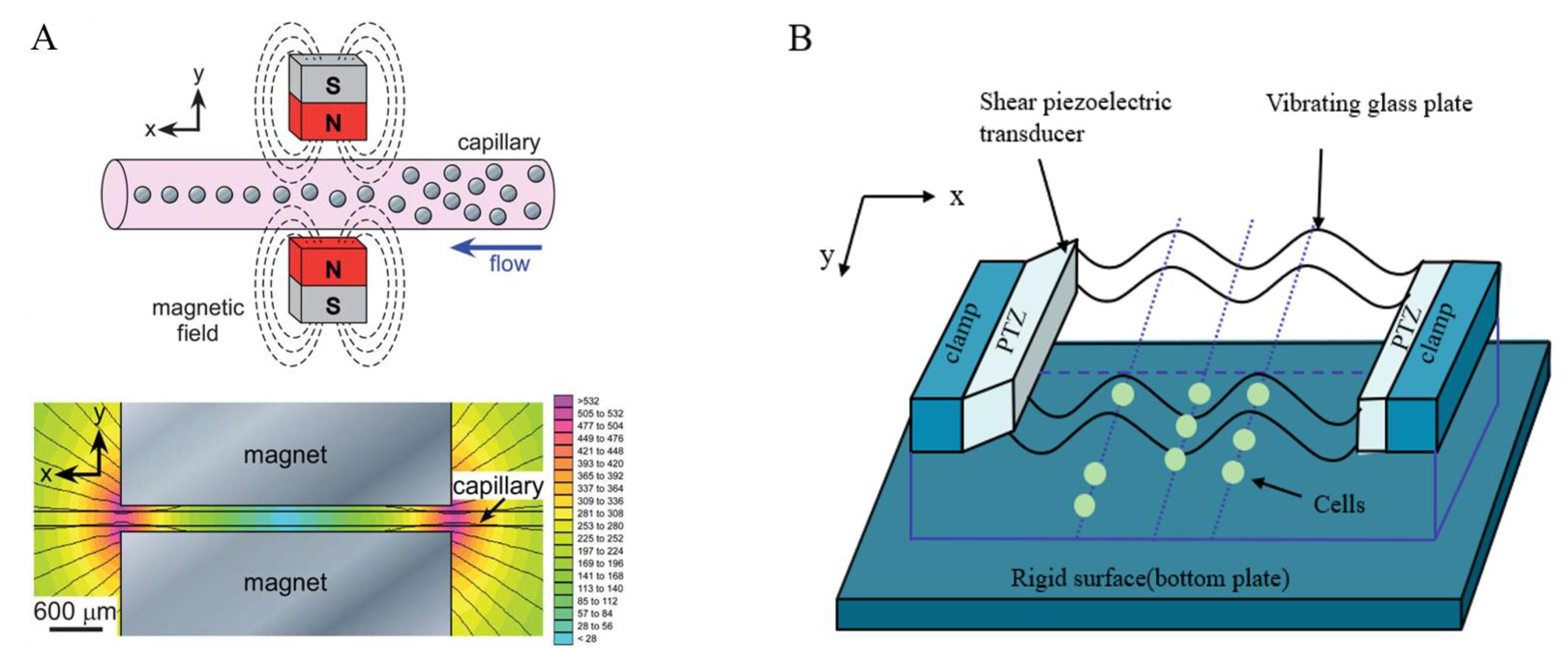
2.6. The Magnetic Field Method
2.7. The Ultrasound Method
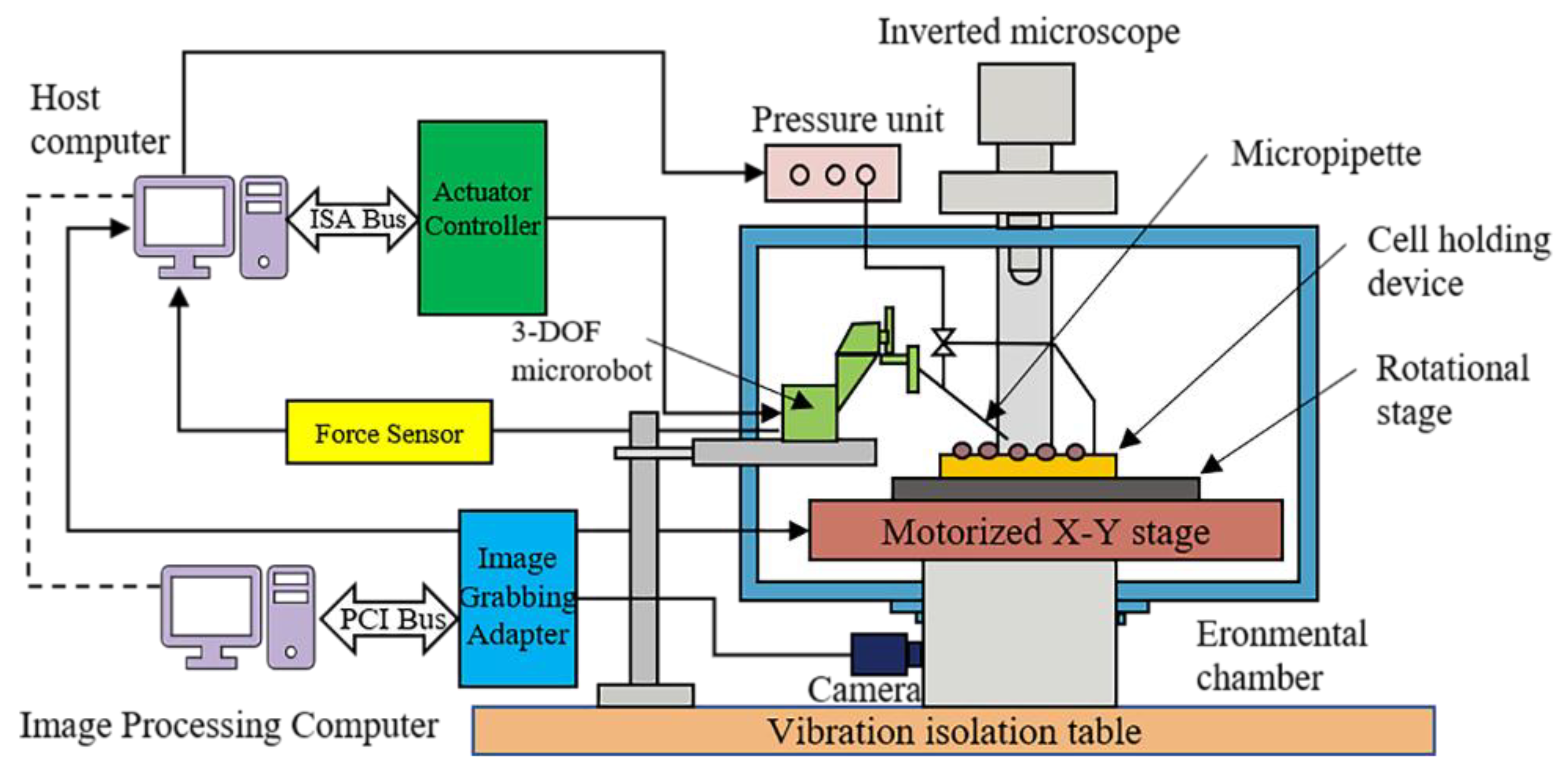
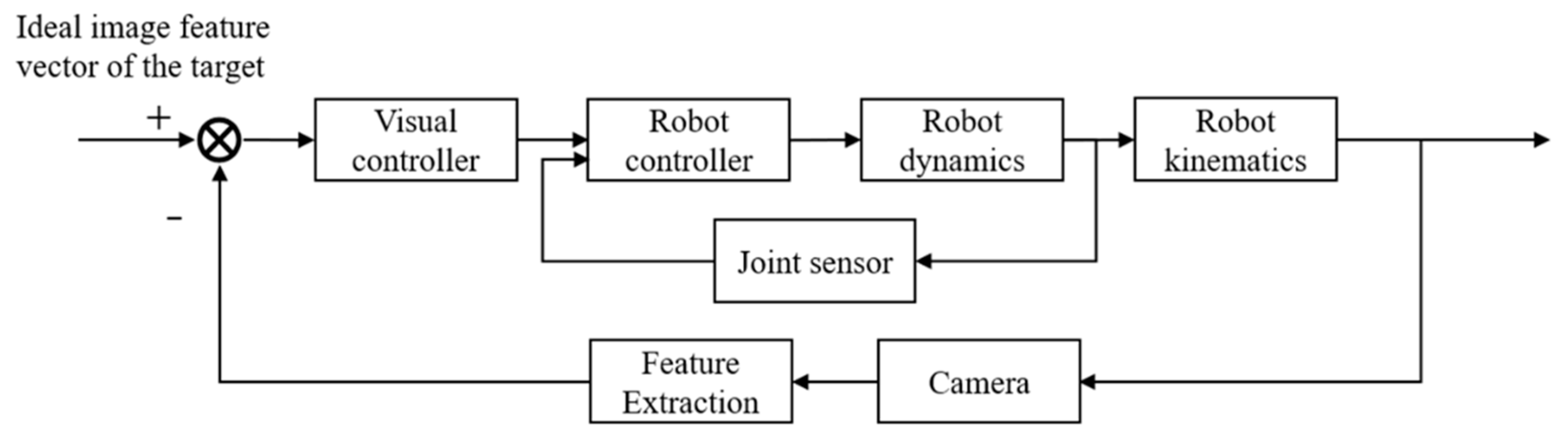
3. Microscopic Visual Servoing System
- Because a closed loop is formed in the image space, image-based visual servoing control is insensitive to calibration and spatial model errors to ensure a high level of control precision. The error is calculated directly in a 2D image space, without requiring any 3D reconstruction.
- Image-based visual servoing control is generally required to calculate the depth information of the target, to calibrate the internal and external parameters of the camera, and to perform hand–eye calibration. In particular, the image Jacobian matrix is difficult to obtain, and the inaccuracy of this matrix also makes it difficult to perform stability analysis on the system, which hinders the controller design process and leads to control system instability.
- The selection of image features has a significant effect on how well the control system performs. Selecting image features with high robustness and weak coupling is a key consideration in working with an image-based visual control system.
4. Actuator
5. Microsensor Detection System
6. Characterization of Cell Models
7. Puncture and Injection
8. Conclusions and Prospects
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kuncova, J.; Kallio, P. Challenges in capillary pressure microinjection. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 7, 4998–5001. [Google Scholar] [CrossRef] [PubMed]
- Iritani, A. Micromanipulation of gametes for in vitro assisted fertilization. Mol. Reprod. Dev. 1991, 28, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rols, M.P. Electropermeabilization, a physical method for the delivery of therapeutic molecules into cells. Biochim. Biophys. Acta 2006, 1758, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, K.; Dechev, N.; Burke, R.D.; Park, E.J. Development of an autonomous biological cell manipulator with single-cell electroporation and visual servoing capabilities. IEEE Trans. Biomed. Eng. 2009, 56, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Walther, W.; Stein, U. Viral Vectors for Gene Transfer. Drugs 2000, 60, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Kalia, Y.N.; Naik, A.; Garrison, J.; Guy, R.H. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 619–658. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.S.; Pulkkinen, L.; Kyonggeun, Y. The gene gun: Current application in cutaneous gene therapy. J. Pak. Assoc. Dermatol. 2002, 12, 167–170. [Google Scholar] [CrossRef]
- Sundaram, J.; Mellein, B.R.; Mitragotri, S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys. J. 2003, 84, 3087–3101. [Google Scholar] [CrossRef]
- Unger, E.C.; Hersh, E.; Vannan, M.; McCreery, T. Gene Delivery Using Ultrasound Contrast Agents. Echocardiography 2001, 18, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Lavitrano, M.; Busnelli, M.; Cerrito, M.G.; Giovannoni, R.; Manzini, S.; Vargiolu, A. Sperm-mediated gene transfer. Reprod. Fertil. Dev. 2006, 18, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Song, Y.K.; Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999, 6, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Budker, V.; Wolff, J.A. High Levels of Foreign Gene Expression in Hepatocytes after Tail Vein Injections of Naked Plasmid DNA. Hum. Gene Ther. 1999, 10, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Zanetti, C.; Blatz, A.; Khine, M. Electrophoresis-assisted single-cell electroporation for efficient intracellular delivery. Biomed. Microdevices 2008, 10, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, J.; Nolkrantz, K.; Ryttsén, F.; Lambie, B.A.; Weber, S.G.; Orwar, O. Single-cell electroporation. Anal. Bioanal. Chem. 2010, 397, 3235–3248. [Google Scholar] [CrossRef]
- Gao, X.; Kim, K.-S.; Liu, D. Nonviral gene delivery: What we know and what is next. AAPS J. 2007, 9, E92–E104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, D.; Saltzman, W.M. Synthetic DNA delivery systems. Nat. Biotechnol. 2000, 18, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Wu, T.H.; Clemens, D.L.; Lee, B.Y.; Wen, X.; Horwitz, M.A.; Teitell, M.A.; Chiou, P.Y. Massively parallel delivery of large cargo into mammalian cells with light pulses. Nat. Methods 2015, 12, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, Y.; Yan, L.; Kwok, S.Y.; Li, W.; Wang, Z.; Zhu, X.; Zhu, G.; Zhang, W.; Chen, X.; et al. Poking cells for efficient vector-free intracellular delivery. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Navarro, J.; Risco, R.; Toschi, M.; Schattman, G. Gene Therapy and Intracytoplasmatic Sperm Injection (ICSI)—A Review. Placenta 2008, 29, 193–199. [Google Scholar] [CrossRef]
- Graf, S.F.; Madigou, T.; Li, R.; Chesné, C.; Stemmer, A.; Knapp, H.F. Fully Automated Microinjection System for Xenopus laevis Oocytes With Integrated Sorting and Collection. J. Lab. Autom. 2011, 16, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Villefranc, J.A.; Amigo, J.; Lawson, N.D. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 2007, 236, 3077–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Sar, A.M.; Musters, R.J.P.; van Eeden, F.J.M.; Appelmelk, B.J.; Vandenbroucke-Grauls, C.M.J.E.; Bitter, W. Zebrafish embryos as a model host for the real time analysis of Salmonella typhimurium infections. Cell. Microbiol. 2003, 5, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Görge, G.; Nagel, R. Toxicity of lindane, atrazine, and deltamethrin to early life stages of zebrafish (Brachydanio rerio). Ecotoxicol. Environ. Saf. 1990, 20, 246–255. [Google Scholar] [CrossRef]
- Xu, H.; Yang, M.; Qiu, W.; Pan, C.; Wu, M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ. Toxicol. Chem. 2013, 32, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Figueras, A. Zebrafish: Model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 2012, 946, 253–275. [Google Scholar] [PubMed]
- Xiang, J.; Yang, H.; Che, C.; Zou, H.; Yang, H.; Wei, Y.; Quan, J.; Zhang, H.; Yang, Z.; Lin, S. Identifying tumor cell growth inhibitors by combinatorial chemistry and zebrafish assays. PLoS ONE 2009, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nelson, B.J. Microrobotic cell injection. Proc. IEEE Int. Conf. Robot. Autom. 2001, 1, 620–625. [Google Scholar] [CrossRef]
- Matsuoka, H.; Komazaki, T.; Mukai, Y.; Shibusawa, M.; Akane, H.; Chaki, A.; Uetake, N.; Saito, M. High throughput easy microinjection with a single-cell manipulation supporting robot. J. Biotechnol. 2005, 116, 185–194. [Google Scholar] [CrossRef]
- Ammi, M.; Ferreira, A. Realistic visual and haptic rendering for biological-cell injection. Proc. IEEE Int. Conf. Robot. Autom. 2005, 2005, 918–923. [Google Scholar] [CrossRef]
- Li, X.; Zong, G.; Bi, S. Development of Global Vision System for Biological Automatic Micro-Manipulation System. In Proceedings of the 2001 ICRA IEEE International Conference on Robotics and Automation (Cat. No.01CH37164), Seoul, Korea, 21–26 May 2001; pp. 127–132. [Google Scholar]
- Kapoor, A.; Taylor, R.H. Preliminary Experiments in RobotMuman Cooperative Microinjection. In Proceedings of the 2003 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2003) (Cat. No.03CH37453), Las Vegas, NV, USA, 27–31 October 2003. [Google Scholar]
- Huang, H.; Sun, D.; Mills, J.K.; Li, W.J.; Cheng, S.H. Visual-Based Impedance Control of Out-of-Plane Cell Injection Systems. Science 2009, 6, 543–549. [Google Scholar] [CrossRef]
- Huang, H.; Sun, D.; Mills, J.K.; Li, W.J. Visual-based impedance force control of three-dimensional cell injection system. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Roma, Italy, 10–14 April 2007; pp. 4196–4201. [Google Scholar] [CrossRef]
- Xu, Q. Design, Fabrication, and Testing of an MEMS Microgripper with Dual-Axis Force Sensor. IEEE Sens. 2015, 15, 6017–6026. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Q. Design and Precision Position/Force Control of a Piezo-Driven Microinjection System. IEEE/ASME Trans. Mechatron. 2017, 22, 1744–1754. [Google Scholar] [CrossRef]
- Huang, H.B.; Su, H.; Chen, H.Y.; Mills, J.K. Piezoelectric driven non-toxic injector for automated cell manipulation. Stud. Health Technol. Inform. 2011, 163, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Sun, Y.; Yun, S.; Kim, B.; Hwang, C.N.; Lee, S.H.; Nelson, B.J. Mechanical property characterization of the zebrafish embryo chorion. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 7, 5061–5064. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Hwang, C.N.; Sun, Y.; Lee, S.H.; Kim, B.; Nelson, B.J. Mechanical analysis of chorion softening in prehatching stages of zebrafish embryos. IEEE Trans. Nanobiosci. 2006, 5, 89–94. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Q.; Wang, H.; Sun, T.; Yu, N.; Huang, Q.; Fukuda, T. Automated Fluidic Assembly of Microvessel-Like Structures Using a Multimicromanipulator System. IEEE/ASME Trans. Mechatronics 2018, 23, 667–678. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Gelinas, D.; Ciruna, B.; Sun, Y. A fully automated robotic system for microinjection of zebrafish embryos. PLoS ONE 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.; Xu, Q. Multiple-cell recognition and path planning for robotic microinjection system. In Proceedings of the 2017 36th Chinese Control Conference (CCC), Dalian, China, 26–28 July 2017; pp. 6691–6696. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, C.; Muruganandam, R.; Mathew, J.; Wong, P.C.; Ang, W.T.; Tan, S.Y.M.; Latt, W.T. A fully automated robotic system for three-dimensional cell rotation. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 1707–1712. [Google Scholar] [CrossRef]
- Lu, Z.; Peter, C.Y.C.; Nam, J.H.; Ge, R.; Lin, W. A micromanipulation system with dynamic force-feedback for automatic batch microinjection. 2013, 17, 14–15.
- Huang, H.; Sun, D.; Mills, J.K.; Cheng, S.H. Integrated vision and force control in suspended cell injection system: Towards automatic batch biomanipulation. Proc. IEEE Int. Conf. Robot. Autom. 2008, 3413–3418. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, M.; Zhao, X.; Zhao, B. Autonomous operating process for zebrafish embryo injection. In Proceedings of the 2012 International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Shaanxi, China, 29 August–1 September 2012; pp. 65–70. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Z.; Sun, Y. Orientation control of biological cells under inverted microscopy. IEEE/ASME Trans. Mechatron. 2011, 16, 918–924. [Google Scholar] [CrossRef]
- Sun, Y.; Nelson, B.J. Biological Cell Injection Using an Autonomous MicroRobotic System. Int. J. Robot. Res. 2002, 21, 861–868. [Google Scholar] [CrossRef]
- Wang, W.H.; Liu, X.Y.; Sun, Y. Contact detection in microrobotic manipulation. Int. J. Robot. Res. 2007, 26, 821–828. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, X.; Cheng, X.; Sun, C.; Lu, G. Key technologies of micro-manipulation system oriented complex task. In Proceedings of the CCC 2010 29th Chinese Control Conference, Beijing, China, 29–31 July 2010; pp. 3678–3683. [Google Scholar]
- Huang, H.B.; Sun, D.; Mills, J.K.; Cheng, S.H. Robotic cell injection system with position and force control: Toward automatic batch biomanipulation. IEEE Trans. Robot. 2009, 25, 727–737. [Google Scholar] [CrossRef]
- Leavers, V.F. Which Hough Transform? CVGIP Image Underst. 1993, 58, 250–264. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, C.; Wang, Y. Chord midpoint randomized Hough transform for the cell image segmentation. In Proceedings of the 2011 Cross Strait Quad-Regional Radio Science and Wireless Technology Conference, Harbin, China, 26–30 July 2011; pp. 1446–1450. [Google Scholar] [CrossRef]
- Xie, Y.; Zeng, F.; Xi, W.; Zhou, Y.; Liu, H.; Chen, M. A robot-assisted cell manipulation system with an adaptive visual servoing method. Micromachines 2016, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Mattos, L.; Grant, E.; Thresher, R. Semi-automated blastocyst microinjection. Proc. IEEE Int. Conf. Robot. Autom. 2006, 2006, 1780–1785. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Q.; Cui, M.; Sun, M.; Zhao, X. Robotic Donor Cell Injection in Somatic Cell Nuclear Transfer (SCNT). In Proceeding of the 11th World Congress on Intelligent Control and Automation, Shenyang, China, 29 June–4 July 2014; pp. 2821–2825. [Google Scholar]
- Sun, Y.; Duthaler, S.; Nelson, B.J. Autofocusing in computer microscopy: Selecting the optimal focus algorithm. Microsc. Res. Technol. 2004, 65, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Nathaniel, N.K.C.; Neow, P.A.; Ang, M.H., Jr. Practical issues in pixel-based autofocusing for machine vision. In Proceedings of the 2001 ICRA. IEEE International Conference on Robotics and Automation (Cat. No.01CH37164), Seoul, Korea, 21–26 May 2001; pp. 2791–2796. [Google Scholar]
- Sun, M.; Zong, G.; Yu, Z.; Bi, S.; Yu, J. Automatic focusing system of micro-vision based on image analysis. J. Beijing Univ. Aeronaut. Astronaut. 2005, 31, 192–196. [Google Scholar]
- Ren, S.-G.; Li, J.-W.; Xie, L.L. Automatic focusing technique based on gray scale difference method. Opto-Electron. Eng. 2003, 2, 015. [Google Scholar]
- Wang, Z.; Latt, W.T.; Tan, S.Y.M.; Ang, W.T. Visual servoed three-dimensional cell rotation system. IEEE Trans. Biomed. Eng. 2015, 62, 2498–2507. [Google Scholar] [CrossRef]
- Zhuang, S.; Lin, W.; Gao, H.; Shang, X.; Li, L. Visual servoed zebrafish larva heart microinjection system. IEEE Trans. Ind. Electron. 2017, 64, 3727–3736. [Google Scholar] [CrossRef]
- Feng, L.; Song, B.; Zhang, D.; Jiang, Y.; Arai, F. On-chip Tunable Cell Rotation Using Acoustically Oscillating Asymmetrical Microstructures. Micromachines 2018, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fan, Z.; Ma, Z.; Zhao, H.; Guo, Y.; Hong, K.; Li, Y.; Liu, H.; Wu, D. Design and experimental research of a novel stick-slip type piezoelectric actuator. Micromachines 2017, 8, 150. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, X.; Che, X.; Fang, Y. Modeling of quantitative microinjection and adaptive control. In Proceedings of the 30th Chinese Control Conference CCC 2011, Chicago, IL, USA, 22–24 July 2011; pp. 6087–6092. [Google Scholar]
- Hom, C.L.; Shankar, N. A finite element method for electrostrictive ceramic devices. Int. J. Solids Struct. 1996, 33, 1757–1779. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, K.-T.; Roberts, K.P.; Bischof, J.C.; Nelson, B.J. Mechanical property characterization of mouse zona pellucida. IEEE Trans. Nanobiosci. 2003, 2, 279–286. [Google Scholar] [CrossRef]
- Sun, Y.; Nelson, B.J. MEMS capacitive force sensors for cellular and flight biomechanics. Biomed. Mater. 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Y.; Wang, W.; Lansdorp, B.M. Vision-based cellular force measurement using an elastic microfabricated device. J. Micromech. Microeng. 2007, 17, 1281–1288. [Google Scholar] [CrossRef]
- Liu, X.; Kim, K.; Zhang, Y.; Sun, Y. Nanonewton force sensing and control in microrobotic cell manipulation. Int. J. Robot. Res. 2009, 28, 1065–1076. [Google Scholar] [CrossRef]
- Kim, D.-H.; Yun, S.; Kim, B. Mechanical force response of single living cells using a microrobotic system. In Proceedings of the IEEE International Conference on Robotics and Automation, New Orleans, LA, USA, 26 April–1 May 2004; Volume 5, pp. 5013–5018. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zappe, S.; Bernstein, R.W.; Sahin, O.; Chen, C.C.; Fish, M.; Scott, M.P.; Solgaard, O. Micromachined silicon force sensor based on diffractive optical encoders for characterization of microinjection. Sens. Actuators A Phys. 2004, 114, 197–203. [Google Scholar] [CrossRef]
- Muntwyler, S.; Beyeler, F.; Nelson, B.J. Three-axis micro-force sensor with tunable force range and sub-micronewton measurement uncertainty. Proc. IEEE Int. Conf. Robot. Autom. 2010, 3165–3170. [Google Scholar] [CrossRef]
- Feng, J.-Y.; Ye, X.-Y.; Chen, F.; Shang, Y.-F. Interferometric displacement measurement of microcantilevers based on integrated dual gratings. Guangxue Jingmi Gongcheng/Opt. Precis. Eng. 2012, 20. [Google Scholar] [CrossRef]
- Ergenc, A.F.; Olgac, N. Micro-pipette Motion Detection by using Optical Fiber Sensors. In Proceedings of the IEEE 31st Annual Northeast Bioengineering Conference, Hoboken, NJ, USA, 2–3 April 2005; pp. 258–259. [Google Scholar]
- Karimirad, F.; Shirinzadeh, B.; Zhong, Y.; Smith, J.; Mozafari, M.R. Modelling a Precision Loadcell using Neural Networks for Vision–Based Force Measurement in Cell Micromanipulation. In Proceedings of the 2013 IEEE/ASME International Conference on Advanced Intelligent Mechatronics, Wollongong, Australia, 9–12 July 2013; pp. 106–110. [Google Scholar]
- Tan, Y.; Sun, D.; Huang, W.; Cheng, S.H. Mechanical modeling of biological cells in microinjection. IEEE Trans. Nanobiosci. 2008, 7, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sit, P.S.; Spector, A.A.; Lue, A.J.C.; Popel, A.S.; Brownell, W.E. Micropipette aspiration on the outer hair cell lateral wall. Biophys. J. 1997, 72, 2812–2819. [Google Scholar] [CrossRef] [Green Version]
- Spector, A.A.; Brownell, W.E.; Popel, A.S. A model for cochlear outer hair cell deformations in micropipette aspiration experiments: An analytical solution. Ann. Biomed. Eng. 1996, 24, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sun, D.; Liu, C.; Cheng, S.H.; Liu, Y.H. A force control based cell injection approach in a bio-robotics system. In Proceedings of the 2009 IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; pp. 3443–3448. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Han, M.L.; Vidyalakshmi, J.; Shee, C.Y.; Ang, W.T. Automatic control of mechanical forces acting on cell biomembranes using a vision-guided microrobotic system in computer microscopy. J. Microsc. 2009, 236, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Yanagimachi, R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995, 52, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Dai, C.; Liu, J.; Wang, X.; Luu, D.K.; Zhang, Z.; Ru, C.; Zhou, C.; Tan, M.; Pu, H.; et al. A Flexure-Guided Piezo Drill for Penetrating the Zona Pellucida of Mammalian Oocytes. IEEE Trans. Biomed. Eng. 2018, 65, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Mills, J.K.; Sun, D. A universal piezo-driven ultrasonic cell microinjection system. Biomed. Microdevices 2011, 13, 743–752. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Hou, L.; Mu, L.; Zhu, L. Femtoliter micro injector using digital microfluidic control. Conf. Microfluid. BioMEMS Med. Microsyst. II 2004, 5345, 220–229. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, W.; Beebe, D.J. Microfluidic valve with cored glass microneedle for microinjection. Lab Chip 2003, 3, 164. [Google Scholar] [CrossRef]
- Kim, J.A.; Cho, K.; Shin, M.S.; Lee, W.G.; Jung, N.; Chung, C.; Chang, J.K. A novel electroporation method using a capillary and wire-type electrode. Biosens. Bioelectron. 2008, 23, 1353–1360. [Google Scholar] [CrossRef]
- Sharma, S.; Parvez, N.; Sharma, P.K. Iontophoresis—Models and Applications: A Review. Afr. J. Basic Appl. Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Fernandes, R.; Gertsenstein, M.; Perumalsamy, A.; Lai, I.; Chi, M.; Moley, K.H.; Greenblatt, E.; Jurisica, I.; Casper, R.F.; et al. Automated microinjection of recombinant BCL-X into mouse zygotes enhances embryo development. PLoS ONE 2011, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ge, H.; Wang, Y. Survey on the Methods of Image Segmentation Research. Comput. Eng. Sci. 2009, 31, 58–61. [Google Scholar]
- Canny, J. A Computational Approach to Edge Detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, PAMI-8, 679–698. [Google Scholar] [CrossRef]
- Duda, R.O.; Hart, P.E. Use of the Hough transformation to detect lines and curves in pictures. Commun. ACM 1972, 15, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Morales, D.A.; Bengoetxea, E.; Larrañaga, P. Selection of human embryos for transfer by Bayesian classifiers. Comput. Biol. Med. 2008, 38, 1177–1186. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, Q.; Tian, L.; Wu, Z. Object Detection and Tracking for a Vision Guided Automated Suspended Cell Injection Process. In Proceedings of the 2010 IEEE International Conference on Mechatronics and Automation, Xi’an, China, 4–7 August 2010; pp. 1760–1764. [Google Scholar]
- Liu, X.; Fernandes, R.; Jurisicova, A.; Casper, R.F.; Sun, Y. In situ mechanical characterization of mouse oocytes using a cell holding device. Lab Chip 2010, 10, 2154. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Mattos, L.; Grant, E.; Thresher, R. Speeding up video processing for blastocyst microinjection. IEEE Int. Conf. Intell. Robot. Syst. 2006, 5825–5830. [Google Scholar] [CrossRef]
- Wang, W.H.; Liu, X.Y.; Sun, Y. High-throughput automated injection of individual biological cells. IEEE Trans. Autom. Sci. Eng. 2009, 6, 209–219. [Google Scholar] [CrossRef]
- Wang, W.H.; Liu, X.Y.; Sun, Y. Autonomous Zebrafish Embryo Injection Using a Microrobotic System. In Proceedings of the 2007 IEEE International Conference on Automation Science and Engineering, Scottsdale, AZ, USA, 22–25 September 2007; pp. 363–368. [Google Scholar]
- Zong, G.H.; Sun, M.L.; Bi, S.S.; Dong, D. Research on wavelet based autofocus evaluation in micro-vision. Chin. J. Aeronaut. 2006, 19, 239–246. [Google Scholar] [CrossRef]
- Yu, B.; Yang, Z.; Tian, F.; Dong, J.; Jiang, B. Definition Evaluation of Auto Focus in Micro-vision Based on the Macro-micro Dual-drive. Trans. Chin. Soc. Agric. Mach. 2010, 6, 199–203. [Google Scholar]
- Chen, L.-G.; Wang, M.-Y.; Yang, Z.-L.; Rong, W.-B. Fast autofocus method for microscopic computer vision. Guangxue Jingmi Gongcheng/Opt. Precis. Eng. 2010, 18. [Google Scholar] [CrossRef]
- Zhou, L.P.; Sun, Z.J.; Zhang, Q. Auto-focusing and control of micro-vision system. Opt. Precis. Eng. 2013, 21, 807. [Google Scholar] [CrossRef]
- Zhang, Y.; Ballas, C.B.; Rao, M.P. Towards ultrahigh throughput microinjection: MEMS-based massively-parallelized mechanoporation. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 594–597. [Google Scholar] [CrossRef]
- Anis, Y.H.; Holl, M.R.; Meldrum, D.R. Automated selection and placement of single cells using vision-based feedback control. IEEE Trans. Autom. Sci. Eng. 2010, 7, 598–606. [Google Scholar] [CrossRef]
- Aoyama, H.; Chiba, N.; Fuchiwaki, O.; Misaki, D.; Usuda, T. Non-contact Bio Cell Manioulation by Nonlinear Micro Flow Around the Vibrated Pipette on Micro Robot. In Proceedings of the 21st Annual Meeting of the American Society for Precision Engineering, ASPE 2006, Monterey, CA, USA, 15–20 October 2006. [Google Scholar]
- Erdil, E.; Topalli, K.; Esmaeilzad, N.S.; Zorlu, Ö.; Kulah, H.; Aydin Civi, O. Reconfigurable nested ring-split ring transmitarray unit cell employing the element rotation method by microfluidics. IEEE Trans. Antennas Propag. 2015, 63, 1163–1167. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, C.; Muruganandam, R.; Ang, W.T.; Tan, S.Y.M.; Latt, W.T. Three-dimensional cell rotation with fluidic flow-controlled cell manipulating device. IEEE/ASME Trans. Mechatron. 2016, 21, 1995–2003. [Google Scholar] [CrossRef]
- Leung, C.; Lu, Z.; Zhang, X.P.; Sun, Y. Three-dimensional rotation of mouse embryos. IEEE Trans. Biomed. Eng. 2012, 59, 1049–1056. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Xiao, X. A novel flexure-based dual-arm robotic system for high-throughput biomanipulations on micro-fluidic chip. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 1531–1536. [Google Scholar] [CrossRef]
- Shin, Y.K.; Kim, Y.; Kim, J. Automated microfluidic system for orientation control of mouse embryos. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 496–501. [Google Scholar] [CrossRef]
- Huang, L.; Tu, L.; Zeng, X.; Mi, L.; Li, X.; Wang, W. Towards on-chip single cell manipulation of trap and rotation. In Proceedings of the 2016 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Paris, France, 18–22 July 2016. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Gong, Z.; Li, Y.-M. Micromanipulation by Means of optical Tweezers and Dielectrophoresis Technologies. Acta Laser Biol. Sin. 2007, 16, 119. [Google Scholar]
- Ouyang, M.; Zhang, G.; Li, W.J.; Liu, W.K. Self-induced rotation of pigmented cells by dielectrophoretic force field. In Proceedings of the 2011 IEEE International Conference on Robotics and Biomimetics, Karon Beach, Thailand, 7–11 December 2011; pp. 1397–1402. [Google Scholar] [CrossRef]
- Park, J.; Jung, S.-H.; Kim, Y.-H.; Kim, B.; Lee, S.-K.; Ju, B.; Lee, K.-L. An integrated bio cell processor for single embryo cell manipulation. In Proceedings of the 2004 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) (IEEE Cat. No.04CH37566), Sendai, Japan, 28 September–2 October 2004; pp. 242–247. [Google Scholar] [CrossRef]
- Jen, C.-P.; Chen, T.-W. Trapping of cells by insulator-based dielectrophoresis using open-top microstructures. Microsyst. Technol. 2009, 15, 1141–1148. [Google Scholar] [CrossRef]
- Hunt, T.P.; Westervelt, R.M. Dielectrophoresis tweezers for single cell manipulation. Biomed. Microdevices 2006, 8, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Kawaji, A.; Luangjarmekorn, P.; Fukuda, T.; Itoigawa, K. Three-dimensional bio-micromanipulation under the microscope. In Proceedings of the 2001 ICRA. IEEE International Conference on Robotics and Automation (Cat. No.01CH37164), Seoul, Korea, 21–26 May 2001; Volume 1, pp. 604–609. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lan, K.-C.; Chen, M.-K.; Wang, M.-H.; Jang, L.-S. Adjustable trapping position for single cells using voltage phase-controlled method. Biosens. Bioelectron. 2013, 49, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Mills, J.K. Development of a cell orientation control system for mouse embryo using electro-rotation. In Proceedings of the 2014 IEEE International Conference on Mechatronics and Automation, Tianjin, China, 3–6 August 2014; pp. 1085–1090. [Google Scholar] [CrossRef]
- Holzapfel, C.; Vienken, J.; Zimmermann, U. Rotation of cells in an alternating electric field theory and experimental proof. J. Membr. Biol. 1982, 67, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Benhal, P.; Chase, J.G.; Gaynor, P.; Oback, B.; Wang, W. AC electric field induced dipole-based on-chip 3D cell rotation. Lab Chip 2014, 14, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Basic Theory of Dielectrophoresis and Electrorotation. IEEE Eng. Med. Biol. Mag. 2003, 22, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, P.; Bian, S.; Shi, G.; Liu, P.; Zong, S.; Wang, W. A novel BioMEMS device for efficient on-chip single cell loading and 3D rotation. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 490–493. [Google Scholar] [CrossRef]
- Huang, L.; Tu, L.; Zeng, X.; Mi, L.; Li, X.; Wang, W. Study of a microfluidic chip integrating single cell trap and 3D stable rotation manipulation. Micromachines 2016, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.H.B.; Krenn, B.E.; Van Driel, R.; Kanger, J.S. Micro magnetic tweezers for nanomanipulation inside live cells. Biophys. J. 2005, 88, 2137–2144. [Google Scholar] [CrossRef]
- Feng, L.; Turan, B.; Ningga, U.; Arai, F. Three dimensional rotation of bovine oocyte by using magnetically driven on-chip robot. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 4668–4673. [Google Scholar] [CrossRef]
- Winkleman, A.; Gudiksen, K.L.; Ryan, D.; Whitesides, G.M.; Greenfield, D.; Prentiss, M. A magnetic trap for living cells suspended in a paramagnetic buffer. Appl. Phys. Lett. 2004, 85, 2411–2413. [Google Scholar] [CrossRef]
- Floyd, S.; Pawashe, C.; Sitti, M. Two-dimensional contact and noncontact micromanipulation in liquid using an untethered mobile magnetic microrobot. IEEE Trans. Robot. 2009, 25, 1332–1342. [Google Scholar] [CrossRef]
- Rodríguez-Villarreal, A.I.; Tarn, M.D.; Madden, L.A.; Lutz, J.B.; Greenman, J.; Samitier, J.; Pamme, N. Flow focussing of particles and cells based on their intrinsic properties using a simple diamagnetic repulsion setup. Lab Chip 2011, 11, 1240–1248. [Google Scholar] [CrossRef]
- Oberti, S.; Neild, A.; Dual, J. Manipulation of micrometer sized particles within a micromachined fluidic device to form two-dimensional patterns using ultrasound. J. Acoust. Soc. Am. 2007, 121, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Läubli, N.; Shamsudhin, N.; Ahmed, D.; Nelson, B.J. Controlled Three-dimensional Rotation of Single Cells Using Acoustic Waves. Procedia CIRP 2017, 65, 93–98. [Google Scholar] [CrossRef]
- Kim, D.-H.; Haake, A.; Sun, Y.; Neild, A.P.; Ihm, J.-E.; Dual, J.; Hubbell, J.A.; Ju, B.-K.; Nelson, B.J. High-throughput cell manipulation using ultrasound fields. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 4, 2571–2574. [Google Scholar] [CrossRef]
- Becattini, G.; Mattos, L.S.; Caldwell, D.G. A fully automated system for adherent cells microinjection. IEEE J. Biomed. Heal. Inf. 2014, 18, 83–93. [Google Scholar] [CrossRef]
- Liu, J.; Siragam, V.; Gong, Z.; Chen, J.; Fridman, M.D.; Leung, C.; Lu, Z.; Ru, C.; Xie, S.; Luo, J.; et al. Robotic adherent cell injection for characterizing cell-cell communication. IEEE Trans. Biomed. Eng. 2015, 62, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sun, D.; Liu, C.; Cheng, S.H. An adaptive impedance force control approach for robotic cell microinjection. In Proceedings of the 2008 IEEE/RSJ International Conference on Intelligent Robots and Systems, Nice, France, 22–26 September 2008; pp. 907–912. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Q. Position and force switching control of a piezo-driven microinjection system. In Proceedings of the 2016 35th Chinese Control Conference (CCC), Chengdu, China, 27–29 July 2016; pp. 6050–6055. [Google Scholar] [CrossRef]
- Tǎtar, O.; Mândru, D.; Ardelean, I. Development of mobile minirobots for in pipe inspection tasks. Mechanika 2007, 68, 60–64. [Google Scholar]
- Gong, F.F.; Shen, H.M.; Wang, Y.N. Structures and defects induced during annealing of sputtered near-equiatomic NiTi shape memory thin films Structures and defects induced during annealing of sputtered near-equiatomic NiTi shape memory thin films. Appl. Phys. Lett. 2013, 2656, 1–4. [Google Scholar] [CrossRef]
- Ishihara, H.; Aral, F.; Fukuda, T. Micro mechatronics and micro actuators. IEEE/ASME Trans. Mechatron. 1996, 1, 68–79. [Google Scholar] [CrossRef]
- Clark, A.E. Chapter 7 Magnetostrictive rare earth-Fe2 compounds. In Handbook of Ferromagnetic Materials; Elsevier: Amsterdam, The Netherlands, 1980; Volume 1, pp. 531–589. ISBN 1574-9304. [Google Scholar]
- Petit, L.; Lebrun, L.; Briot, R.; Gonnard, P. Estimation of available performances of ultrasonic motors. In Proceedings of the SPIE—The International Society for Optical Engineering, Lyon, France; 7 June 1996; p. 628. [Google Scholar]
- Kim, D.H.; Kim, B.; Kang, H. Development of a piezoelectric polymer-based sensorized microgripper for microassembly and micromanipulation. Microsyst. Technol. 2004, 10, 275–280. [Google Scholar] [CrossRef]
- Arai, F.; Kawaji, A.; Sugiyama, T.; Onomura, Y.; Ogawa, M.; Fukuda, T.; Iwata, H.; Itoigawa, K. 3D micromanipulation system under microscope. In Proceedings of the MHA’98 1998 International Symposium on Micromechatronics and Human Science.-Creation of New Industry-(Cat. No.98TH8388), Nagoya, Japan, 25–28 Novomber 1998; pp. 127–134. [Google Scholar] [CrossRef]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrozza, M.C.; Eisinberg, A.; Menciassi, A.; Campolo, D.; Micera, S.; Dario, P. Towards a force-controlled microgripper for assembling biomedical microdevices. J. Micromech. Microeng. 2000, 10, 271–276. [Google Scholar] [CrossRef]
- Zhang, R.; Chu, J.; Wang, H.; Chen, Z. A multipurpose electrothermal microgripper for biological micro-manipulation. Microsyst. Technol. 2013, 19, 89–97. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Fung, C.K.M.; Elhajj, I.; Li, W.J.; Xi, N. A 2-D PVDF force sensing system for micro-manipulation and micro-assembly. In Proceedings of the 2002 IEEE International Conference on Robotics and Automation (Cat. No.02CH37292), Washington, DC, USA, 11–15 May 2002; pp. 1489–1494. [Google Scholar]
- Xie, Y.; Sun, D.; Tse, H.Y.G.; Liu, C.; Cheng, S.H. Force sensing and manipulation strategy in robot-assisted microinjection on zebrafish embryos. IEEE/ASME Trans. Mechatron. 2011, 16, 1002–1010. [Google Scholar] [CrossRef]
- Pelham, R.J.; Wang, Y.-L. High Resolution Detection of Mechanical Forces Exerted by Locomoting Fibroblasts on the Substrate. Mol. Biol. Cell 1999, 10, 935–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembo, M.; Wang, Y.L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 1999, 76, 2307–2316. [Google Scholar] [CrossRef]
- Yang, M.T.; Sniadecki, N.J.; Chen, C.S. Geometric considerations of micro- To nanoscale elastomeric post arrays to study cellular traction forces. Adv. Mater. 2007, 19, 3119–3123. [Google Scholar] [CrossRef]
- Ghibaudo, M.; Di Meglio, J.-M.; Hersen, P.; Ladoux, B. Mechanics of cell spreading within 3D-micropatterned environments. Lab Chip 2011, 11, 805–812. [Google Scholar] [CrossRef]
- Sniadecki, N.J.; Anguelouch, A.; Yang, M.T.; Lamb, C.M.; Liu, Z.; Kirschner, S.B.; Liu, Y.; Reich, D.H.; Chen, C.S. Magnetic microposts as an approach to apply forces to living cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14553–14558. [Google Scholar] [CrossRef] [Green Version]
- Kleinke, D.K.; Uras, H.M. A magnetostrictive force sensor. Rev. Sci. Instrum. 1994, 65, 1699–1710. [Google Scholar] [CrossRef]
- Greminger, M.A.; Nelson, B.J. Vision-Based Force Measurement. IEEE Trans. Pattern Anal. Mach. Intell. 2004, 26, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-D.; Xu, D.; Shi, Y.-L.; Zhang, Z.-T. Development of Vision-Based Force Measurement. In Proceedings of the 31st Chinese Control Conference, Hefei, China, 25–27 July 2012; pp. 3769–3773. [Google Scholar]
- Xie, Y.; Sun, D.; Liu, C. Penetration Force Measurement and Control in Robotic Cell Microinjection. In Proceedings of the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems, St.Louis, MI, USA, 11–15 October 2009; pp. 4701–4706. [Google Scholar]
- Wang, G.; Xu, Q. Design and development of a piezo-driven microinjection system with force feedback. Adv. Robot. 2017, 31, 1349–1359. [Google Scholar] [CrossRef]
- Hiramoto, Y. Mechanical properties of sea urchin eggs. I. Surface force and elastic modulus of the cell membrane. Exp. Cell Res. 1963, 32, 59–75. [Google Scholar] [CrossRef]
- Nakamura, S.; Hiramoto, Y. Mechanical properties of the cell surface in starfish eggs. Dev. Growth Differ. 1978, 20, 317–327. [Google Scholar] [CrossRef]
- Ladjal, H.; Hanus, J.-L.; Ferreira, A. Methodologies of dynamic cell injection techniques using FEM biomechanical modeling. In Proceedings of the 2008 2nd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Scottsdale, AZ, USA, 19–22 October 2008; pp. 631–636. [Google Scholar] [CrossRef]
- Ergenc, A.F.; Li, M.W.; Toner, M.; Biggers, J.D.; Lloyd, K.C.K.; Olgac, N. Rotationally oscillating drill (Ros-Drill©) for mouse ICSI without using mercury. Mol. Reprod. Dev. 2008, 75, 1744–1751. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, M.; Feng, X.; Wang, Y.N.; Zhao, B.; Zhao, X. Automatic Operating Process for Zebrafish Embryo Injection. Int. J. Intell. Mechatron. Robot. 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Yang, Y.; Chen, J. Injection Volume Control by Thermal Way in Transgenic DNA Micro-Injection System. Chin. J. Mech. Eng. 2004, 17, 20–21. [Google Scholar] [CrossRef]





| Experimental Operations | Technical Parts Involved | Main Tasks | Key Problems to Resolve |
|---|---|---|---|
| 1. Immobilize zebrafish embryos and detect their positions | Cell immobilization, cell detection, and tracking | • Cell immobilization [40,43,44,45,46] • Cell detection [41,47,48,49,50,51,52,53] • Microinjection needle detection [39,48,53,54,55] | To avoid damaging the cell structure and improve the operational efficiency |
| Rapid automatic focusing | • Image sharpness evaluation function [53,56] • Focus position search • Image sharpness global maximization search strategy [57,58,59] | ||
| 2. Recognize cell postures based on microscopic visuals and adjust the cell postures | Cell posture adjustment | Contact [42,60] and non-contact: micro-fluid, dielectric electrophoresis, magnetic field method, ultrasonic method | To resolve the problem in cell posture adjustment during the pre-puncturing stage, so that the injection needle can be kept away from the first polar body and guided to the ideal injection site |
| Visual servoing control | • Position-based visual servoing control [60,61] • Image-based visual servoing control [39] • Trajectory planning [41] | ||
| 3. Perform rapid and effective puncture and quantitative injection of cells using a holding pipette and an injection needle-driving device | Actuator | • Piezoelectric ceramics [62,63,64] • Electrostrictive ceramics [65] • Other types of actuators are shown in Table 3 | To ensure that the changes in the relationship between the applied force and chorion deformations caused by chorion softening during zebrafish embryo development do not affect the puncturing mechanism |
| Sensor detection | • Micro-force sensor [38,66,67,68,69,70,71,72] • Micro-displacement sensor [73,74] • Visual sensor [75] | ||
| Cell models | • Young’s modulus [37,76] • Shear modulus [77,78] • Other cell models [64,79,80] | ||
| Puncturing mechanism | • Pulse puncturing [81] • Drilling movement [36,82] • Lateral vibration movement [83] | ||
| Microinjection | • Capillary pressure injection [1] • Pulse pressure injection [84,85] • Balanced pressure injection [45] • Capillary electrophoresis [86] • Capillary iontophoresis [87] |
| Method | Operational Principles | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|
| Mechanical contact method | Continuously hold and release the cell to adjust its position and posture | • Easy to operate • No additional equipment required | • Time consuming • Inefficient | [103] | |
| Use an injection needle to pluck the cells that are held in the holding tube | • Simple to operate • No additional equipment required | • Cell vulnerability | [104] | ||
| Use a rotating device and a visual servoing system together to position the rotating cell at three points | • The ability to automatically adjust the cell position and posture | • Slightly lower operational efficiency | [60] | ||
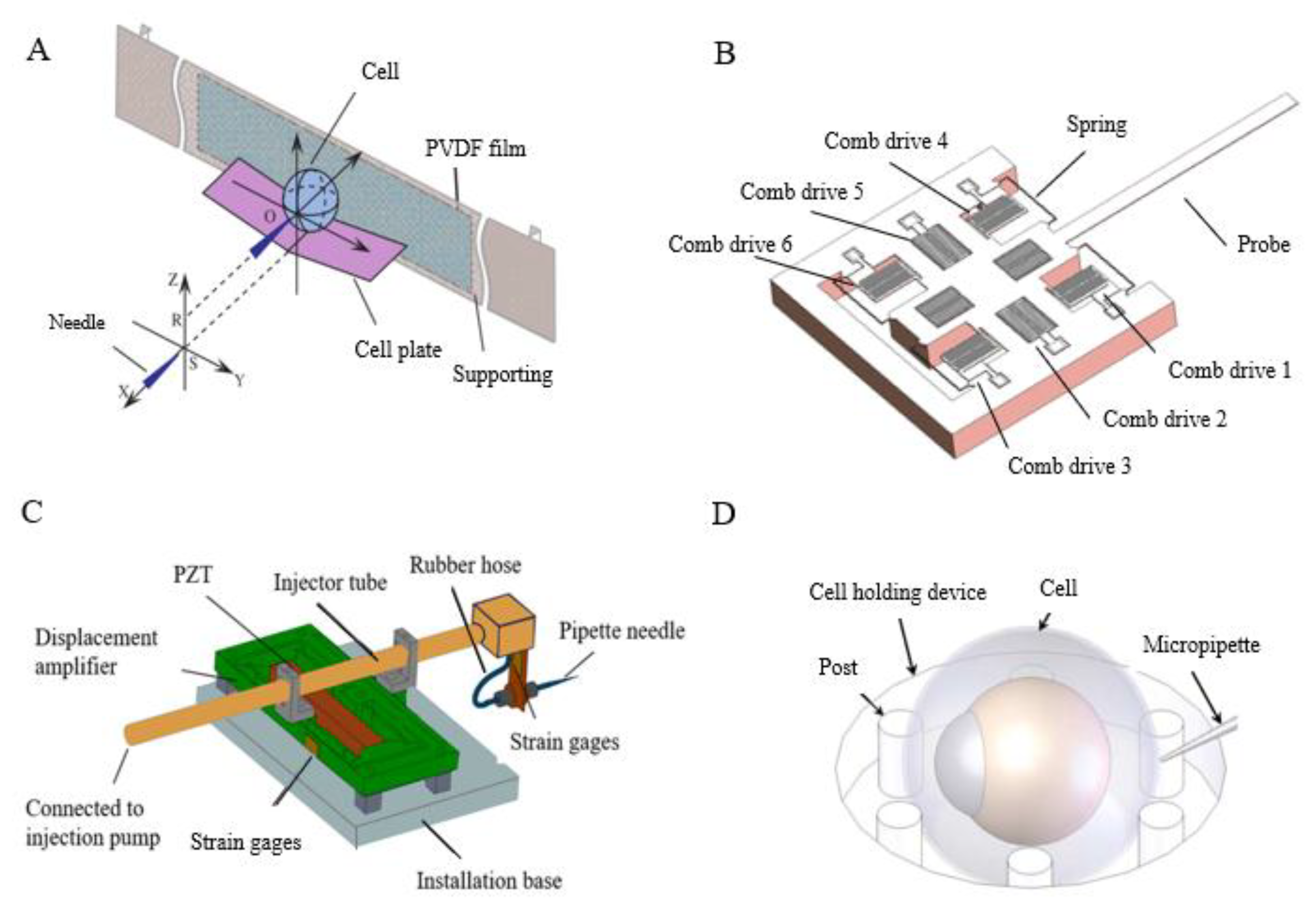
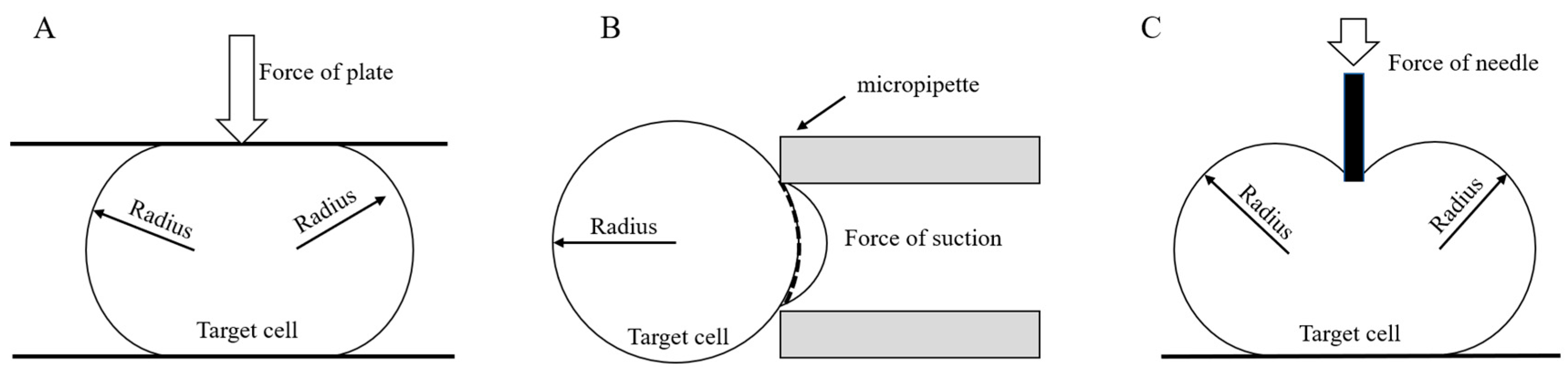
| Non-contact method | Microfluidics | Fluid flow characteristics and interaction between forces | • Minor damage to cells • Rotation of cells to any position | • Complicated debugging • Low experimental efficiency | [42,105,106,107,108,109,110] |
| Dielectrophoresis | Electric field force | • Fast operation • High positioning accuracy | • The influence of electric fields on the cells makes it difficult to set up a system | [111,112,113,114,115,116,117,118,119,120,121,122,123,124] | |
| Magnetic field | Magnetic force | • Easy to operate and control | • The influence of magnetic fields on the cells makes it difficult to set up a system. | [125,126,127,128,129] | |
| Ultrasonic | The action of acoustic radiation | • Easy to operate | • High local pressure and heat | [62,130,131] | |
| Type | Operational Principles | Performance Features | Precision | References |
|---|---|---|---|---|
| Direct current motor | Electromagnetic effect | • Fast response but large force and displacement | Submicron | [137] |
| Piezoelectric ceramics | Piezoelectric effect | • Applicable in a wide range of frequencies but insensitive to temperature • No magnetic field influence but exhibits hysteresis | Sub-nanometer | [62,63,64,134,135] |
| Electrostrictive ceramic | Electrically induced effect | • Fast response but small force and displacement | Sub-nanometer | [65] |
| Shape memory alloy | Metal phase change | • Slow response and small force and displacement | Nano | [138] |
| Magnetostrictive material | Magnetic effect | • Good reliability, simple driving mode but exhibits hysteresis, low precision, poor response, and a tendency to overheat | Sub-nanometer | [139] |
| Giant magnetostrictive material | Magnetic effect | • Fast response but large force and displacement | Sub-nanometer | [140] |
| Ultrasonic motor | Piezoelectric effect Ultrasonic oscillation | • Fast response speed but large force and displacement | 10 nm (linear) Seconds (rotary type) | [141] |
| Type | Detection Principle | Advantages | Disadvantages | Precision | References |
|---|---|---|---|---|---|
| Piezoelectric sensor | Piezoelectric effect of piezoelectric materials | • Wide band • High sensitivity • High signal-to-noise ratio • Simple structure | • Poor output direct current response • Unsuitable for static measurement | μN–sub μN | [142] |
| Piezoresistive sensor | The relationship between force and resistance | • Proven detection method and good frequency response | • Modest signal-to-noise ratio • Complex structure • Temperature-sensitive | mN–sub mN | [143,144] |
| Capacitive transducer | The relationship between force and capacitance change between plates | • Simple structure • Good stability • High sensitivity | • Highly nonlinear strain | μN–sub μN | [66,69] |
| Strain gauge | The relationship between the shape variable and stress | • Simple structure | • Modest detection accuracy | mN | [145] |
| Polyvinylidene fluoride force transducer | Piezoelectric effect | • High linearity • High signal-to-noise ratio • Suitable for dynamic force induction | • Cannot work under high temperature | sub μN | [146,147,148] |
| Polydimethylsiloxane (PDMS) patch force transducer | Deviate from PDMS posts | • Easy to fabricate and can be used to study different types of cell characteristics | • Difficult to prepare | nN | [68,149] |
| Cantilever-based force sensor | Beam deflection | • Easy to fabricate and allows vision-enabled measurement of structural deformation | • Low sensitivity and precision • Difficult to detect static forces | nN | [150,151] |
| Cantilever force transducer with an indentation probe | Beam deflection | • Simple structure • High resolution, can be used to study the force response of cells under large deformation | • Complex mechanical structure | nN–pN | [71,152,153,154] |
| Miniature camera-based force sensor | Change in diffraction efficiency | • High resonance frequency | • Difficult to fabricate • Complex optical setup | N/A | [38] |
| Magnetic effect-based sensor | The compressive magnetic effect of magnetic materials | • High measurement accuracy | • Prone to be affected by the surrounding magnetic field | nN | [155] |
| Vision-based sensor | The relationship between stress and image deviation | • Non-contact measurement | • Strict requirements for image processing precision | mN–μN | [156,157] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Sun, H.; Sha, X.; Gu, L.; Zhan, Z.; Li, W.J. A Review of Automated Microinjection of Zebrafish Embryos. Micromachines 2019, 10, 7. https://doi.org/10.3390/mi10010007
Zhao Y, Sun H, Sha X, Gu L, Zhan Z, Li WJ. A Review of Automated Microinjection of Zebrafish Embryos. Micromachines. 2019; 10(1):7. https://doi.org/10.3390/mi10010007
Chicago/Turabian StyleZhao, Yuliang, Hui Sun, Xiaopeng Sha, Lijia Gu, Zhikun Zhan, and Wen J. Li. 2019. "A Review of Automated Microinjection of Zebrafish Embryos" Micromachines 10, no. 1: 7. https://doi.org/10.3390/mi10010007




