A Novel Fabricating Process of Catalytic Gas Sensor Based on Droplet Generating Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.1.1. Droplet Generating Method Based on Pulse Inertia Force
2.2.2. Manufacturing Process of the Pellistor
3. Results and Discussion
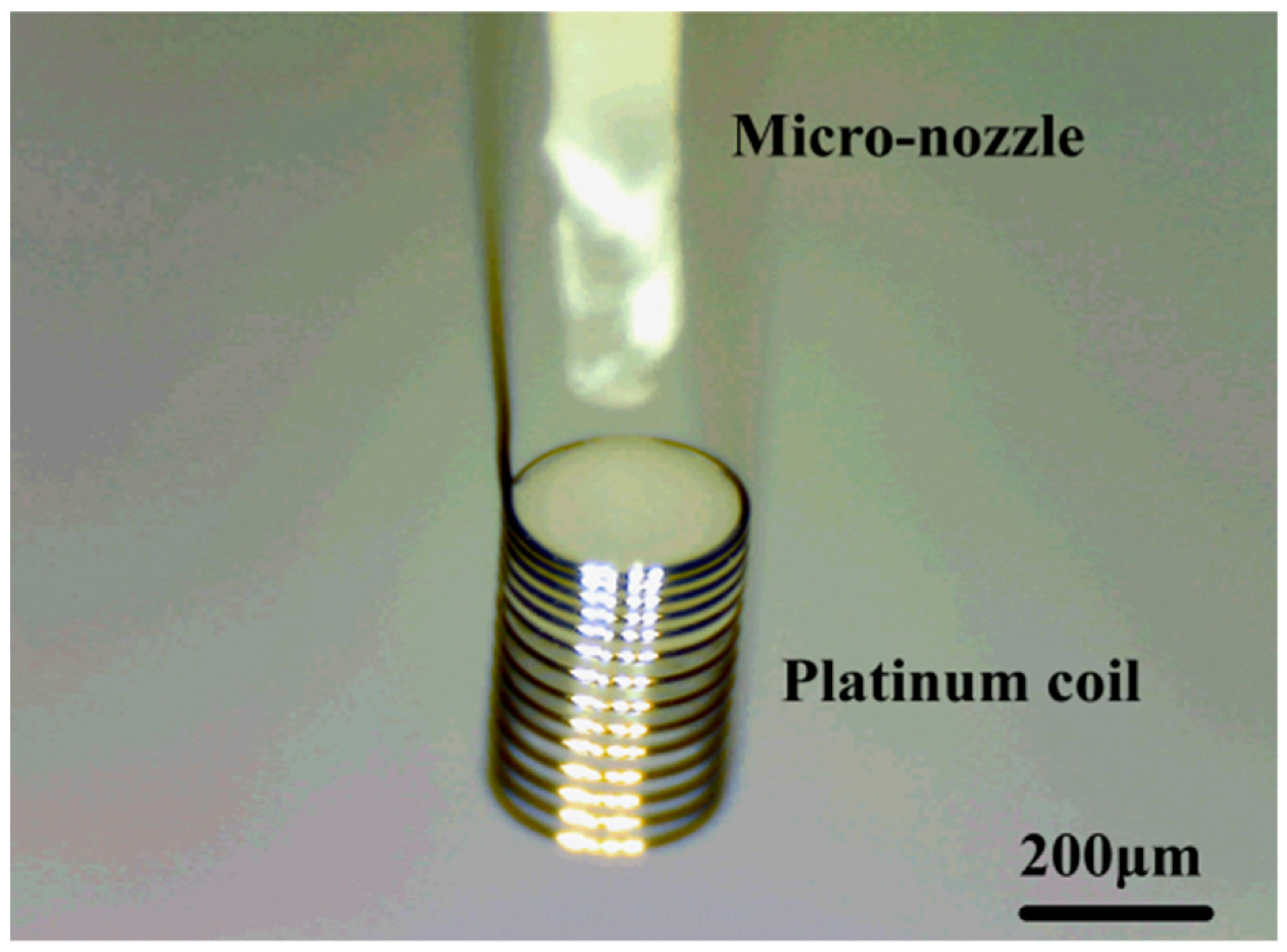
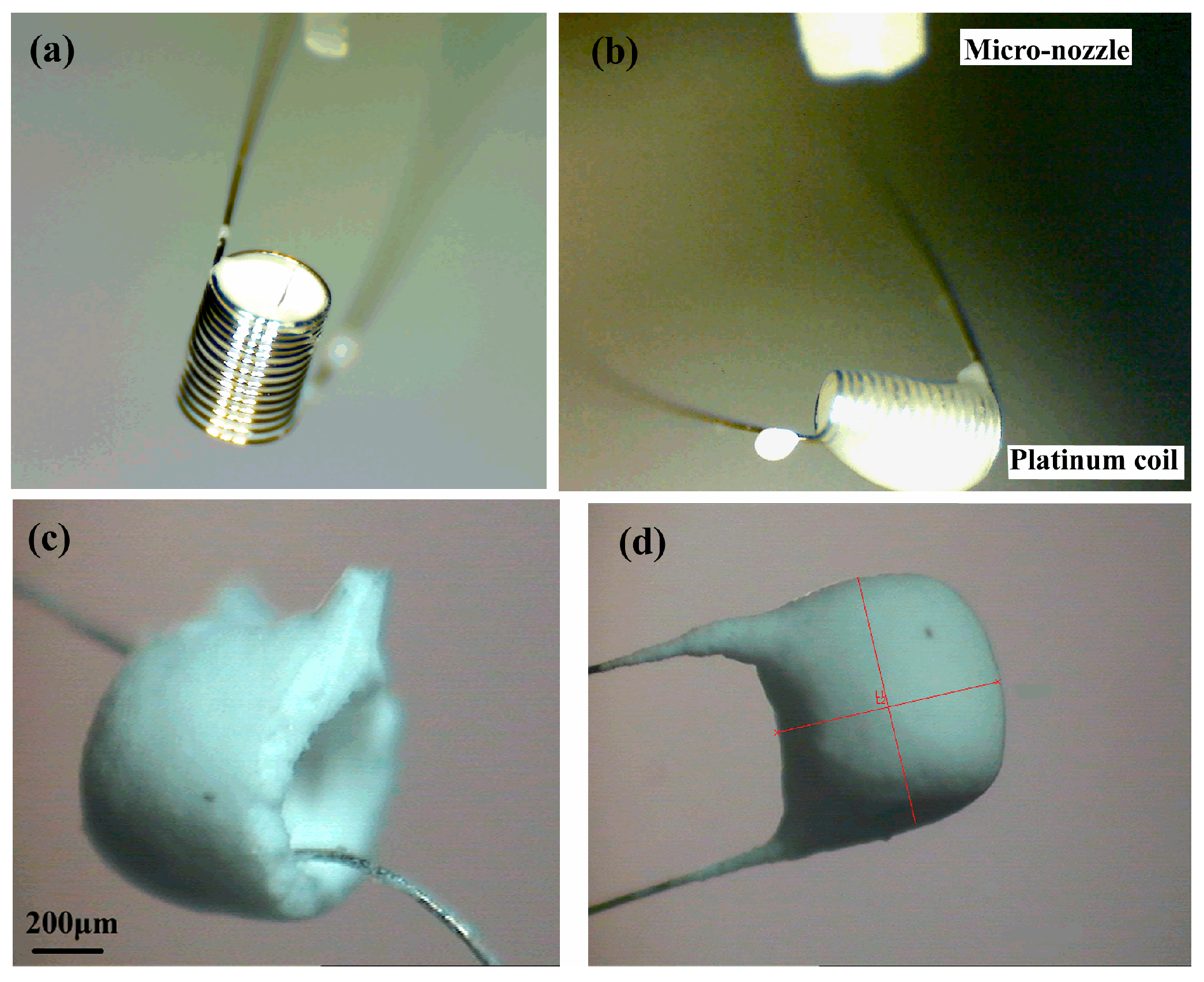
3.1. Pattern of the Pellistor
3.2. Performance of the Pellistor
3.2.1. Flameless Catalytic Combustion
3.2.2. Activation of the Pellistor
3.2.3. Sensitivity of the Pellistor
3.2.4. Power Consumption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, M.; Yadav, B.C.; Ranjan, A.; Sonker, R.K.; Kaur, M. Detection of liquefied petroleum gas below lowest explosion limit (LEL) using nanostructured hexagonal strontium ferrite thin film. Sens. Actuators B 2017, 249, 96–104. [Google Scholar] [CrossRef]
- Fonollosa, J.; Solorzano, A.; Marco, S. Chemical sensor systems and associated algorithms for fire detection: A review. Sensors 2018, 18, 553. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, A.; Alijani, V.; Sangaletti, L.; D’Arsiè, L. Advanced promising routes of carbon/metal oxides hybrids in sensors: A review. Electrochim. Acta 2018, 266, 139–150. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Dong, H.P.; Xia, S.H. A novel micro-pellistor based on nanoporous alumina beam support. J. Electron. 2012, 29, 469–472. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, M.M.; Zhang, D.; Gao, Z. Improving the Performance of Catalytic Combustion Type Methane Gas Sensors Using Nanostructure Elements Doped with Rare Earth Cocatalysts. Sensors 2011, 11, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.T. Solid state gas sensors. Meas. Sci. Technol. 1997, 8, 223–227. [Google Scholar] [CrossRef]
- Famuyiro, S. Use of combustible gas detectors in Safety Instrumented Systems-A practical application case study. J. Loss Prevent Proc. 2018, 54, 333–339. [Google Scholar] [CrossRef]
- Ma, H.; Qin, S.; Wang, L.; Wang, G.; Zhao, X.; Ding, E. The study on methane sensing with high-temperature low-power cmos compatible silicon microheater. Sens. Actuators B 2017, 244, 17–23. [Google Scholar] [CrossRef]
- Weinberg, F.J. Combustion temperature: The future? Nature 1971, 233, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Kumar, A.; Huang, Y.; Chan, S.; Fan, X. Flameless combustion with liquid fuel: A review focusing on fundamentals and gas turbine application. Appl. Energy 2017, 193, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Tajima, K.; Shin, W.; Izu, N.; Matsubara, I.; Murayama, N. Effect of pt/alumina catalyst preparation method on sensing performance of thermoelectric hydrogen sensor. J. Mater. 2006, 41, 2333–2338. [Google Scholar] [CrossRef]
- Brauns, E.; Morsbach, E.; Kunz, S.; Baeumer, M.; Lang, W. Temperature modulation of a catalytic gas sensor. Sensors 2014, 14, 20372–20381. [Google Scholar] [CrossRef] [PubMed]
- Brauns, E.; Morsbach, E.; Kunz, S.; Bäumer, M.; Lang, W. A fast and sensitive catalytic gas sensors for hydrogen detection based on stabilized nanoparticles as catalytic layer. Sens. Actuators B 2014, 193, 895–903. [Google Scholar] [CrossRef]
- Bársony, I.; Ádám, M.; Fürjes, P.; Lucklum, R.; Hirschfelder, M.; Kulinyi, S. Efficient catalytic combustion in integrated micropellistors. Meas. Sci. Technol. 2009, 20, 124009. [Google Scholar] [CrossRef]
- Sofija, V.M.; Frédéric, C.; Jürgen, V.; Jan, G.P.; David, N. Droplet generation and characterization using a piezoelectric droplet generator and high speed imaging techniques. Crop Prot. 2015, 69, 18–27. [Google Scholar]
- Wang, T.; Lin, J.; Lei, Y.; Guo, X.; Fu, H.; Zhang, N. Dominant factors to produce single droplet per cycle using drop-on-demand technology driven by pulse electromagnetic force. Vacuum 2018, 156, 128–134. [Google Scholar] [CrossRef]
- Ma, M.; Wei, X.; Shu, X.; Zhang, H. Producing solder droplets using piezoelectric membrane-piston-based jetting technology. J. Mater. Process. Tech. 2019, 263, 233–240. [Google Scholar] [CrossRef]
- Wang, H.C.; Hou, L.Y.; Zhang, W.Y. A drop-on-demand droplet generator for coating catalytic materials on microhotplates of micropellistor. Sens. Actuators B 2013, 183, 342–349. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Lei, Y.; Guo, X.; Fu, H. Droplets generator Formation and control of main and satellite droplets. Colloids Surf. A 2018, 558, 303–312. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Yu, Y.; Xu, J.; Sun, J.; Lu, G. Enhanced sensing performance of catalytic combustion methane sensor by using Pd nanorod/γ-Al2O3. Sens. Actuators B 2011, 160, 1091–1097. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Zhang, T.; Wang, H.; Tang, C.; Zhang, L. A Novel Fabricating Process of Catalytic Gas Sensor Based on Droplet Generating Technology. Micromachines 2019, 10, 71. https://doi.org/10.3390/mi10010071
Wu L, Zhang T, Wang H, Tang C, Zhang L. A Novel Fabricating Process of Catalytic Gas Sensor Based on Droplet Generating Technology. Micromachines. 2019; 10(1):71. https://doi.org/10.3390/mi10010071
Chicago/Turabian StyleWu, Liqun, Ting Zhang, Hongcheng Wang, Chengxin Tang, and Linan Zhang. 2019. "A Novel Fabricating Process of Catalytic Gas Sensor Based on Droplet Generating Technology" Micromachines 10, no. 1: 71. https://doi.org/10.3390/mi10010071



